Abstract
Since 2006, over 600 biodegradable vascular scaffolds (BVS) have been implanted worldwide in clinical trials such as ABSORB cohort A and B, ABSORB Extend and ABSORB II RCT. Due to completely changed construction and mechanical properties of BVS, the choice of proper scaffold diameter and its implantation differ significantly from those used in the case of metal stents (bare metal stent (BMS) or drug eluting stent (DES)). Furthermore, all data concerning BVS efficacy and safety come from clinical trials, conducted in a selected group of patients. In 2012 BVS ABSORB™ was approved as the first biodegradable scaffold for the treatment of coronary artery disease in EU and other countries, with a limitation of use only for experienced and trained interventional cardiologists. As one of the most experienced clinical centers in Europe and the first one that in 2006 implanted BVS ABSORB™ in Poland we have a great pleasure and honor to share our experience with interventional cardiologists who would like to prepare for BVS ABSORB™ implantation in their centers. In this article we wanted to summarize the clinical data from already finished and ongoing trials, give a short overview of patient selection, and provide a detailed description of the implantation process with tips which could be helpful during BVS use.
Keywords: biodegradable vascular scaffold, percutaneous coronary intervention
Introduction
Stents made of bioresorbable material (poly-L-lactic acid – PLLA) were used for the first time in humans in a clinical trial conducted by Japanese scientists at the end of the 20th century. Between August 1998 and April 2000 a total of 50 patients (63 de novo lesions) were treated with implantation of 84 Igaki-Tamai® stents (bioresorbable, not drug-eluting). The results of a 10-year observation were published in 2012. There was one cardiac death, 6 deaths due to other reasons and 4 myocardial infarctions [1, 2]. The proportion of patients free from all-cause death, cardiac death or adverse cardiovascular events after 10 years was 87%, 98% and 50%, respectively. The frequency of repeated revascularization of the target vessel (target lesion) was 16% (16%) after 1 year, 18% (22%) after 5 years and 28% (38%) after 10 years from stent implantation. There were also 2 cases of definite in-stent thrombosis (according to ARC criteria) including one case of subacute and 1 case of very late thrombosis. The analysis of the control intravascular ultrasound (IVUS) studies allowed us to determine that the time of total stent absorption approximated 3 years [2–5]. The study began a breakthrough, which is currently considered as the “fourth revolution in invasive cardiology” [6]. This was mainly driven by the series of ABSORB studies (cohort A and B, ABSORB Extend, ABSORB II) conducted in 2006. The first of them, the ABSORB (cohort A and B) study, was aimed at clinical assessment of everolimus-eluting biodegradable vascular solutions (BVS) in the treatment of patients with de novo lesions in the native coronary arteries (Figure 1). The first phase of the study conducted in 2006 (cohort A) included 30 patients in 4 centers (6 patients were treated in the Catheterization Laboratory of the University Hospital in Cracow, the others in the Netherlands, Denmark and New Zealand) who during long-term observation (after 6 months and after 2 years) had control coronary angiography by means of additional methods of coronary artery imaging (IVUS, optical coherence tomography – OCT). A subgroup of patients underwent a non-invasive multislice computed tomography (MSCT) scan after 18 months. Current reports show good results of treatment after 5 years from BVS implantation [7, 8]. The MSCT examination performed after 18 months demonstrated that the mean degree of stenosis in the BVS was 19 ±9%. The proportion of adverse cardiovascular events during 2-year observation was very low – 3.3% (one patient with non-Q type myocardial infarction underwent a repeated PCI procedure of the previously treated vessel; the degree of stenosis at the site of the previously implanted BVS did not exceed 50% on quantitative angiography). There were no cases of in-stent thrombosis. Control coronary angiographies after 2 years from BVS implantation disclosed a late lumen loss of 0.48 ±0.28 mm and a mean stenosis degree of 27%, which is similar to observations from control examination after 6 months. The OCT study showed that 34.5% of the BVS struts underwent complete degradation, while other struts were at different levels of biodegradation and remained in complete apposition [9, 10]. There were no new adverse cardiovascular events during 5-year observation and dual antiplatelet therapy was discontinued in all patients without any influence on the frequency of major adverse cardiac events (MACE) [8]. The second phase of the study (cohort B) started in 2009 and included 101 patients (13 in the Cracow center). The polymer was slightly modified to increase its radial force and delay the first phase of degradation. The first 45 patients from cohort B were allocated to the group in which control coronary angiography was planned for 6 months and 2 years after the BVS implantation. In the other 56 patients these studies were planned to occur 1 year and 2 years after the procedure. Stenosis at the margins of the BVS was detected in 1 patient 6 months after stent implantation (restenosis rate of 2.4%). Control IVUS and OCT studies showed a decrease of the scaffold area by 2%, late lumen loss of 0.19 ±0.18 mm and percent stenosis of 5.4%. Additionally, the OCT examination demonstrated BVS coverage with neointima in 96.8% of cases. A malapposition of at least one strut initially observed in 12 patients was confirmed in only 3 cases after 6 months [11]. In the next 56 patients from cohort B of the ABSORB study control coronary angiography, IVUS and OCT studies were performed 12 months and 3 years after BVS implantation. The analysis of results of these examinations showed decrease of the scaffold area by 16.8% on IVUS and 20% on OCT, late lumen loss of 0.27 ±0.32 mm and percent stenosis of 1.94% (IVUS). The OCT examination demonstrated BVS coverage with neointima in 96.69% of cases. A malapposition of at least one strut initially observed in 18 patients was confirmed in only 4 cases after 12 months [12]. Among 101 patients there was a total of 3 cases of postprocedural increase of the necrotic markers and 3 cases of repeated revascularization. Therefore the rate of adverse cardiovascular events after 12 months was 5.9%. The results of ongoing control studies after 3 years of observation in both groups of patients are expected soon. After promising initial results of everolimus-eluting biodegradable scaffold implantations other studies of the ABSORB series were initiated – the Extend and the ABSORB II studies. The ABSORB Extend study is a non-randomized, single arm trial in which 2.5 mm and 3.0 mm diameter and 18 and 28 mm long BVS are implanted into maximally 2 coronary arteries. The study began in 2010 andincluded 13 patients from our center. Its ending is planned for the year 2015. The ABSORB II study, on the other hand, was initiated in 2011 and is the first randomized, single-blind trial, in which patients are randomly assigned to implantation of the ABSORB™ or the everolimus-eluting metallic stent XIENCE™.
Fig. 1.
Biodegradable vascular scaffold used respectively in cohort A and in cohort B of the ABSORB study
The concept of “regenerative therapy of coronary vessels” using the ABSORB™ scaffold
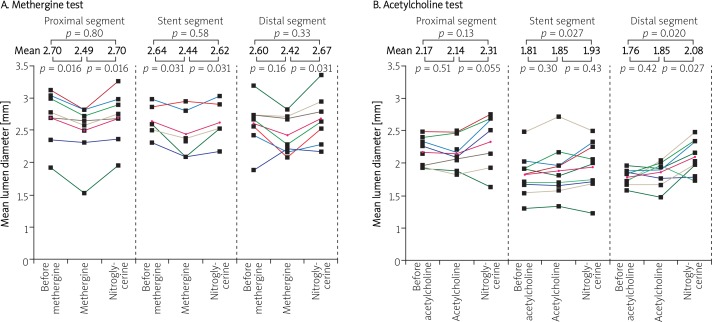
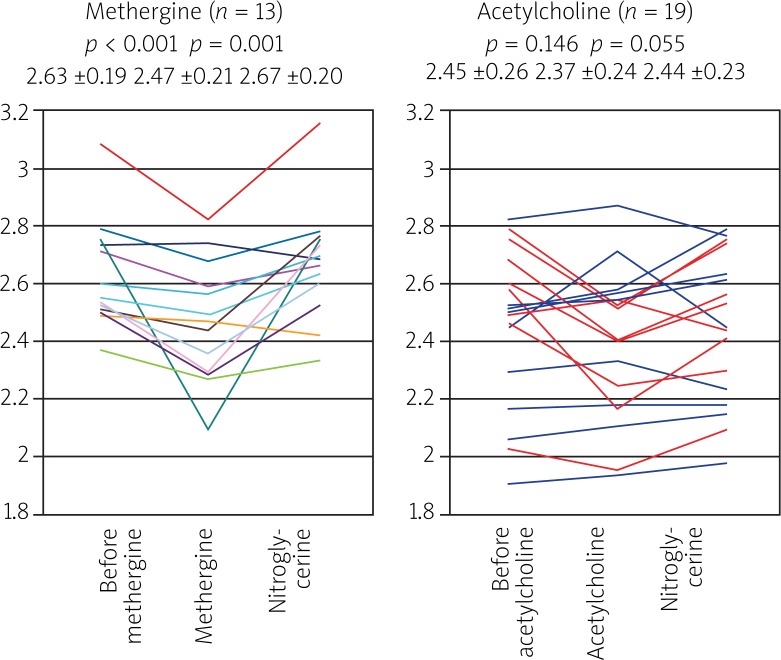
Available results of the ABSORB series of studies indicate that BVS: 1) provides a mechanical support for the coronary artery wall after the percutanous coronary intervention (PCI) procedure, 2) its biodegradation leads to attenuation of a chronic inflammatory reaction in the vascular wall, 3) may facilitate repeated percutaneous or surgical revascularization in case of atherosclerosis progression and 4) permits noninvasive control of patients with the use of imaging modalities such as MSCT or magnetic resonance imaging (MRI) without the risk of artifacts. Particularly interesting findings came from the examination of the vasomotor function after implantation of the ABSORB™ scaffold. Two years after BVS implantation patients from cohort A underwent testing with the use of acetylcholine (Ach) and methergine. Vasodilatation exceeding 3% of the vessel diameter after drug administration was found in 5 patients from the group subjected to the Ach test (n = 9). After administration of nitroglycerin (NTG) there was a significant enlargement of the segments covered with the scaffold as well as the distal segment. Patients who received methergine (n = 7) demonstrated contraction of the proximal segment of the vessel and the segment with implanted BVS with return to their normal dimensions after administration of NTG (Figure 2). The study confirmed return of vasomotor activity of the vascular segment with the implanted stent [9]. These observations were confirmed by tests performed in patients from cohort B, where return of vasomotor function of the treated coronary arteries was observed 12 months after BVS implantation (Figure 3) [12]. For these reasons the procedure of ABSORB™ DES BVS implantation has become a new method of invasive cardiology aimed not only at dilation of the narrowed coronary arteries (percutaneous angioplasty), but also at regeneration of the vessel, and therefore the term “PCI” is slowly being replaced by the term “vascular regenerative therapy – VRT” [6]. The term “regenerative therapy” means that with the use of the drug eluting stent (DES) BVS it will be possible to implant a vascular scaffold preventing acute vascular occlusion and the recoil phenomenon during angioplasty. In the following months, the scaffold releasing an anti-proliferative drug will prevent excessive in-stent neointimal hyperplasia and negative remodeling of the vessel at the site of implantation. However, after about 2 years the struts of resorbed scaffold will be replaced by proteoglycans, and after the next 2 years full integration of the DES BVS with the vessel wall will occur with properly functioning smooth muscle cells within the vascular wall. These observations (total replacement of the polymer by a tissue) were confirmed by the imaging studies (OCT) in patients from cohort A of the ABSORB study. Additionally there was an increase of the vessel lumen and positive remodeling at the site of the implanted DES BVS with return of the proper vasomotor function dependant on the endothelial response to acetylcholine and nitroglycerin.
Fig. 2.
The results of tests with methergine and acetylcholine in the proximal segment, in the segment with implanted BVS and in the distal segment, also after NTG administration – cohort A of the ABSORB study 2 years after PCI [9]
Fig. 3.
The results of tests with methergine and acetylcholine in the proximal segment, in the segment with implanted BVS and in the distal segment, also after NTG administration – cohort B of the ABSORB study 12 months after PCI (patients with vasodilatation after Ach are marked blue) [12]
Other technologies used in the construction of bioresorbable stents
Together with the advent of a biodegradable scaffold made of PLLA, research on the use of other materials undergoing absorption was undertaken. Currently, there are ongoing clinical trials with stents made of tyrosine polymers, acetylsalicylic acid (ASA) and metals: magnesium and iron. The DESolve NX study, which began in 2012, included 120 patients in 15 centers (3 in Poland) who received Novolimus-eluting DESolve™ stents. This stent is also made of lactic acid polymers. During long-term observation the patients will be subjected to control angiography 6 and 24 months after stent implantation and to control imaging studies (IVUS, OCT) aimed at assessment of the bioabsorption rate. The ReZolve™ stent made of sirolimus-eluting tyrosine polymer (REVA Medical, San Diego, California, USA) is an example of another stent currently being evaluated in clinical studies. Pre-clinical studies showed that this polymer has radial force, elasticity and visibility on X-ray similar to metallic stents. The RESTORE study began in 2012 and is planned to recruit 50 patients (including 2 centers in Poland). The assessment will include the occurrence of adverse cardiovascular events during 5-year observation. The Lekton Magic (Biotronic) stent is another bioresorbable stent made of magnesium alloy (WE 43) and a small percentage of other metals, including rare ones (less than 5%). The product of biodegradation of this stent is magnesium, which is completely indifferent to the body. The PROGRESS study published in 2007 aimed at the assessment of the efficacy and safety of biodegradable magnesium stent implantation in coronary arteries in humans. Late lumen loss during 4-month observation was 1.08 ±0.49 mm and percent stenosis increased to 48.4 ± 17.0%. The proportion of patients who suffered from adverse cardiovascular events after 4 months was 23.8% and repeated target lesion revascularization was necessary in 45% of patients after 12 months. During long-term observation there were no deaths, myocardial infarctions or episodes of in-stent thrombosis [13–15]. Currently, studies are being conducted on the revised concept of the stent, because it seems that the original stent was undergoing degradation too quickly and was not able to influence negative remodeling of the vessel during the first months after implantation. Additionally, it is planned to construct a drug-eluting stent that would control neointimal hyperplasia during the initial post-implantation phase.
The role of quantitative angiography (QCA on-line) in sizing of the ABSORB® scaffold
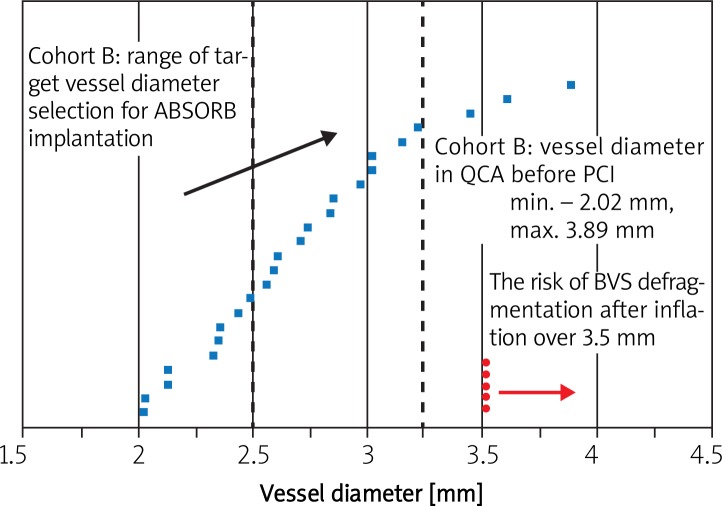
According to the producer's recommendations it is necessary to perform quantitative angiography (QCA on-line) for the sizing of the scaffold. This recommendation is based on the specific properties of the stent, which in contrast to metallic stents does not have malleable or ductile properties and is therefore not as stretchable as metallic stents and cannot be expanded beyond the specified limit. According to the protocol of the ABSORB cohort A study sizing of the first generation of anti-mitotic everolimus-eluting fully biodegradable stent was based on the visual angiographic assessment of the vessel size performed by an experienced operator. The next step (cohort B) also allowed visual vessel assessment, but the estimated vessel size had to fit within the range of 2.5-3.3 mm. A post-hoc quantitative angiography (QCA) analysis by an independent core laboratory demonstrated that only 48% of stents were properly sized (Figure 4). In most cases the stent was either over- or undersized (at that time only a 3.0 mm diameter stent was available) in comparison to average vessel diameter assessed with QCA (the RVD was < 2.5 mm in 40% of patients and > 3.3 mm in 17% of patients) [16]. While the implantation of a 3.0 mm diameter scaffold in smaller vessels (< 2.5 mm) was not associated with greater late lumen loss (LL) on angiography and OCT, or worse clinical results in comparison to larger vessels (> 2.5 mm), the use of scaffolds over 3.0 mm in diameter in too large vessels (> 3.3 mm) more often resulted in lack of adhesion of the scaffold to the vessel wall (malapposition) on the OCT [17, 18]. Therefore in another study – ABSORB Extend – the protocol recommended the use of QCA on-line in the selection of the scaffold diameter. An algorithm used to assess the vessel with a newly developed term, maximal lumen diameter in the reference segments (D-max), was proposed. After initial analysis using a standard protocol based on the reference vessel diameter (RVD), the assessment based on the QCA on-line should include the measurement of maximal lumen diameter (D max) in segments proximal and distal to the lesion at sites where anchoring of the implanted stent is planned. The distance between points of measurement should correspond to the length of the implanted device. Comparison of the stent size and vessel diameter based on visual assessment in cohort B (101 patients) and sizing based on D max measurements with QCA in the initial 101 patients from the ABSORB Extend study who also received 3.0 mm diameter scaffolds showed a higher percentage of compliance with the measurements obtained in the core laboratory (Figure 5). It was also found that D max is better in selection of the scaffold size in comparison to standard QCA measurement of the reference vessel diameter. The chance of oversizing was lower in the case of the measurement based on the D max assessment in comparison to the assessment based on RVD (2.9% vs. 16.7%, p = 0.002). Comparison of the QCA analysis in both studies showed a higher percentage of properly determined vessel diameters (69.4% vs. 47.1%, p = 0.001), a trend towards decrease of implantations in small (< 2.5 mm) vessels (26.9% vs. 39.2%, p = 0.057) and a significant decrease of implantations in large (> 3.3 mm) vessels (3.7% vs. 16.7%, p = 0.002) in the ABSORB Extend study in comparison to cohort B [16]. In our center, the QCA analysis used in the assessment of the vessel diameter before the implantation of biodegradable stents is usually done by means of a three dimensional QCA analysis (3D QCA) with CAAS 5 software (Pie Medical, Maastricht, The Netherlands), which minimizes the phenomenon of length shortening dependent on the projection used (so-called foreshortening) (Figure 6). Based on the delineated contour it is possible to accurately and reliably measure the length of an 18 mm or 28 mm long device which is to be implanted and to assess the proximal and distal D max values without the risk of shortening of the distance between both measurement points [19]. After the introduction of 2.5 mm and 3.5 mm diameter scaffolds to the protocol of the ABSORB Extend study, the algorithms of QCA and D max assessment in the ABSORB II study were adjusted to the new stent sizes. The currently proposed scheme used in selection of different ABSORB™ diameters is presented in Table 1. It should be noted that in the event of discrepant values of proximal and distal reference diameters, which can occur in the case of cone-shape vessels, the selection of the device size is based on the physician's decision.
Fig. 4.
Analysis by means of quantitative angiography (QCA) regarding sizing of the ABSORB™ scaffold in the ABSORB study – cohort A
Fig. 5.
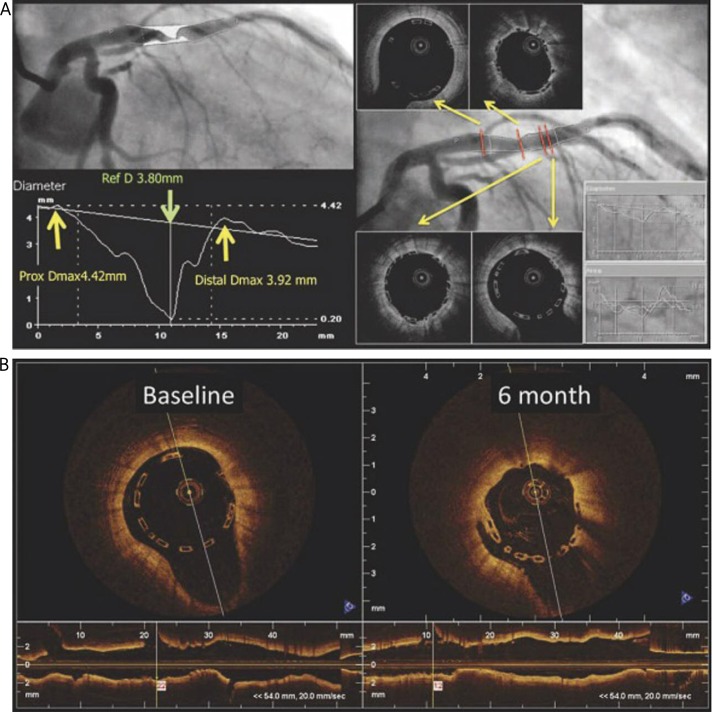
An example from the ABSORB cohort B: implantation of the 3.0 mm diameter BVS into the vessel with RVD diameter of 3.8mm (D max values in the proximal and distal references – 4.42 and 3.92mm respectively) (A). Undersizing of the vessel diameter leads to spontaneous malapposition at both ends of the BVS (B) at baseline (left) and after 6 months on OCT (with permission of Dr Gomez-Lara J) [17]
Fig. 6.
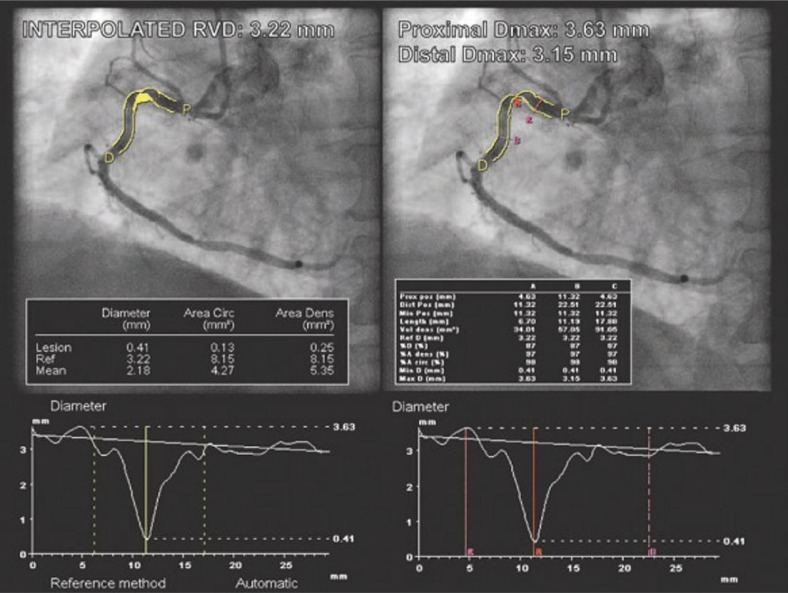
Comparison of the QCA measurements for the same lesion using a commonly used algorithm based on the reference vessel diameter (RVD) (left) and on D max (right) – an example from ABSORB cohort B. RVD was calculated for 3.22 mm. Based on this QCA measurement, implantation of a 3.0 diameter BVS would be acceptable (the implantation range is between 2.5 mm and 3.3 mm). However, the use of D max measurement gave a value of proximal reference diameter of 3.63 mm, which excluded the possibility of BVS implantation (with permission of Dr Vasim Faroog) [16]
Table 1.
Ranges of target artery diameters and the ABSORB ™ system diameters used for implantation
| Proximal and distal diameter of the target artery [mm] | Diameter of the ABSORB BVS™ system [mm] |
|---|---|
| ≥ 2.0 and ≤ 3.0 | 2.5 |
| ≥ 2.5 and ≤ 3.3 | 3.0 |
| ≥ 3.0 and ≤ 3.8 | 3.5 |
Preparation for ABSORB™ scaffold implantation
Preparation for implantation should include not only the vessel diameter, but also the morphology of the atherosclerotic plaque, particularly in terms of calcifications and the possibility to reach the culprit lesion with the device by assessment of the vessel tortuosity before the stenosis and the presence of possible calcifications in this part of the vessel. It should be noted that the device has not been studied in the following types of lesions: ostial lesions, lesions with side branches over 2 mm in diameter, lesions in the left main stem or in occluded arteries. Before the implantation it is necessary to appropriately prepare the plaque by effective predilation to obtain residual stenosis below 40%. Usually it is recommended to use balloons with diameter 0.5 mm smaller or equal to the size of the planned device and semi- or non-compliant characteristics. Currently there are no data regarding the use of other devices such as plaque modifying balloons or devices used for directional coronary atherectomy or rotablation. The length of the scaffold should include at least two millimeters of the “healthy” vessel before and after the stenosis. In the case of long stenosis requiring implantation of more than one scaffold it is acceptable to implant two scaffolds for one lesion at maximum. Maximal length of the dilated segment should not exceed 48 mm for two scaffolds of 28 mm (the difference includes 4 mm reserve for stents overlap and 4 mm for healthy margins of the vessel). Initial predilations should encompass the whole atherosclerotic segment of the vessel but damage to the adjacent areas should be avoided. The first scaffold should be placed distally. This should be followed by introduction of the second scaffold with the presence of the previously mentioned 1-4 mm overlap. Such a sequence of implantation permits one to avoid damage to the newly implanted scaffold when crossing with the non-dilated system through the lumen of the initially implanted scaffold. It is advised to use the same diameter of the device, but in case of discrepant references it is acceptable to use larger diameters for the proximal segment at the discretion of the operator. In the case of single-stage multivessel angioplasty the number of implanted scaffolds is limited by the total allowable dose of everolimus which is contained in the 94 mm of the scaffold. Because of that it is acceptable to implant seven 12 mm long DES BVS, five 18 mm long scaffolds or three 28 mm long devices at maximum.
The implantation
The introductory system is compatible with 6F or larger catheters with minimal internal diameter of 0.070″(1.8 mm). It is not acceptable to use catheters facilitating the introduction of stents such as the child in mother, 5 in 6 catheter or 6 in 7 catheter, because of the lower than required internal lumen diameter. There is only one compatible device, the 7 in 8 catheter, but it requires the use of an 8 F system. Introduction of the scaffold to the target location should be gentle, which is done by application of constant tension on the introductory catheter. The struts of the ABSORB™ are in-visible to X-ray radiation, and therefore the proper introduction of the scaffold should be guided by the presence of platinum markers placed on both ends of the scaffold, which correspond to markers on the unexpanded balloon. Forcing any resistance in the course of introduction and especially Dotter technique should be avoided. In case of significant resistance the introductory system should be gently withdrawn into the guiding catheter after confirmation of their collinear positions. In case of any resistance in the guiding catheter it is recommended to remove the whole system together with the guiding catheter. In the event of an unsuccessful attempt to withdraw the scaffold into the guiding catheter it is forbidden to re-introduce the scaffold into the artery and a new device should be used. After introduction of the scaffold to its target location, the implantation should last at least 30 s with gradual pressure increase by 2 atm every 5 s without exceeding the maximal acceptable pressure determined by the RBP value described in the leaflet. After implantation the balloon should be completely emptied and the introductory system should be gently withdrawn.
Postdilation of the ABSORB™ scaffold
After expansion of the ABSORB™ scaffold control angiography should be performed to assess the residual stenosis. It should be kept in mind that larger dimensions of the ABSORB™ struts in comparison to the struts of metallic stents cause weakening of the vessel contours contrast, which is why the dimension of the stented segment may appear smaller despite adequate scaffold expansion. Therefore, any subsequent postdilations should be performed only in case of evident residual stenosis. In case of doubts, visualization of apposition of the scaffold struts to the vessel wall may be additionally assessed using IVUS or OCT. If postdilation is required, adequately sized non-compliant balloons should be used. Inflation pressure should remain in accordance with the characteristic of the balloon expandability to avoid unacceptable dilation of the scaffold, which is 0.5 mm over the nominal value. It is crucial to remain cautious during introduction of any devices such as guidewires, balloons and IVUS or OCT probes through a newly implanted device to avoid damage to the scaffold. In case of vessel dissection requiring stenting it is acceptable to use a second properly sized scaffold or everolimus-eluting metallic stent.
Summary – a step-by-step implantation
Everolimus-eluting bioresorbable ABSORB™ scaffold is indicated for the treatment of de novo lesions in native coronary arteries of 2.0-3.8 mm diameter. It is a temporary device undergoing absorption and permitting a return of the proper vessel function. In contrast to angioplasty with the use of metallic stents, which is mainly aimed at optimal stent expansion with the use of high-pressure inflation and postdilation, to obtain an optimal result of ABSORB™ scaffold implantation it is necessary to correctly choose the diameter of the scaffold and to perform optimal initial dilation of the atherosclerotic plaque. Particular attention should be paid to:
- Adequate assessment of the vessel diameter using QCA analysis and measurement of maximal vessel lumen diameter (D max) in the proximal and distal reference segments in relation to the target location of the ABSORB™ scaffold. In comparison to the reference vessel diameter typically calculated with the algorithm, D max is a real value of maximal vessel diameter at sites of the target location of the scaffold margins.
-
Both D max values in the range:2.0-3.0 mm – ABSORB™ 2.5 mm should be chosen,2.5-3.3 mm – ABSORB™ 3.0 mm should be chosen,3.0-3.8 mm – ABSORB™ 3.5 mm should be chosen.
- One of the values outside of the range – the choice of the scaffold diameter depends on the physician's decision; it is recommended to choose larger diameters.
- The use of other imaging techniques such as IVUS and OCT is not required, but may be useful in case of doubts regarding the reference diameter of the vessel, which is a target for implantation of the ABSORB™ system and in the assessment of the atherosclerotic plaque and its calcifications.
-
The choice of the ABSORB™ length: the scaffold should cover at least 2 mm of the “healthy” vessel before and after the stenosis. In case of long stenosis it is acceptable to implant maximally two scaffolds into one lesion. Length measurement should include an overlap of 1-4 mm.
The introductory system is compatible with 6 F or larger catheters with minimal internal diameter of 0.070”(1.8 mm). Particular attention should be paid to collinear positioning of the guiding catheter.
- Preparation of the atherosclerotic plaque with adequate predilations:
- Predilation with a balloon catheter having 0.5 mm smaller diameter than the planned ABSORB™ diameter.
- Predilation with a balloon catheter equal to the diameter of the planned scaffold (with non-compliant high pressure balloon), when there is no effect of the first predilation or there is persistent residual stenosis > 40%.
- In case of long stenosis predilations should include the whole atherosclerotically modified segment of the vessel and injury to the adjusting areas should be avoided.
- Introduction of the device to the target lesion:
- Patients with tortuous segments and significant visible calcifications should not be treated, particularly if these are located proximally to the lesion, because it creates a risk of scaffold slippage off the balloon.
- The scaffold should be carefully introduced to the target lesion under constant tension on the introductory system and any forceful crossing of the resistance and particularly Dotter technique should be avoided. Multiple attempts to cross the lesion can cause damage to the scaffold or its slippage off the balloon.
- The struts of the ABSORB™ scaffold are invisible to X-ray radiation and therefore proper placement of the scaffold in the lesion should be guided by platinum markers present on the scaffold (two on each end of the scaffold), which correspond to the markers on the unexpanded balloon.
- In case of significant resistance an introductory system should be gently withdrawn into the guiding catheter. Repeated introduction of the retracted scaffold is forbidden and a new device should be used. In case of any resistance on the guiding catheter it is recommended to remove the whole system together with the guiding catheter.
Implantation of the scaffold: progressive inflation by 2 atm every 5 s – minimal total time of inflation is 30 s, RBP should not be exceeded.
In case of implantation of two ABSORB™ scaffolds the first one should be placed distally. The second scaffold should be implanted with a 1-4 mm overlap.
In case of vessel dissection requiring stenting it is acceptable to use a second scaffold with adequate diameter or everolimus-eluting metallic stent.
- Postdilation. The vessel diameter at the site of the implanted scaffold may appear smaller on control angiography. Postdilation should be used only in cases of evident residual stenosis or scaffold deflection. Postdilation should be performed using high pressure low compliant balloon catheters, whose size should be adjusted to the reference and which should be expanded cautiously according to the balloon characteristic not to exceed the maximal limit of the scaffold size:
- Absorb™ 2.5 mm – postdilation with 2.75 mm or 3.0 mm balloons not exceeding the 3.0 mm balloon size (nominal pressure at maximum),
- ABSORB™ 3.0 mm – postdilation with 3.25 mm or 3.5 mm balloon not exceeding the 3.5 mm balloon size,
- ABSORB™ 3.5 mm – postdilation with 3.75 mm or 4.0 mm balloon not exceeding 4.0 mm diameter.
Additional imaging studies – IVUS and OCT – are not required, but may be useful in case of doubts regarding the optimal apposition of the scaffold to the vascular wall.
Dual antiplatelet treatment is recommended for a minimum of 6 months or longer depending on clinical indications.
Special precautions. It should be noted that the device should not be implanted into lesions which cannot be adequately prepared with inflations due to the presence of excessive calcifications or when the balloon used for predilation cannot be fully expanded or when the result of dilation is unsatisfactory. The safety of other methods such as plaque modifying balloons and directional or rotational atherectomy is not known and the use of these techniques was not permitted in the clinical studies. It is not allowed to implant scaffolds into bifurcating lesions with side branches exceeding or equal to 2 mm diameter. Effective management of side branch occlusion is unknown and the “kissing balloons” technique was not allowed in the currently used study protocols. There is also no information regarding lesions located in distal segments, the left main stem, chronic occlusions or coronary artery bypass grafting. Because of lack of data it is not advised to use this device in pregnant women, during lactation or in patients wishing to have children. Contraindications to use of the ABSORB include allergy to components of the scaffold (poly-L-lactic acid, poly-D-lactic acid, everolimus) or contraindications to antiplatelet or antithrombotic treatment.
Summary
The results of clinical studies conducted so far indicate a huge change that is taking place in interventional cardiology due to the introduction of biodegradable vascular scaffolds – the ABSORB™. It is expected that in the coming years BVS will become a common alternative to currently implanted metallic stents, including antiproliferative drug-eluting ones. This is supported, especially in Poland, by the research program POLAR ACS 1 (Polish ABSORB™ Registry), in which a growing number of interventional cardiology centers will have an opportunity to use DES BVS in daily clinical practice [20]. Therefore interventional cardiologists should acquire knowledge on the details and differences of BVS implantation during percutaneous coronary intervention.
References
- 1. http://www.abbott.com/news-media/press-releases/2011Jan10.htm.
- 2.Nishio S, Kosuga K, Igaki K, et al. Long-term (> 10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation. 2012;125:2343–2353. doi: 10.1161/CIRCULATIONAHA.110.000901. [DOI] [PubMed] [Google Scholar]
- 3.Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-L-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 4.Tamai H. Biodegradable stents, an update and work in progress; 2003. Presentation at TCT. [Google Scholar]
- 5.Tamai H. Biodegradable stents, four-year follow-up; 2004. Presentation at TCT. [Google Scholar]
- 6.Depukat R, Dudek D. Biodegradable stents – 4th revolution of the interventional cardiology. Postep Kardiol Inter. 2009;5:144–147. [Google Scholar]
- 7.Dudek D, Onuma Y, Ormiston JA, et al. Four-year clinical follow-up of the ABSORB everolimus-eluting bioresorbable vascular scaffold in patients with de novo coronary artery disease: the ABSORB trial. Eurointervention. 2012;7:1060–1061. doi: 10.4244/EIJV7I9A168. [DOI] [PubMed] [Google Scholar]
- 8.Nieman K, Dudek D, Ormiston J, et al. ABSORB cohort a trial: five year clinical and MSCT results of the ABSORB bioresorbable everolimus eluting vascular scaffold. Circulation. 2011;124:A10570. [Google Scholar]
- 9.Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 10.García-García HM, Gonzalo N, Pawar R, et al. Assessment of the absorption process following bioabsorbable everolimus-eluting stent implantation: temporal changes in strain values and tissue composition using intravascular ultrasound radiofrequency data analysis. A substudy of the ABSORB clinical trial. EuroIntervention. 2009;4:443–448. doi: 10.4244/eijv4i4a77. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, Onuma Y, Ormiston JA, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122:2301–2312. doi: 10.1161/CIRCULATIONAHA.110.970772. [DOI] [PubMed] [Google Scholar]
- 12.Serruys PW, Onuma Y, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58:1578–1588. doi: 10.1016/j.jacc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Erbel R, Di Mario C, Bartunek J, et al. PROGRESS-AMS (Clinical Performance and Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869–1875. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]
- 14.Waksman R, Erbel R, Di Mario C, et al. PROGRESS-AMS (Clinical Performance Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009;2:312–320. doi: 10.1016/j.jcin.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Ghimire G, Spiro J, Kharbanda R, et al. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention. 2009;4:481–484. doi: 10.4244/jv4i4a82. [DOI] [PubMed] [Google Scholar]
- 16.Farooq V, Gomez-Lara J, Brugaletta S, et al. Proximal and distal maximal luminal diameters as a guide to appropriate deployment of the ABSORB everolimus-eluting bioresorbable vascular scaffold: a sub-study of the ABSORB Cohort B and the on-going ABSORB EXTEND Single Arm Study. Catheter Cardiovasc Interv. 2012;79:880–888. doi: 10.1002/ccd.23177. [DOI] [PubMed] [Google Scholar]
- 17.Diletti R, Onuma Y, Farooq V, et al. Six-month clinical outcome following the implantation ofthe bioresorbable everolimus eluting vascular scaffold in vessels smaller or larger than 2.5 mm. J Am Coll Cardiol. 2011;58:258–264. doi: 10.1016/j.jacc.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Lara J, Diletti R, Brugaletta S, et al. Angiographic maximal luminal diameter and appropriate deployment of the everolimus-eluting bioresorbable vascular scaffold as assessed by optical coherence tomography. EuroIntervention. 2012;8:214–224. doi: 10.4244/EIJV8I2A35. [DOI] [PubMed] [Google Scholar]
- 19.Legutko J, Rzeszutko L, Partyka L, et al. Comparison of three-dimensional and two-dimensional quantitative coronary analysis measuring intracoronary lengths in patients undergoing diagnostic coronary angiography. Postęp Kardiol Inter. 2012;8:31–41. [Google Scholar]
- 20. http://www.abbott.com/news-media/press-releases/abbott-announces-international-launch-of-the-absorb-bioresorbable-vascular-scaffold.htm.