Abstract
Hereditary persistence of fetal hemoglobin (HPFH) is a benign condition in which the normal shutoff of fetal hemoglobin (Hb F) production fails to occur. In the G gamma beta+ type of HPFH, erythrocytes of adult heterozygotes contain approximately equal to 20% Hb F, which is almost exclusively of the G gamma-globin variety, without increased levels of gamma-globin chains from the nearby A gamma-globin gene. Unlike some forms of HPFH, no major deletions in the globin gene cluster have been found by genomic blotting in the G gamma beta+ variety. We report here a family with this condition, from which cosmid clones of the beta-globin gene cluster from the G gamma beta+ HPFH allele have been obtained. Sequencing around the fetal genes has identified a point mutation 202 base pairs 5' to the G gamma-globin gene that is present in genomic DNA of 3/3 unrelated individuals with G gamma beta+ HPFH but in none of more than 100 non-HPFH individuals. Although the mutation could represent a tightly linked polymorphism, its location in a region suggested by recent data to be important in tissue-specific control of gene expression suggests the possibility that the -202 mutation accounts for the phenotype. The sequence created resembles elements of other eukaryotic promoters known to be important for efficient transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Balsley J. F., Rappaport E., Schwartz E., Surrey S. The gamma-delta-beta-globin gene region in G gamma-beta +-hereditary persistence of fetal hemoglobin. Blood. 1982 Apr;59(4):828–831. [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. A sensitive new prenatal test for sickle-cell anemia. N Engl J Med. 1982 Jul 1;307(1):30–32. doi: 10.1056/NEJM198207013070105. [DOI] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Felsenfeld G. Specific factor conferring nuclease hypersensitivity at the 5' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):95–99. doi: 10.1073/pnas.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Baty D., Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983 Apr 25;11(8):2447–2464. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M., Gelinas R., Tatsis B., Murray J., Yagi M., Mueller R., Stamatoyannopoulos G. Restriction endonuclease mapping of gamma-delta-beta-globin region in G gamma (beta)+ HPFH and a Chinese A gamma HPFH variant. Am J Hum Genet. 1983 Jul;35(4):611–620. [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Fromm M., Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–481. [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T., Turner P., Moulton A. D., Nienhuis A. W. Differences in human alpha-, beta- and delta-globin gene expression in monkey kidney cells. Cell. 1982 Aug;30(1):173–183. doi: 10.1016/0092-8674(82)90023-x. [DOI] [PubMed] [Google Scholar]
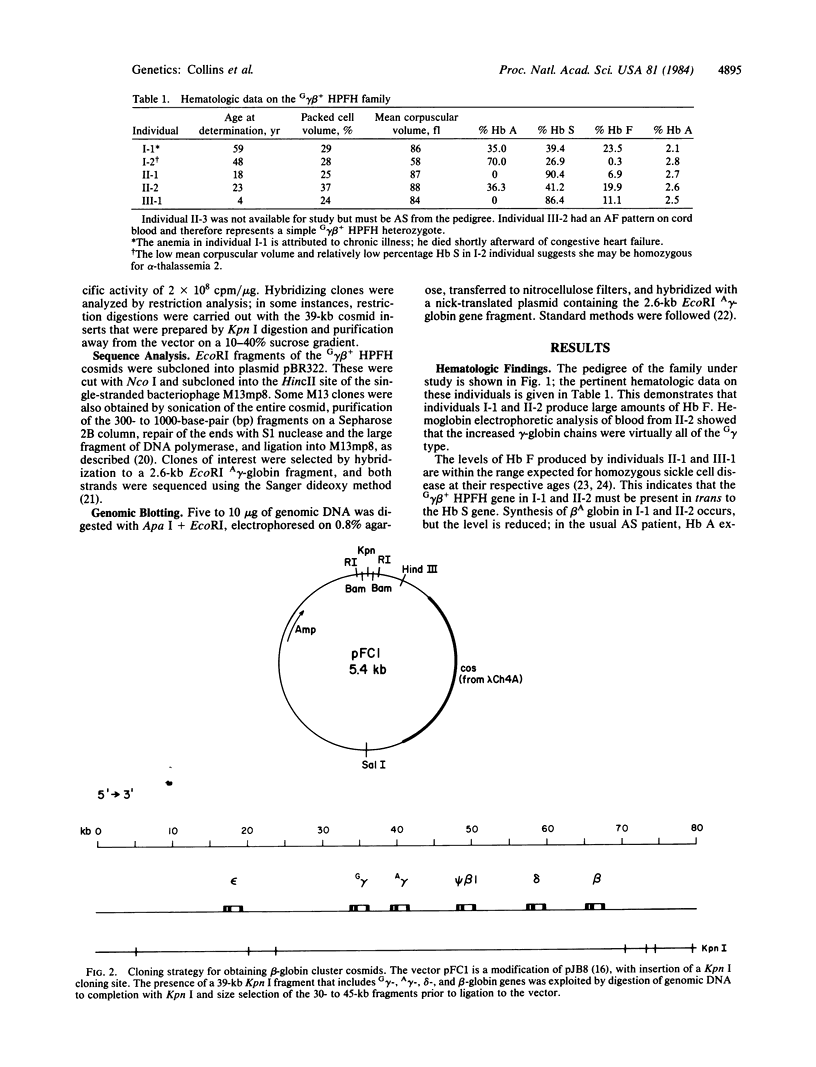
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Wood W. G., Clegg J. B., Weatherall D. J. Restriction endonuclease maps of the beta-like globin gene cluster in the British and Greek forms of HPFH, and for one example of G gamma beta + HPFH. Br J Haematol. 1982 Mar;50(3):415–422. doi: 10.1111/j.1365-2141.1982.tb01936.x. [DOI] [PubMed] [Google Scholar]
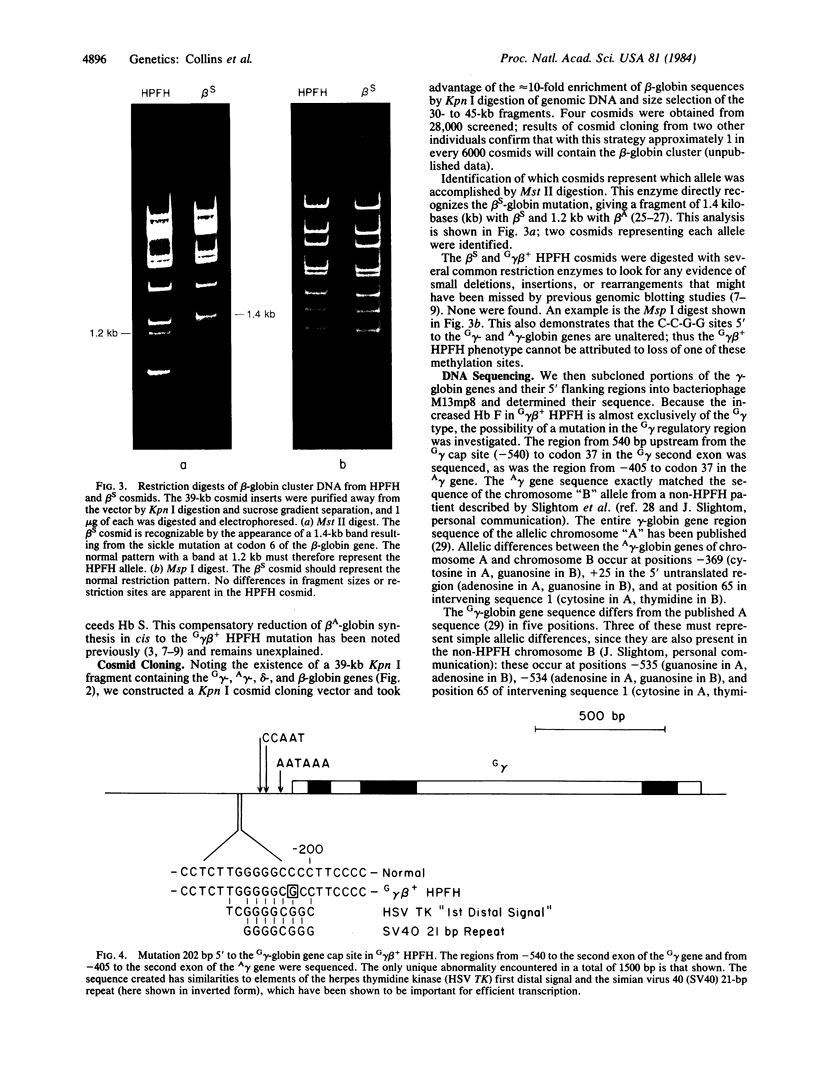
- Kan Y. W., Dozy A. M., Trecartin R., Todd D. Identification of a nondeletion defect in alpha-thalassemia. N Engl J Med. 1977 Nov 17;297(20):1081–1084. doi: 10.1056/NEJM197711172972002. [DOI] [PubMed] [Google Scholar]
- Kolata G. Globin gene studies create a puzzle. Science. 1984 Feb 3;223(4635):470–471. doi: 10.1126/science.6197757. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Noguchi C. T., Turner P. H., Schechter A. N., Heller P., Nienhuis A. W. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [PubMed] [Google Scholar]
- Mason K. P., Grandison Y., Hayes R. J., Serjeant B. E., Serjeant G. R., Vaidya S., Wood W. G. Post-natal decline of fetal haemoglobin in homozygous sickle cell disease: relationship to parenteral Hb F levels. Br J Haematol. 1982 Nov;52(3):455–463. doi: 10.1111/j.1365-2141.1982.tb03915.x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Little P. F., Kazazian H. H., Jr, Boehm C. D. Improved detection of the sickle mutation by DNA analysis: application to prenatal diagnosis. N Engl J Med. 1982 Jul 1;307(1):32–36. doi: 10.1056/NEJM198207013070106. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Taramelli R., Comi P., Mazza U., Saglio G., Camaschella C., Izzo P., Cao A., Galanello R. Molecular comparison of delta beta-thalassemia and hereditary persistence of fetal hemoglobin DNAs: evidence of a regulatory area? Proc Natl Acad Sci U S A. 1982 Apr;79(7):2347–2351. doi: 10.1073/pnas.79.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey M. E., Wood W. G., Weatherall D. J., Perrine R. P. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. Br J Haematol. 1978 Nov;40(3):415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Rovera G., Magarian C., Borun T. W. Resolution of hemoglobin subunits by electrophoresis in acid urea polyacrylamide gels containing Triton X-100. Anal Biochem. 1978 Apr;85(2):506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- Serjeant G. R. Fetal haemoglobin in homozygous sickle cell disease. Clin Haematol. 1975 Feb;4(1):109–122. [PubMed] [Google Scholar]
- Serjeant G. R., Grandison Y., Lowrie Y., Mason K., Phillips J., Serjeant B. E., Vaidya S. The development of haematological changes in homozygous sickle cell disease: a cohort study from birth to 6 years. Br J Haematol. 1981 Aug;48(4):533–543. doi: 10.1111/j.1365-2141.1981.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Murnane M. J., deRiel J. L., Forget B. G. Heterogeneity in the molecular basis of hereditary persistence of fetal haemoglobin. Nature. 1980 May 29;285(5763):335–337. doi: 10.1038/285335a0. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Milner P. F., Summer M. E., Nallaseth F. S., Fadel H. E., Reindollar R. H., McDonough P. G., Wilson L. B. Use of restriction endonucleases for mapping the allele for beta s-globin. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3628–3631. doi: 10.1073/pnas.79.11.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. G., Clegg J. B., Weatherall D. J. Hereditary persistence of fetal haemoglobin (HPFH) and delta beta thalassaemia. Br J Haematol. 1979 Dec;43(4):509–520. doi: 10.1111/j.1365-2141.1979.tb03784.x. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Weatherall D. J., Clegg J. B., Hamblin T. J., Edwards J. H., Barlow A. M. Heterocellular hereditary persistence of fetal haemoglobin (heterocellular HPFH) and its interaction with beta thalassaemia. Br J Haematol. 1977 Aug;36(4):461–473. doi: 10.1111/j.1365-2141.1977.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]