Abstract
We present a MEMS affinity sensor that can potentially allow long-term continuous monitoring of glucose in subcutaneous tissue for diabetes management. The sensing principle is based on detection of viscosity changes due to affinity binding between glucose and poly(acrylamide-ran-3-acrylamidophenylboronic acid) (PAA-ran-PAAPBA), a biocompatible, glucose-specific polymer. The device uses a magnetically driven vibrating microcantilever as a sensing element, which is fabricated from Parylene and situated in a microchamber. A solution of PAA-ran-PAAPBA fills the microchamber, which is separated from the surroundings by a semi-permeable membrane. Glucose permeates through the membrane and binds reversibly to the phenylboronic acid moiety of the polymer. This results in a viscosity change of the sensing solution, which is obtained by measuring the damped cantilever vibration using an optical lever setup, allowing determination of the glucose concentration. Experimental results demonstrate that the device is capable of detecting glucose at physiologically relevant concentrations from 27 mg/dL to 324 mg/dL. The glucose response time constant of the sensor is approximately 3 min, which can be further improved with device design optimization. Excellent reversibility and stability are observed in sensor responses, as highly desired for long-term, stable continuous glucose monitoring.
Keywords: Affinity binding, Biocompatibility, Biosensors, Continuous glucose monitoring, Diabetes, MEMS, Viscometry
1. Introduction
Close monitoring of daily physiological glucose levels reduces the risk of complications caused by conditions such as hypoglycemia or hyperglycemia. This is generally achieved by continuous glucose monitoring (CGM) systems, which involve either non-invasive or minimally invasive detection of glucose. Currently, subcutaneously implanted enzymatic electrochemical detection is the prevailing CGM technique, and is the basis for a number of commercially available devices, such as Medtronic MiniMed Paradigm [1], Freestyle Navigator [2], and DexCom Seven [3].
While electrochemical methods are sensitive and specific for glucose detection, they also suffer significant drawbacks. Firstly, the irreversible consumption of glucose in electrochemical detection induces a potential change in the equilibrium glucose concentration in the tissue, and thus, affects the actual measured glucose level. Furthermore, the rate of glucose consumption is diffusion-limited. Any changes in diffusion layers due to biofouling (e.g., by cell deposition and capsule formation) on the sensor surface affects the diffusion rate, and, thus, the device sensitivity. In addition, hydrogen peroxide production and interference from electrode-active chemicals may cause erosion of the sensor electrodes and deactivation of functional enzymes, compromising the device accuracy, reliability and longevity [4]. As a result, electrochemical CGM sensors generally exhibit large drifts over time, and require frequent calibration by finger pricks (typically at least once every 12 h) [5,6]. This lack of reliability has been severely hindering CGM applications to practical diabetes management.
To overcome the drawbacks of electrochemical detection, alternative glucose sensing techniques have been under active investigation. In particular, methods that use non-consumptive, competitive affinity binding of glucose have shown great promise [7–9]. A widely used technique exploits the solution of a polysaccharide (e.g., dextran) crosslinked by a glucose-binding protein (e.g., concanavalin A, or Con A) [7]: glucose binds competitively to Con A and causes reversible de-crosslinking of the dextran–Con A complex, which can be detected via the resulting changes in solution properties, such as fluorescence [8] or viscosity [8,9]. As it is based on equilibrium binding in which glucose is not consumed, affinity sensing is not susceptible to electroactive interferents. More importantly, affinity sensing is considerably more tolerant to biofouling. That is, the deposition of biological material (e.g., cells and proteins) on the implanted affinity sensor surface results only in an increased equilibration time without any changes in measurement accuracy. Consequently, affinity glucose sensors can be highly stable and low-drift.
Unfortunately, Con A is immunogenic and cytotoxic [9], and degrades with time [10]. Alternatively affinity sensing systems utilizing synthetic glucose-responsive polymers have the potential to address these issues [11]. Polymers containing boronic acid groups have been recognized for forming cyclic ester in alkaline media rapidly and reversibly with diols in glucose at physiological pH values [12]. This property has been exploited to develop a variety of glucose sensors based on different mechanisms, such as fluorescence [13], volume change [14], and conductometry [15]. Recently, we developed a novel boronic acid-based affinity sensing system that uses the polymer poly(acrylamide-ran-3-acrylamidophenylboronic acid) (PAA-ran-PAAPBA) [16]. In the system, glucose reversibly forms strong ester bonds with the phenylboronic acid moiety on the backbone of PAA-ran-PAAPBA, resulting in crosslinking of the polymer and an increase in the viscosity of the solution.
MEMS technology promises to allow batch-fabrication of low-cost implantable sensors that integrate multiple functional components for metabolic monitoring. Such devices are miniaturized, leading to improved measurement time response and minimized invasiveness. MEMS or related technologies have been applied for glucose sensors that are based on electrochemical [17,18], impedance [19], eletrophoretic [20], thermal [21,22], optical [23] and colorimetric [24] detection methods. MEMS-based devices have also exploited microdialysis [25] and glucose-induced hydrogel swelling [14]. In addition, we previously reported a MEMS affinity glucose sensor [26]. The sensor was based on viscometric measurements of a Con A-based affinity system, demonstrating the potential of non-consumptive, equilibrium-based glucose detection. However, the sensor suffered from limitations associated with Con A, and due to material limitations and osmotic pressure imbalances, exhibited limited mechanical reliability, poor reversibility, and significant drifts.
In this paper, we present a MEMS affinity glucose sensor that effectively addresses these problems, and can thus potentially allow long-term continuous glucose monitoring. The device uses a magnetically driven vibrating microcantilever as a sensing element, which is fabricated from the polymer Parylene and situated in a microchamber. A solution of the biocompatible polymer PAA-ran-PAAPBA fills the microchamber, which is separated from the surroundings by a semi-permeable membrane. Glucose permeates through the membrane and binds reversibly to the phenylboronic acid moiety of the polymer. This results in a viscosity change of the sensing solution, which is obtained by measuring the damped cantilever vibration, allowing determination of the glucose concentration.
2. Viscometric affinity glucose sensing with a biocompatible polymer
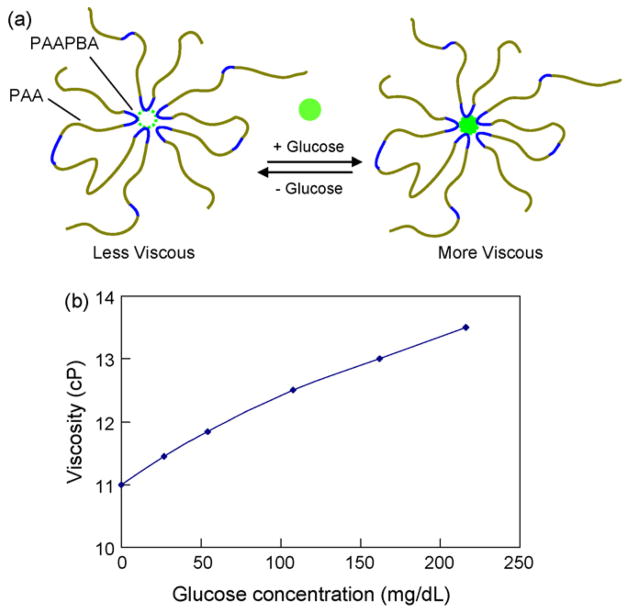
Our CGM device is based on an affinity sensing system utilizing the biocompatible polymer PAA-ran-PAAPBA. This section describes the main characteristics of this system; more details can be found elsewhere [16,27]. PAA-ran-PAAPBA is an amphiphilic copolymer containing two components, hydrophilic polymer segment polyacrylamide (PAA) and hydrophobic polymer segment poly(3-acrylamidophenylboronic acid) (PAAPBA) (Fig. 1a). Here, PAAPBA is the glucose-sensitive component, containing boronic acid groups which can form cyclic boronate esters in aqueous media after binding with glucose. PAA, which is water soluble, serves to improve the water solubility of the hydrophobic PAAPBA segment. In addition to being a water soluble component, PAA provides an added neighbor coordinating effect by carbonyl oxygen and boron chelating which enhances the binding between the boronic acid and carbohydrates [27]. The polymer is synthesized by a free radical polymerization process, which is described in detail elsewhere [16,27].
Fig. 1.
Biocompatible, glucose-sensitive polymer poly(acrylamide-ran-3-acrylamidophenylboronic acid) (PAA-ran-PAAPBA) [16,27]. (a) The polymer composition and mechanism of interaction with glucose. (b) Glucose-induced viscosity change of a 1.9% PAA-ran-PAAPBA solution in PBS buffer (pH 7.4).
A solution of PAA-ran-PAAPBA undergoes a viscosity change when interacting with glucose molecules. That is, when glucose is added to the PAA-ran-PAAPBA solution, the phenylboronic acid moieties in the polymer are able to reversibly form strong ester bonds with the glucose at a ratio of two to one on the PAA-ran-PAAPBA polymer backbone, resulting in the crosslinking of the polymer and hence a conformation change. This leads to a shorter distance between polymer chains and a further aggregation of the polymer, thus resulting in an increase in the viscosity of the polymer solution (Fig. 1b), which can be detected by our MEMS sensor. The viscosity change is reversible due to the reversibility of the polymer–glucose binding, and is highly specific to glucose over other saccharides as experimentally verified elsewhere [16,27].
3. Device design
The MEMS affinity glucose sensor is based on a cantilever, made of the polymer Parylene, situated inside a microchamber (Fig. 2). The microchamber is formed between a cavity etched into the substrate and a cellulose acetate (CA) semi-permeable membrane, and is filled with a PAA-ran-PAAPBA polymer solution. The Parylene cantilever is anchored onto the substrate at one end, with its free end coated with a permalloy thin film, which is protected by an additional Parylene layer to prevent the permalloy from corrosion by the polymer solution. The semi-permeable membrane prevents the polymer from escaping, while allowing glucose to diffuse into and out of the microchamber. While not part of the device, a test cell is incorporated on the other side of the membrane for introduction of glucose solution samples for device characterization.
Fig. 2.
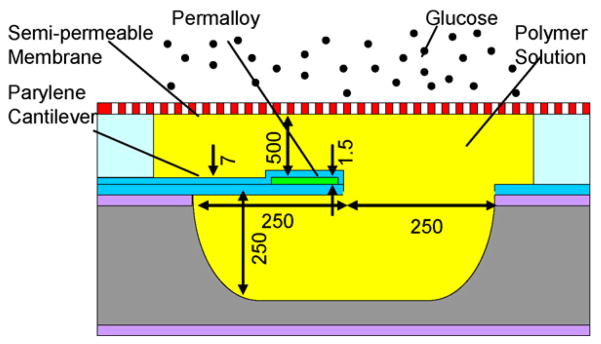
MEMS affinity CGM sensor design. A cantilever vibrates in a glucose-sensitive polymer solution inside a microchamber. Glucose permeates through a semi-permeable membrane, changing the solution viscosity and vibration damping. (Dimensions for a prototype device are given in micrometers).
The sensor is placed in two mutually orthogonal magnetic fields. When the sensor is oriented horizontally, these include a vertical electromagnetic (EM) field generated by a solenoid and a horizontal magnetic field from a permanent magnet (PM). The PM magnetizes the permalloy thin film, exciting a magnetic field in the permalloy film along the cantilever length. A torque thus is generated on this magnetized permalloy film attempting to align the cantilever with the EM field. This torque is distributed along the length of the cantilever, with a magnitude proportional to the product of the permalloy volume, the EM field intensity, and the magnetization of the permalloy, and causes the cantilever to bend. Thus, a time-dependent EM field produces a time-dependent torque, resulting in vibration of the cantilever. In addition, the vibration-induced flow of the polymer solution in general imparts hydrodynamic inertia and damping on the cantilever. Because of the direct dependency of the flow–structure interaction on the viscosity of the polymer solution, the viscosity of the sensing solution can be obtained by measuring the damped cantilever vibration, allowing the determination of the glucose concentration.
4. Fabrication process
To fabricate the device (Fig. 3), a Parylene layer (5 μm) was deposited by chemical vapor deposition onto a SiO2-coated silicon wafer into which small cavities were etched (Fig. 3a). The small cavities allowed the Parylene to be anchored over an increased surface area for improved adhesion. A 100 nm copper seed layer was then deposited on the Parylene, followed by the deposition and patterning a 1.5 μm photoresist layer defining the permalloy deposition area (150 μm long and 200 μm wide). A permalloy thin film was then deposited by electroplating, followed by the removal of the photoresist and unused copper, and the deposition of a second Parylene layer (2 μm) (Fig. 3b). The two Parylene layers, along with the underlying SiO2 layer, were then patterned to define a cantilever (250 μm in both length and width) (Fig. 3c). The cantilever was finally released by gas-phase XeF2 etching of the silicon underneath (forming a cavity approximately 500 μm × 500 μm × 250 μm in dimensions) and removal of the SiO2 directly beneath the cantilever (Fig. 3d). Following wafer dicing, a chip bonded to a poly(dimethylsiloxane) (PDMS) sheet in which a hole was fabricated by replica molding to define a microchamber with inlet and outlet channels (approximate 30 μL in dimensions), which was in turn bonded to a semi-permeable membrane (regenerated CA, with a molecular weight cutoff of 3500 Da; Fisher) using an adhesive (Devcon epoxy adhesive). Another PDMS sheet, in which a test cell (volume: 500 μL) was fabricated along with inlet and outlet channels and wells for introduction of glucose solution, was finally adhesive bonded to the CA membrane. A fabricated device is shown in Fig. 4.
Fig. 3.
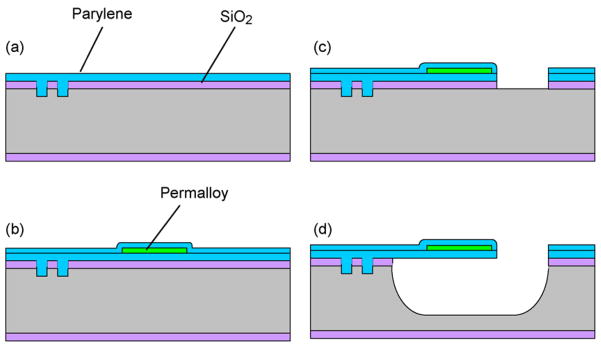
Fabrication process: (a) etching small anchoring cavities in silicon and depositing a Parylene layer; (b) electroplating permalloy and passivating it with Parylene; (c) patterning the Parylene and permalloy layers to define a cantilever; and (d) etching silicon and SiO2 to release the cantilever.
Fig. 4.
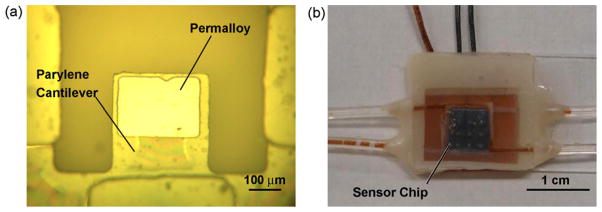
A fabricated MEMS CGM sensor: (a) before, and (b) after packaging.
5. Experimental method
Chemical and reagents used in the experiments include PAA-ran-PAAPBA that is synthesized using a previously described method [16], and D-(+)-glucose (Sigma–Aldrich). Phosphate buffer saline (PBS), pH 7.4, was prepared from potassium phosphate (20 mM), NaCl (150 mM) and NaN3 (0.05%). PAA-ran-PAAPBA (284 mg, with 1.9% of PAAPBA in the polymer) was dissolved in PBS (6 mL) to obtain the sensing solution. Glucose stock solution (1 M) was prepared by dissolving glucose (1.8 g) in PBS to 10 mL. A series of glucose solutions (27 mg/dL, 54 mg/dL, 108 mg/dL, 216 mg/dL, and 324 mg/dL) were prepared by further diluting the stock solution with PBS. To prepare polymer solutions that were mixed with glucose before being loaded into the microchamber, PAA-ran-PAAPBA solution (1 mL, 0.45 mg/dL) and glucose (6 μL, 1 M) were mixed to obtain 108 mg/dL glucose concentration in PAA-ran-PAAPBA solution.
All experiments with the MEMS CGM device were conducted at 37 °C with closed-loop temperature control by placing the device on an ultra-thin kapton heater with temperature measured by a k-type thermocouple. This was necessary so as to minimize temperature-dependent viscosity changes in the fluids, and provide a physiologically relevant glucose monitoring condition. In all experiments unless otherwise noted, the device’s microchamber was filled with a glucose-free polymer solution (1.9%), while glucose solutions of varying concentrations were introduced into the test cell. Glucose permeated through the semi-permeable membrane and bound to the polymer, until this process reached equilibrium. Because the volume of the test cell was 20 times than that of the chamber, it was reasonable to assume that the equilibrium glucose concentration equaled the initial glucose concentration in the test cell.
The cantilever vibration was driven by a hand-wound solenoid (4000 turns of a 200 μm diameter copper wire on a 2.5 μm diameter plastic core), which under a 5 Vrms driving voltage generated an electromagnetic of approximately 0.8 kA/m perpendicular to the cantilever surface. A permanent magnet with field strength of 500 kA/m was placed parallel to the cantilever surface to magnetize the permalloy film. The cantilever vibration was detected by an optical-lever system, which is described elsewhere [26]. Briefly, a laser beam was directed onto and reflected off of the free end of the cantilever. Detection of the reflected laser beam using a position sensitive detector allowed determination of the cantilever deflection.
6. Results and discussion
This section presents experimental results from a fabricated device. We first investigate the device’s time response to glucose concentration changes. The equilibration process in which glucose permeates through the semi-permeable membrane and binds with the polymer is evaluated. Finally, steady-state vibration measurements involving glucose permeation through the semi-permeable membrane at varying concentrations are presented, including a simulated glucose variation measurement to demonstrate the reversibility and stability in device responses.
6.1. Time response
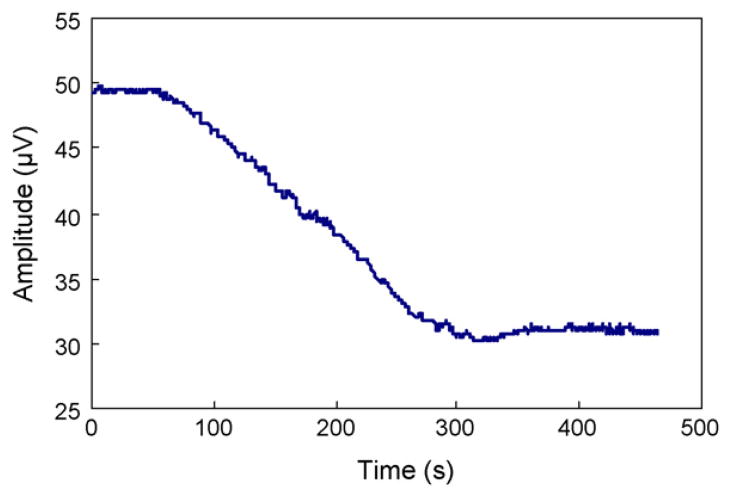
To characterize the time response of the sensor to glucose concentration change, the time constant of an experiment involving glucose permeation through the membrane and binding to the polymer was obtained. The chamber of the device was initially filled with glucose-free polymer solution, while the test cell was filled with PBS buffer. Glucose solution (108 mg/dL) was then introduced into the test cell. The cantilever vibration amplitude, which was proportional to the amplitude of the voltage output of the position sensitive detector, at a fixed frequency (28 Hz) was obtained over time (Fig. 5). It can be seen that the amplitude decreased gradually with time, corresponding to a steady increase in the damping on the cantilever vibration due to glucose binding-induced viscosity increase. The cantilever vibration amplitude finally saturated to a constant level, reflecting that the cantilever vibration had reached steady state and the process of glucose permeation and binding had reached equilibrium. The time constant of this process was determined to be approximately 3 min. This is appropriate for CGM applications, considering 5–15 min of response time for commercially available systems and a 5 min detection requirement for general clinical treatment.
Fig. 5.
The variation with time of the cantilever vibration amplitude at 28 Hz upon introduction a 108 mg/dL glucose solution to the device. Glucose was initially absent from the polymer solution in the microchamber.
6.2. Evaluation of glucose permeation and equilibrium binding through the membrane
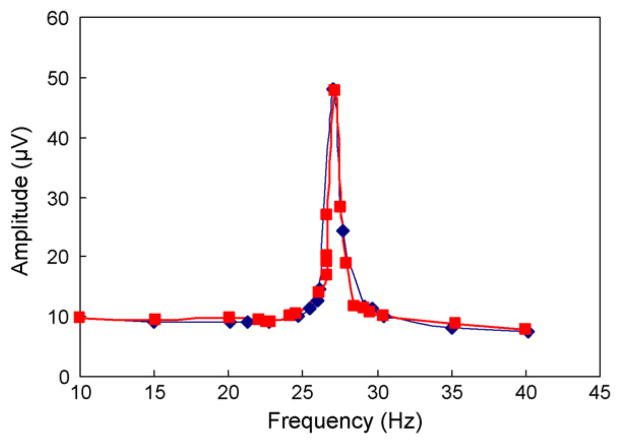
We compare the saturated amplitude frequency response of the cantilever, obtained from the experiment above after glucose permeation and binding had reached equilibrium, with results from a comparison experiment. In the comparison experiment, the microchamber and test cell were both filled with a polymer solution that was mixed with glucose at 108 mg/dL. Thus, the glucose concentrations inside and outside the microchamber were pre-equilibrated at the predetermined value. The cantilever vibration amplitude frequency responses obtained from these two experiments are shown in Fig. 6. We observe that the responses exhibit resonances at almost identical frequencies (27.0 Hz and 27.2 Hz) with nearly the same amplitudes (48 μV and 47.6 μV). The amplitudes at other frequencies also agree within 6%. The small discrepancies between the two responses can be attributed to the required separate preparation of the samples used in the two experiments. These experiments confirm that the process of glucose permeation through the membrane and binding to the polymer indeed achieved equilibrium, and the device would be capable of accurately determining glucose concentrations in its implanted environment.
Fig. 6.
Frequency dependent amplitude of the cantilever vibration obtained after glucose permeation and binding had reached equilibrium (squares), as compared to that from a comparison experiment (diamonds) in which glucose concentrations inside and outside the microchamber were pre-equilibrated.
6.3. Steady-state response at varying glucose concentrations
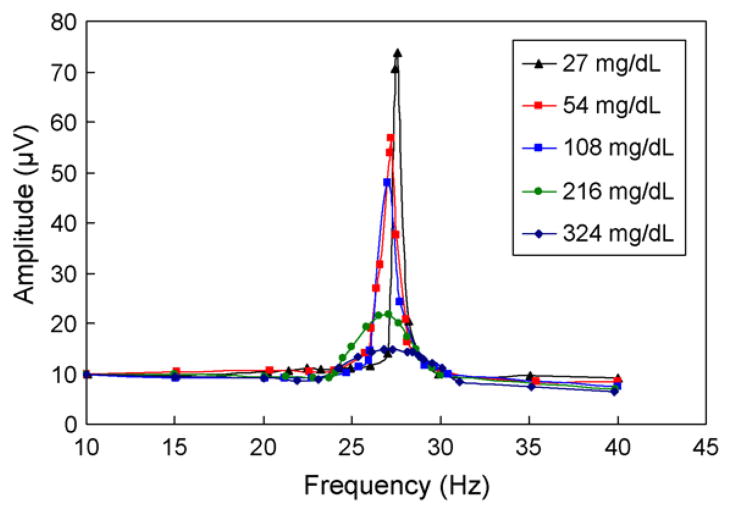
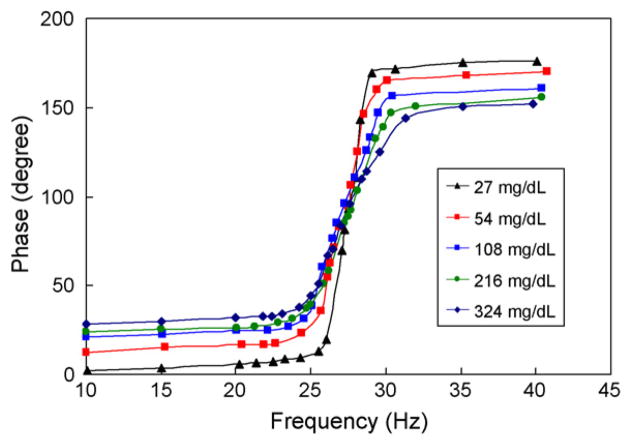
To investigate the dependence of cantilever vibration characteristics on the excitation frequency, the steady-state cantilever vibration was then measured at varying, physiologically relevant glucose concentrations. The vibration exhibited resonance behavior at all glucose concentrations tested (Figs. 7 and 8). As the glucose concentration increased from 27 mg/dL to 324 mg/dL, the resonance peak of the vibration amplitude decreased consistently by about 70% (Fig. 7). This was accompanied by a downward shift of the resonance frequency by about 0.77 Hz and an attenuation of vibration Q-factor from 29 to 7. These observations indicate a significant increase in vibrational damping, which is consistent with increased viscosity of the polymer solution at higher glucose concentrations. In addition, there was a significant change in the phase lag between the cantilever vibration and the magnetic excitation for the cantilever vibration (Fig. 8). For example, at 10 Hz, the phase lag increased from 2.2° at 27 mg/dL to 28.3° at 324 mg/dL. Based on the resolution of our phase measurements (0.01°), this implies that our device would be able to resolve glucose concentrations at about 0.1 mg/dL resolution.
Fig. 7.
Frequency dependent amplitude of the cantilever vibration at physiologically relevant glucose concentrations.
Fig. 8.
Frequency dependent phase lag of the cantilever vibration at physiologically relevant glucose concentrations.
6.4. Simulated glucose variation measurements
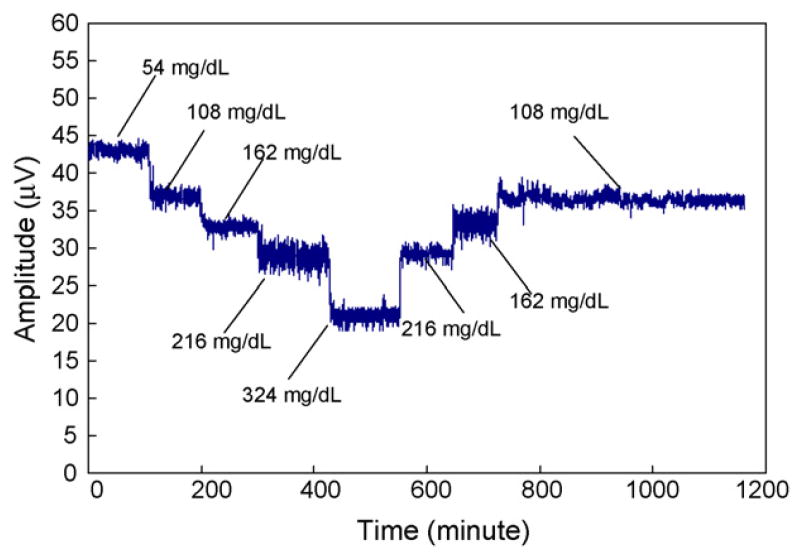
Measurements of a time-dependent sequence of glucose variations were made to simulate possible glucose concentration changes in the interstitial fluid of a diabetes patient, and also evaluate the reversibility and stability of the MEMS affinity glucose sensor. In these measurements (Fig. 9), while a glucose concentration of 108 mg/dL represented a stable daily glucose level, glucose concentrations of 54 mg/dL and 324 mg/dL were used respectively to simulate glucose levels before and after intake of food. In addition, two intermediate glucose concentrations, 162 mg/dL and 216 mg/dL, were also measured. The measured vibration amplitude at 28 Hz varied from 43 μV at 54 mg/dL to 20 μV at 324 mg/dL, and remained to be 37 μV at 108 mg/dL. (The noise observed in the data can be mostly attributed to environmental disturbances to the optical setup.) In particular, when the device was exposed to a glucose concentration after experiencing another sample that was either higher or lower in concentration, virtually the same amplitude was consistently obtained. For example, the average amplitudes at 108 mg/dL over the two periods, approximately defined by the intervals of [110,200] and [800,1100] min, were respectively 36.83 μV and 36.36 μV, which agree within 1.3%. Similarly, the reversibility was within 1.06% and 1.13% for the measurement data at 162 and 216 mg/dL glucose concentrations, respectively. This indicates an excellent reversibility of our sensor in response to glucose concentration variations. Moreover, exposing the device to a glucose concentration over an extended period allowed us to assess the drift in the device response. For example, for the [800,1100] min period during which the glucose concentration was maintained at 108 mg/dL, the vibration amplitude was steady at 36.36 μV with a standard deviation of 0.427 μV. That is, the drift in the device response was about 0.17 μV, or, 0.5% per hour. The drift can be further reduced by minimizing osmotic effects across the membrane, ultimately allow a stability appropriate for long-term continuous glucose monitoring.
Fig. 9.
Frequency-dependent amplitude of the cantilever vibration in response to a sequence of glucose concentrations.
7. Conclusions
In this paper, a MEMS affinity CGM sensor that uses a bio-compatible glucose-responsive copolymer has been presented. The device consists of a Parylene microcantilever which vibrates under magnetic excitation inside a microchamber. The chamber, based on PDMS, is filled with a solution of PAA-ran-PAAPBA, a biocompatible polymer that specifically recognizes glucose by affinity binding. The chamber is sealed with a cellulose acetate semi-permeable membrane, which prevents the polymer from escaping while allowing permeation of glucose into and out of the chamber. Affinity binding between the polymer and glucose results in the crosslinking of the polymer and an increase in the viscosity of the solution. Thus, by measuring the damped cantilever vibration in the solution, the glucose concentration can be determined. Experimental results have shown that a fabricated MEMS CGM device responded to glucose concentration variations at a time scale of 3 min. This is shorter when compared with time responses of commercially available electrochemical CGM sensors, and can be further reduced by design optimization. Additionally, comparative experiments confirmed that the process of glucose permeation through the membrane and binding to the polymer was able to equilibrate the glucose concentrations inside and outside the chamber, making the MEMS sensor suitable for an implanted setting. Moreover, the MEMS sensor response obtained at varying, physiologically relevant glucose concentrations indicated that the device was capable of resolving glucose concentration changes by measurement of viscosity. Specifically, over a glucose concentration range of 27–324 mg/dL, the phase frequency response could allow a glucose concentration resolution of 0.1 mg/dL. Finally, the MEMS device was tested with a time-dependent sequence of glucose variations to simulate possible glucose concentration changes in the interstitial fluid of a diabetes patient. The measurement data indicated that the device response was highly reversible (within 1.2%) and stable (within 0.5%/h). These results demonstrate the potential of our MEMS sensor for use as a subcutaneously implanted device for stable and reliable continuous monitoring of glucose in practical diabetes management.
Acknowledgments
We gratefully acknowledge financial support from NSF (grant # ECCS-0702101) and the Columbia Diabetes and Endocrinology Research Center (NIH grant # DK63068-05). X.H. also appreciates partial support from a National Scholarship from the China Scholarship Council.
Biographies
Xian Huang is a PhD candidate in Mechanical Engineering at Columbia University. He received his BS and MS degrees in Measurement and Control Technology and Instrumentation from Tianjin University, Tianjin, China, in 2004 and 2007, respectively. His research interests are in the development of implantable miniature biosensors for diagnostic and therapeutic applications.
Siqi Li is a PhD student at the Department of Chemistry and Biochemistry in University of South Carolina. He received his BE in Polymer Materials and Engineering from Hefei University of Technology in 1998, MS in Polymer Chemistry and Physics from University of Science and Technology of China in 2001, MS in Organic Chemistry from University of Iowa in 2004. His research interests are the development of glucose sensing polymer for continuous glucose monitoring, polymer and nanoparticle self-assembly and nanoparticle bioconjugations for cancer therapy.
Jerome Schultz received his BS (1954) and MS (1956) in Chemical Engineering from Columbia University, and his PhD in Biochemistry from the University of Wisconsin (1958). He started his career as a group leader in the pharmaceutical industry (Lederle Laboratories) then joined the University of Michigan, where he was Chairman of the Department of Chemical Engineering. He spent two years at the National Science Foundation as Deputy Director of the Engineering Centers Program. In 1987 he joined the University of Pittsburgh as Director of the Center for Biotechnology and Bioengineering, and was the Founding Chairman of the Department of Bio-engineering. In 2003 he spent a year at NASA’s Ames Research Center as a Senior Scientist in their Fundamental Biology Program. In 2004 he joined the faculty at the University of California, Riverside as a distinguished professor and Chairman of the Bioengineering Department. His research interests include biosensors, membrane transport phenomena, transport processes in tissues, pharmacokinetics, and immobilized enzymes. He is a member of the National Academy of Engineering, a Fellow of the Biomedical Engineering Society, a Fellow of the American Association for the Advancement of Sciences, Editor of Biotechnology Progress, and is a Founding Fellow and former President of the American Institute for Medical and Biological Engineering.
Qian Wang received a BS (1992) and PhD (1997) in Chemistry from Tsinghua University. After postdoctoral researches with Prof. Manfred Schlosser at University of Lausanne and Prof. M.G. Finn at the Scripps Research Institute, he started as an assistant professor at University of South Carolina in 2003. He has been an associate professor since 2008 and the Robert L. Sumwalt professor of Chemistry since 2009 at University of South Carolina. His research interest focuses on the development of novel biomaterials and the understanding of cell behavior using self-assembled materials as scaffold (see also: http://www.chem.sc.edu/faculty/wang/).
Qiao Lin received his PhD in Mechanical Engineering from the California Institute of Technology in 1998 with thesis research in robotics. He conducted postdoctoral research in microelectromechanical systems (MEMS) at the Caltech Micromachining Laboratory from 1998 to 2000, and was an assistant professor of Mechanical Engineering at Carnegie Mellon University from 2000 to 2005. He has been an associate professor of Mechanical Engineering at Columbia University since 2005. His research interests are in designing and creating integrated micro/nanosystems, in particular MEMS and microfluidic systems, for biomedical applications.
References
- 1.Mastrototaro JJ. The minimed continuous glucose system. Diabetes Technology & Therapeutics. 2000;2:13–18. doi: 10.1089/15209150050214078. [DOI] [PubMed] [Google Scholar]
- 2.Wilson DM, Beck RW, Tamborlane WV, et al. The accuracy of the FreeStyle Navigator Continuous Glucose Monitoring System in children with type 1 diabetes. Diabetes Care. 2007;30:59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danne T, Lange K, Kordonouri O. Real-time glucose sensors in children and adolescents with type-1 diabetes. Hormone Research. 2008;70:193–202. doi: 10.1159/000151592. [DOI] [PubMed] [Google Scholar]
- 4.Heller A. Implanted electrochemical glucose sensors for the management of diabetes. Annual Review of Biomedical Engineering. 1999;1:153–175. doi: 10.1146/annurev.bioeng.1.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Tempels FWA, Wiese G, Underberg WJM. On-line coupling of size exclusion chromatography and capillary electrophoresis via solid-phase extraction and a Tee–split interface. Journal of Chromatography B. 2006;839:30–35. doi: 10.1016/j.jchromb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Liu CL, Hofstadler SA, Bresson JA, et al. On line dual microdialysis with ESI-MS for direct analysis of complex biological samples and microorganism lysates. Analytical Chemistry. 1998 May;70(9):1797–1801. doi: 10.1021/ac971193k. [DOI] [PubMed] [Google Scholar]
- 7.Ballerstadt R, Ehwald R. Suitability of aqueous dispersions of dextran and Concanavalin A for glucose sensing in different variants of the affinity sensor. Biosensors & Bioelectronics. 1994;9:557–567. [Google Scholar]
- 8.Schultz JS, Mansouri S, Goldstein IJ. Affinity sensor—a new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care. 1982;5(3):245–253. doi: 10.2337/diacare.5.3.245. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka T, Oh-Hashi F, Sakurai Y. Immunogenicity and amplifier cell production by tumor vaccines enhanced by Concanavalin A. Gann. 1982;73:193–205. [PubMed] [Google Scholar]
- 10.Matsuura H, Ranganathan S, Yamamoto M, et al. Studies on the effect of Concanavalin A on the metabolism of low density lipoproteins in skin fibroblasts. Japanese Journal of Medicine. 1987;26:323–325. doi: 10.2169/internalmedicine1962.26.323. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka K, Matsumoto A. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin-release. Journal of the American Chemical Society. 1998;120(48):12694–12695. [Google Scholar]
- 12.James TD, Sandanayake KRAS, Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angewandte Chemie. 1996;35:1910–1922. (International edition in English) [Google Scholar]
- 13.Yan J, Fang H, Wang B. Boronolectins and fluorescent boronolectins: an examination of the detailed chemistry issues important for the design. Medicinal Research Reviews. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 14.Lei M, Baldi A, Nuxoll E, et al. A hydrogel-based implantable micromachined transponder for wireless glucose measurement. Diabetes Technology & Therapeutics. 2006;8:112–122. doi: 10.1089/dia.2006.8.112. [DOI] [PubMed] [Google Scholar]
- 15.Arnold FH, Zheng W, Michaels AS. A membrane-moderated, conductimetric sensor for the detection and measurement of specific organic solutes in aqueous solutions. Journal of Membrane Science. 2000;167:227–239. [Google Scholar]
- 16.Li S, Davis EN, Anderson J, et al. Development of boronic acid grafted random copolymer sensing fluid for continuous glucose monitoring. Biomacro-molecules. 2009;10:113–118. doi: 10.1021/bm8009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai XX, Glidle A, Cooper JM. Miniaturized electroanalytical sensor systems in micromachined structures. Electroanalysis. 2000;12(9):631–639. [Google Scholar]
- 18.Park SI, Jun SB, Park S, et al. Application of a new Cl-plasma-treated Ag/AgCl reference electrode to micromachined glucose sensor. IEEE Sensors Journal. 2003;3(3):267–273. [Google Scholar]
- 19.Chinami K, Akira Y, Keishin K, et al. Study on sensing system configuration by MEMS technology. IEIC Technical Report. 2004;104:13–18. [Google Scholar]
- 20.Wang J, Chatrathi MP, Tian BM, et al. Microfabricated electrophoresis chips for simultaneous bioassays of glucose, uric acid, ascorbic acid, and acetaminophen. Analytical Chemistry. 2000;72(110):2514–2518. doi: 10.1021/ac991489l. [DOI] [PubMed] [Google Scholar]
- 21.Xie B, Ramanathan K, Danielsson B. Mini/micro thermal biosensors and other related devices for biochemical/clinical analysis and monitoring. Trends in Analytical Chemistry. 2000;19:340–349. [Google Scholar]
- 22.Bataillard P. Calorimetric sensing in bioanalytical chemistry: principles, applications and trends. Trends in Analytical Chemistry. 1993;12:387–394. [Google Scholar]
- 23.Pu C, Zhu ZH, Lo YH. A surface-micromachined optical self-homodyne polarimetric sensor for noninvasive glucose monitoring. IEEE Photonics Technology Letters. 2000;12(2):190–192. [Google Scholar]
- 24.Paranjape M, Garra J, Brida S, et al. A PDMS dermal patch for non-intrusive transdermal glucose sensing. Sensors and Actuators a-Physical. 2003 May;104(3):195–204. [Google Scholar]
- 25.Lin Y, Matson DW, Kurath DE, et al. Microfluidic devices on polymer substrates for bioanalytical applications. In: Ehrfeld W, editor. Microreaction Technology: Industrial Prospects. Springer-Verlag; 2000. pp. 451–460. [Google Scholar]
- 26.Zhao Y, Li S, Davidson A, et al. A MEMS viscometric sensor for continuous glucose monitoring. Journal of Micromechanics and Microengineering. 2007;17:2528–2537. doi: 10.1088/0960-1317/23/5/055020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Huang X, Davis EN, et al. Development of novel glucose sensing fluids with potential application to microelectromechanical systems-based continuous glucose monitoring. Journal of Diabetes Science and Technology. 2008;2(6):1066–1074. doi: 10.1177/193229680800200615. [DOI] [PMC free article] [PubMed] [Google Scholar]