Abstract
Historically, treatment of patients with cancer using chemotherapeutic agents has been associated with debilitating and systemic toxicities, poor bioavailability, and unfavorable pharmacokinetics. Nanotechnology-based drug delivery systems, on the other hand, can specifically target cancer cells while avoiding their healthy neighbors, avoid rapid clearance from the body, and be administered without toxic solvents. They hold immense potential in addressing all of these issues which has hampered further development of chemotherapeutics. Furthermore, such drug delivery systems will lead to cancer therapeutic modalities which are not only less toxic to the patient but also significantly more efficacious. In addition to established therapeutic modes of action, nanomaterials are opening up entirely new modalities of cancer therapy, such as photodynamic and hyperthermia treatments. Furthermore, nanoparticle carriers are also capable of addressing several drug delivery problems which could not be effectively solved in the past and include overcoming formulation issues, multi-drug-resistance phenomenon and penetrating cellular barriers that may limit device accessibility to intended targets such as the blood-brain-barrier. The challenges in optimizing design of nanoparticles tailored to specific tumor indications still remain; however, it is clear that nanoscale devices carry a significant promise towards new ways of diagnosing and treating cancer. This review focuses on future prospects of using nanotechnology in cancer applications and discusses practices and methodologies used in the development and translation of nanotechnology-based therapeutics.
Keywords: Nanoparticles, Drug Development, Cancer
Defining Oncology Applications for Nanotechnology Constructs
Moving a prospective new drug to the clinic is an arduous task. Despite decades of experience and well-defined best practices for evaluating small molecules as drug candidates, only one of every 5,000 to 10,000 prospective formulations reaches FDA approval and only 5% of oncology drugs entering Phase I clinical trials are approved [1].
Recently, chemists, pharmaceutical scientists, biologists, biomedical engineers and oncologists have turned to nanotechnology in their quest for innovation and improvement of the success rate in drug development. Nanotechnology is defined by the National Nanotechnology Initiative (http://www.nano.gov) as research and technology development at the atomic, molecular or macromolecular scale leading to the controlled creation and use of structures, devices and systems with a length scale of approximately 100 nanometers (Figure 1). Examples of nanoparticle platforms are included in Figures 1 and 2. The multi-functional constructs based on novel nanomaterials can be delivered directly to the tumor site and eradicate cancer cells selectively. An appropriate nano-construct design allows for improved drug efficacy at lower doses as compared to the small molecule drug treatment, wider therapeutic window, and lower side effects. In addition to established therapeutic modes of action, nanomaterials are opening up entirely new modalities of cancer therapy, such as photodynamic and hyperthermia treatments. Furthermore, nanoparticle carriers are also capable of addressing several drug delivery problems which could not be effectively solved in the past and include overcoming multi-drug-resistance phenomenon and penetrating cellular barriers that may limit device accessibility to intended targets, blood-brain-barrier, among others. PEGylated- liposomal doxorubicin (Doxil®, Caelyx®), liposomal daunorubicin (DaunoXome®), liposomal cytarabine (DepoCyt®), and paclitaxel albumin-bound particles (Abraxane®) are the only members of this relatively new class of agents that are approved in the United States (US)[2-7].
Figure 1.
Definition of nanotechnology and examples of nanotechnology platforms used in drug development. This figure was obtained with permission from McNeil et al, Nanotechnology for the Biologist. Journal of Leukocyte Biology, Volume 78, pages 585-594, 2005 (Figure 3 of this paper).
Figure 2.
Collage of nanomedical particles and devices developed by members of the NCI Alliance for Nanotechnology in Cancer. This figure was obtained with permission from Hinkal et al, Cancer Therapy Through Nanomedicine. IEEE Nanotechnology Magazine, Volume 5(2), pages 6-12, June 2011 (Figure 1 of this paper).(Photo courtesy of the NCI Alliance for Nanotechnology in Cancer, Nanotechnology Image Library).
The challenges in optimizing design of nanoparticles tailored to specific tumor indications still remain; however, it is clear that nanoscale devices carry significant promise towards new ways of diagnosing and treating cancer. This review focuses on future prospects of using nanotechnology in cancer applications and discusses practices and methodologies used in the development and translation of nanotechnology-based therapeutics.
Design Trends for a Successful In Vivo Carrier
General Concepts
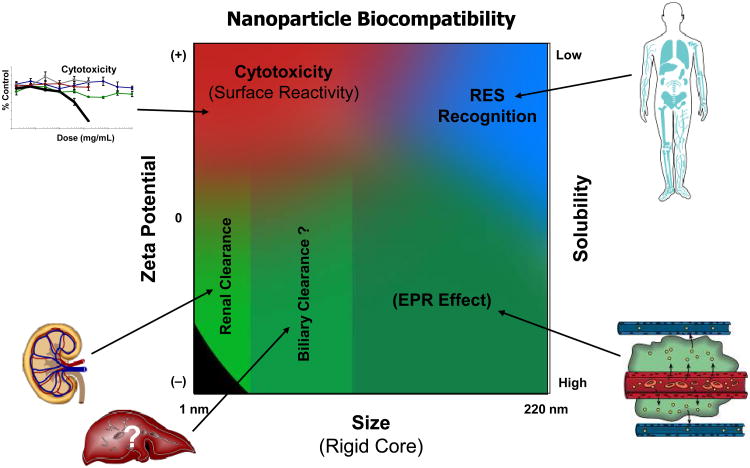
The therapeutic effects of many anti-cancer drugs and the outcome of anti-cancer therapies could be significantly improved if: 1) delivery of the drug occurs specifically to tumors (cancer cells) or preferably inside specific organelles in cells and 2) reduction of drug toxic side-effects is achieved. In the case of poorly soluble drug candidates the solubility/bioavailability problem could also be overcome. Various pharmaceutical nanocarriers (e.g., liposomes, polymeric micelles, and polymeric nanoparticles) have been used in preparing novel dosage formulations with good bioavailability and specific drug delivery to tumors. Zeta potential, size, cationic surface charge and solubility are factors that affect the biocompatibility of these nanocarriers (Figure 3). These factors influence the cytotoxicity (surface reactivity), clearance process (renal or biliary), MPS (mononuclear phagocyte system)/RES (reticuloendothelial system) recognition and EPR (enhanced permeability and retention) effect.
Figure 3.
Nanoparticle biocompatibility trends. The zeta potential, size, and solubility affect the cytotoxicity (surface reactivity), clearance process (renal or biliary), MPS/RES recognition and EPR effect. This figure was obtained with permission from McNeil. Nanomedicine and Nanobiotechnology, Volume 1(3), pages 264–271, May/June 2009 (Figure 3 of this paper).
Multi-functional nanomedicines which are being developed can combine not only different biological properties (e.g., increased circulation time in blood, ability to accumulate in tumors via the enhanced permeability and retention effect, and stimulus-sensitivity), but also carry a combination of several drugs and diagnostic labels/markers [8]. Targeted nanosystems which have a capability of targeting specifically cell surfaces or intracellular components and as such contribute to enhanced accumulation of the drug in tumor are also emerging [9]. Currently, lipid-based nanomedicines (such as liposomes, lipid-core polymeric micelles, solid lipid nanoparticles) have gained increased interest due to their good biological compatibility, easy control over their composition and properties, and suitability for scale-up to large scale production at reasonable costs [10].
Active Targeting with Ligands and Antibodies
Nearly all cancer nanomedicines utilize some aspect of targeting. Most rely solely upon “passive” targeting, also known as the enhanced permeability and retention (EPR) effect, which allows for the extravasation of nanoparticles from the circulation via abnormal fenestrations in tumor vasculature.
“Active” targeting of nanomedicines provides the additional targeting mechanism of receptor-mediated binding of nanoparticles to surface receptors expressed on tumor cells or blood vessels such as ανβ3-integrins [11], folic acid [12], and prostate specific membrane antigen, also known as PSMA [13]. Research has been conducted on several targeting ligands, including antibodies, aptamers, peptides and small molecules. Studying transferrin-targeted nanoparticles, the Davis group at the California Institute for Technology demonstrated that actively targeted nanoparticles deliver a higher payload to cancer cells than their passively targeted counterparts [14].
A successful, actively-targeted nanomedicine requires a delicate balance of ligand content and surface exposure that minimizes immunological recognition and clearance to provide sufficient nanoparticle circulation time to reach the target cells, while achieving appropriate binding affinity to the surface receptors expressed on tumor cells or blood vessels. The presence of multiple targeting ligands per nanoparticle yields a binding affinity stronger than for the ligand alone, thus enhancing the ligand-receptor binding interaction for the nanoparticles.
The Langer group at MIT and Farokhzad group at Harvard University demonstrated the importance of this balance with their aptamer-functionalized, actively-targeted PLGA-PEG nanoparticles where 5% aptamer surface coverage was ideal for optimal tumor accumulation [13]. These fundamental findings have been advanced by BIND Biosciences, replacing the aptamer with a small molecule targeting ligand better suited for pharmaceutical development, with their PSMA-targeted docetaxel (BIND-014) in a Phase 1 clinical study for a range of solid tumor cancers (ClinicalTrials.gov Identifier: NCT01300533). The efficacy of BIND-014 PSMA-targeted docetaxel nanoparticles in PSMA-expressing human LNCaP prostate cancer xenograft mouse model is presented in Figure 4.
Figure 4.
Efficacy of BIND-014 PSMA-targeted docetaxel nanoparticles in PSMA-expressing human LNCaP prostate cancer xenograft mouse model. Passively targeted docetaxel nanoparticles (PTNP, green) decrease tumor growth rate compared to conventional docetaxel (DTXL, red). BIND-014 (blue) is identical to PTNP in every way except for PSMA-targeting ligand on the surface. The additional active PSMA binding by BIND-014 results in tumor shrinkage of nearly 50%, a vast improvement over DTXL. Mice were treated four times with five mg/kg of DTXL, PTNP or BIND-014 at four day intervals (i.e., Q4D x 4). This figure was obtained with permission from Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105(7):2586-91.
Design Trends for Nanoparticle Intended for Delivery of Special Agents or Distinct Indications: Highly toxic agents
Since many anti-cancer drugs are highly toxic, it is very desirable to develop nanomedicines which limit their toxicity at tumor sites with a minimal toxicity towards normal tissues. Ideally, drugs should be developed which possess toxicity only against diseased or mutated cells. For example, some inhibitors of poly(ADP-ribose) are highly toxic only against cell lines exhibiting certain cancer-associated mutations and do not affect cells without such mutations [15]. However, the development of such drugs with mechanism-based selectivity is in its infancy. Approaches that are more developed are associated with the specific targeting of drug-loaded nanoparticles to tumor cells or providing controlled or on-demand drug delivery. The paradox of targeted delivery is that even with effective targeting, the majority of the administered dose ends up in normal tissues throughout the body resulting in pronounced non-specific off-target toxicity. If targeting efficiency could be increased such that as little as 3% of the injected dose reaches the tumor site, this would allow for the dramatic decrease in the total administered dose, thus sharply diminishing the toxic effect to normal tissues and still providing more drug in the tumor than after “traditional” administration. Thus, the high cytotoxicity of many anti-cancer drugs can be predominantly localized only in the tumor. On-demand drug delivery by mesoporous silica nanoparticles functionalized with a pH-sensitive nano-valve that only opens in acidifying intracellular endosomal compartments is an example of a novel type of nanocarrier capable of controlled drug release [16].
Pharmacologic Characterization and Properties
Pharmacologic Nomenclature
The nomenclature used to describe the pharmacokinetic disposition of carrier-mediated drugs are termed encapsulated or conjugated (drug within or covalently bound to the carrier), released (the active-drug released from the carrier), and sum total (encapsulated or conjugated drug plus released drug) [17, 18]. The released drug has also been called the legacy drug, regular drug, or warhead [17-19]. The released drug consists of a protein bound or free drug. The pharmacokinetic disposition of these nanoparticle agents is dependent upon the carrier and not the parent-drug until the drug is released from the carrier [20]. The drug that remains encapsulated in nanoparticles or linked to a conjugate or polymer is an inactive-prodrug and thus the drug must be released from the carrier to be active [17, 21]. Whether the drug needs to be released outside of the cell in the tumor extracellular fluid (ECF) or within the cell depends on the formulation of the carrier and the mechanism of release [17, 19, 22]. After the drug is released from the carrier, the pharmacokinetic disposition of the drug will be the same as after administration of the non-carrier form of the drug [17, 18]. In certain formulations, there may be unencapsulated drug along with the encapsulated drug; separation and quantitation of each of these species would help explain complex pharmacokinetics. Many formulations use a combination of drugs for cancer. In such cases, the encapsulation/conjugation efficiencies and release profiles of individual drugs may be different depending on the hydrophobicity/hydrophilicity of the drug and the formulation platform. For synergy, appropriate concentration of drugs reaching the desired site of action, such as tumor, is critical for efficacy. As such, performing appropriate pharmacokinetic studies to understand the individual release profiles and fine tuning the nanomaterial platform for enhanced efficacy is critically important. Thus, the pharmacology and pharmacokinetics of these agents are complex and detailed studies must be performed to evaluate the disposition of the encapsulated or conjugated form of the drug, and the released active-drug and metabolites in plasma, tumors and tissues [21].
Systemic, Tissue and Tumor Disposition of Nanoparticles
Nanoparticles can alter both the tissue distribution and the rate of clearance of the drug by making the drug take on the pharmacokinetic characteristics of the carrier [23-25]. Pharmacokinetic parameters of the nanoparticles depend on the physiochemical characteristics of the nanoparticle, such as size, surface charge, shape, nature and density of coating, composition, stability, membrane lipid packing (in case of liposomal particles), steric stabilization, deformability, dose, and route of administration [23]. The primary sites of accumulation of nanoparticles are the tumor, liver, and spleen compared to non-nanoparticle formulations [23, 24, 26-30]. The development of PEGylated nanoparticles was based on the discovery that incorporation of PEG onto the surface of nanoparticles yields preparations with superior prolonged plasma exposures and tumor delivery compared to non-PEGylated nanoparticles [23, 26, 27].
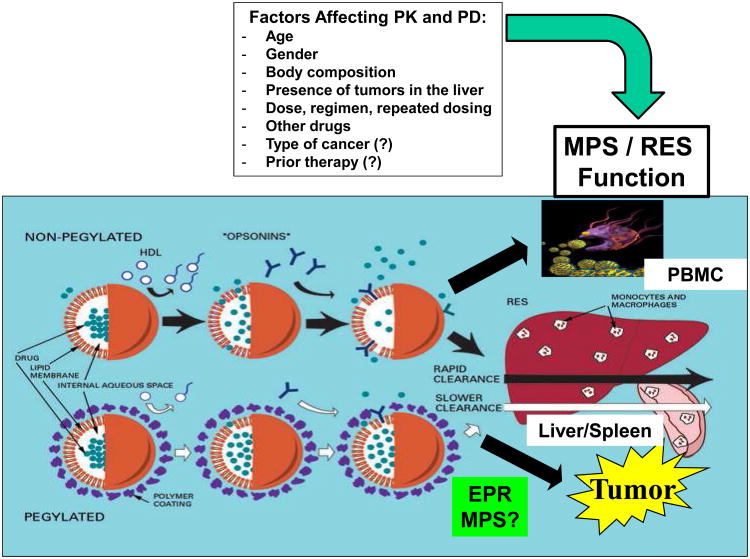
The clearance of nanoparticles has been proposed to occur by uptake of the carrier by the reticuloendothelial system (RES), which is also called the mononuclear phagocytic system (MPS) (Figure 5) [23, 27, 31]. The MPS uptake of cationic or hydrophobic nanoparticles results in their rapid removal from the blood and accumulation in tissues involved in the MPS, such as the liver and spleen. Uptake by the MPS may result in irreversible sequestering of the encapsulated drug in the MPS, where it can be degraded. In addition, the uptake of the nanoparticles by the MPS may result in acute impairment of the MPS and toxicity. The presence of the negatively charged coating on the outside of the nanoparticles does not prevent uptake by the MPS, but simply reduces the rate of uptake (Figure 5) [25-27, 32]. The exact mechanism by which steric stabilization of nanoparticles decreases the rate of uptake by the MPS is unclear [23, 30, 32].
Figure 5.
Summary of the clearance of nanoparticle agents via the mononuclear phagocyte system (MPS). Most of the studies evaluating factors affecting nanoparticle agents have been performed in patients receiving PEGylated and non-PEGylated liposomal agents and thus these carrier systems are depicted in the figure. However, in theory these factors may also affect other nanocarrier systems but need to be evaluated in future studies. Nanoparticle agents are primarily cleared via the monocytes, macrophages and dendritic cells of the MPS that are located in the liver, spleen, and blood. In addition, the MPS cells in the lung and bone marrow also appear to be involved. The tumor delivery of nanoparticle agents is determined by the EPR effect and potentially MPS in tumors. The factors affecting the PK and PD of nanoparticle agents in patients and animal models included age, gender, body composition, tumors in the liver, the dose and regimen, other drugs, type of cancer and prior therapy.
The development of effective chemotherapeutic agents for the treatment of solid tumors depends, in part, on the ability of those agents to achieve cytotoxic drug exposure within the tumor via the EPR effect [33, 34]. In addition, studies suggest that the cells of the MPS may also play a role in the tumor disposition of liposomal agents and in the sensitivity of the tumors to liposomal agents [35-37]. Once in the tumor, the non-ligand targeted PEGylated nanoparticles are localized in the extracellular fluid (ECF) surrounding the tumor cell, but do not enter the cell [38, 39]. Thus, for the nanoparticles to deliver the active form of the anti-cancer agent, the drug must be released from the nanoparticle into the ECF and then diffuse into the cell, or taken up into the cell directly and then released [21]. As a result, the ability of the nanoparticle to carry the anti-cancer agent to the tumor and release it into the ECF is equally important factors in determining the antitumor effect of nanoparticle anti-cancer agents. In general, the kinetics of this local release is unknown as it is difficult to differentiate between the nanoparticle-encapsulated and released forms of the drug in solid tissue, although with the development of microdialysis, this is becoming easier [21].
Factors Affecting Pharmacokinetic and Pharmacodynamic Variability in Patients
There is significant interpatient variability in the pharmacokinetic disposition of nanoparticle and liposomal encapsulated agents in patients [17, 37, 40-42]. It appears that the pharmacokinetic variability of the carrier formulation of a drug is several-fold higher compared with the non-nanoparticle formulation of the drug [17, 41, 42]. Thus, there is a need to identify factors associated with the significant pharmacokinetic variability. Most of the studies evaluating the factors that affect the pharmacokinetic variability of nanoparticle agents in patients have involved liposomal agents. The factors associated with altered pharmacokinetics of PEGylated liposomal agents are age, body composition, gender, presence of tumors in the liver, and changes in and the function of monocytes in blood (Figure 5). There is a 2- to 3-fold lower clearance of PEGylated liposomal doxorubicin (Doxil®) and PEGylated liposomal CKD-602 (S-CKD602) in patients ≥ 60 years of age compared with patients < 60 years of age [43]. Results also suggest that monocytes in blood engulf S-CKD602 which causes the release of CKD-602 from the liposome and toxicity to the monocytes, and that the effects are more prominent in patients < 60 yrs. [44]. Patients with a lean body composition have an increased plasma exposure of encapsulated drug after administration of S-CKD602 (P = 0.02) (Figure 4). It has also been reported that women have a lower clearance of encapsulated drug after administration of PEGylated liposomal agents compared with men. Population pharmacokinetic studies have reported that patients with refractory solid tumors that have primary or metastatic tumors in the liver have a higher clearance of S-CKD602 compared with patients that do not have tumors in their liver [45]. In theory, the presence of tumors in the liver may induce the MPS cells in the liver and thus increase the sequestering of the liposome in the liver which would lead to an increase in systemic clearance of encapsulated drug [45].
Gabizon and colleagues reported that the clearance of Doxil® decreased by approximately 25 to 50% from cycle 1 to 3 (Figure 5) [46]. In addition, La and colleagues reported that this reduction in clearance of Doxil® from cycle 1 to cycle 3 was associated with a reduction in pre-cycle monocyte count [47]. These studies suggest that there is a reduction in the clearance of liposomes over time and thus dose reductions may be needed in subsequent cycles to minimize the risk of toxicity [46]. Interestingly, repeat dose studies of PEGylated liposomal doxorubicin in mice and rats did not report accumulation of drug in plasma suggesting that these preclinical models may not accurately reflect the disposition of PEGylated liposomal agents after repeated dosing [48, 49]. Thus, there is also a need to develop better preclinical animal models for pharmacology and toxicology studies of liposomal and nanoparticle agents.
Future studies need to evaluate the mechanism of clearance of nanoparticle agents and identify the factors associated with pharmacokinetic and pharmacodynamic variability of nanoparticle anti-cancer agents in patients and specifically in tumors [30, 40, 50-53]. Future studies also need to develop phenotypic probes that can be used to predict this variability and individualize therapy with nanoparticle agents.
Translation of Nano-therapeutics – Perception and Reality
The successful translation of anti-cancer nanomedicines from bench to bedside requires significant efforts which include the development of reasonably simple, inexpensive and scalable protocols to prepare such medicines, and control over the biological behavior and pharmacological properties of these preparations. In general, the real use of nanomedicine as an essential part of personalized medicine will also require the development of multiple regulatory guidelines and appropriate training and education [54]. The creation of an “industrial culture” of making nanomedicines including their standardized testing and characterization is another challenge. It appears that nanomedicines currently under development will target the most common cancers, such as breast and prostate cancer [55]. The first generation of anti-cancer nanomedicines is based on their “passive” (EPR-mediated) delivery into tumors, and drugs (such as Doxil®) and diagnostic agents (such as SPION (superparamagnetic iron oxide nanoparticles)-based NMR contrast agents) are now approved and in active clinical trials, respectively [56]. Still, despite a clear understanding that effective anti-cancer nanomedicines should specifically recognize the disease (diseased cells), provide an imaging signal from the affected zone, and effectively deliver drugs in this zone, a clear set of uniform requirements to such preparation is still absent [57]. Despite receiving significant attention [58], rational design of such systems has yet to suggest clear guidelines for clinical translation. Another important, but unresolved, issue for the translation of nanoparticle-based medicines is the role of nanoparticle shape and architecture in their biological behavior and therapeutic properties [59].
Animal models for Preclinical Evaluations of Nanomedicines
As the translation of nanomedicines into clinical practice is still in the early stages, the concordance of preclinical and clinical data with regard to pharmacokinetics, toxicology and efficacy are still unknown. However, the developing paradigms underlying nanoparticle distribution and biocompatibility offer perspective as to what preclinical models are likely to have the greatest translational utility. One important determinant of nanoparticle distribution, is the mononuclear phagocyte system (MPS), previously referred to as reticuloendothelial system, which is responsible for particle sequestration [60]. It appears that the primary MPS organ(s) of particle sequestration is species dependent, with more common laboratory animals (e.g., dog, rat and rabbit) having particle distribution primarily to Kupffer cells of the liver and splenic macrophages, similar to man [61]. In less commonly used preclinical species (e.g., goat and pig) distribution of particles to pulmonary intravascular macrophage is primarily observed [61]. For example, intravenous injection of nanoparticulate iron oxide resulted in high pulmonary intravascular uptake (> 85% of dose) in sheep, calf, pig, goat and cat, while uptake was primarily observed in hepatic Kupffer cells (> 65% of dose) in monkey, hyrax, hamster, rabbit, guinea pig, rat, mouse and chicken [62]. This would support the use of the more traditional species for toxicological and pharmacokinetic evaluation of nanomedicines due to the similar MPS distribution profile. This correspondence in nanoparticle distribution for common laboratory species and man is also supported by a recent allometeric analysis of clearance of a PEGylated TNF bound gold nanoparticle in rat, rabbit and man [63]. Across these species, the TNF clearance-brain-weight product was found to scale proportional to body weight, as has been found for many macromolecular therapeutics, and would suggest common mechanisms of nanomedicine disposition in these species. However, a recent study by Caron and colleagues found that the clearance of a series of PEGylated liposomal anti-cancer agents did not allometrically scale from mice, rats, and dogs to patients [64]. Thus, the ability to scale the pharmacokinetic disposition of nanoparticle agents across species may be nanoparticle and model specific. In the study by Caron, the physiologic co-factors that produced the best scaling of clearance across animal models and patients were factors associated with the MPS, such as monocyte count in blood. Thus, new methods of allometric scaling of nanoparticle agents and the use of MPS characteristics and function need to be evaluated and developed.
The issue of selective tissue distribution and accumulation of nanoparticles is a concern [65]. Accumulation of nanoparticles in organs of the MPS, or selectively targeted tissues, is a common occurrence for nanomedicines. For this reason, repeat-dose tissue distribution studies may be required to identify such tissues, which may then be subjected to greater scrutiny in subsequent toxicity studies [65]. Similarly, nanoparticle biopersistance is also a concern, especially for metals and non-biodegradable polymers, and may necessitate lengthy toxicology studies to identify potential chronic toxicities.
In addition to general pharmacokinetic evaluation, assessing tumor distribution and efficacy of oncology nanomedicines in relevant preclinical cancer models is also crucial. One issue unique to nanomedicine tumor distribution in comparison to small molecules is the dependency upon long systemic circulation and vascular permeability for uptake into the interstitial space. Studies have identified nanoparticle properties associated with long circulation, such as PEGylation and small size, that correlate with increased tumor concentration maxima and total exposure (area-under-the-time-concentration-curve, AUC) [66]. However, there have been no systematic studies evaluating the clinical relevance of vascular permeabilities found in these animal models. Studies have shown that tumor vascular pore size can be highly variable in animal xenografts, ranging from hundreds of nanometers to microns [67]. As nanomedicine tumor permeability is at least partially dependent upon vascular pore size, it is important that the tumor model vasculature resemble the clinical case [68]. In addition to histological type, tumor vascular permeability has also been shown to vary depending on site of tumor implantation, with orthotopic brain tumors, for example, having lower permeability than peripherally implanted tumors [69]. This would suggest a role for the tumor microenvironment in vascular permeability.
Preliminary studies suggest that some clinical tumors contain ultrastructural features, such as fenestrations, similar to those found in animals models [70]. However, other human cancers, such as certain brain tumors, appear to be devoid of these pores [71]. Since the vascular permeability of human cancers in comparison to animal models have not been thoroughly evaluated, the selection of animal models most appropriate for specific cancer types with regard to nanoparticle permeability is difficult. At this time, the same recommendations for small molecule oncology animal model selection would apply to nanomedicines. NCI's developmental therapeutics program after analyzing the clinical and preclinical data sets of several small molecule chemotherapeutics, found that medicines that were successful in multiple xenograft models were more likely to succeed in the clinic [72]. Since histological correlations of treatment success between clinical and preclinical cancers were not observed in most cases, this would suggest that xenograft cancer models are not predictive of specific cancer activity, but rather activity in general. Together with the variability in tumor vascular architecture and nanomedicine permeability discussed above, this would suggest that evaluation of nanomedicines in multiple models is prudent. While veterinary cancers and transgenic models may be more physiologically relevant than xenograft/syngeneic models, they often suffer from low availability or prolonged time in generating tumors. For this reason, xenograft/syngeneic models are the most commonly used models. Syngeneic models, with intact immune systems, would conceivably be an improvement over xenograft models in athymic nude or SCID mice, especially for evaluation of nanomedicines, which are prone to immunological interactions (see below). Likewise, orthotopically implanted tumors, with relevant microenvironments, may also have advantages.
Previous reviews have suggested the current regulatory framework for assessing the safety of small molecules, biologics and devices, is considered sufficient for nanomedicines [65]. While that appears to be true at this time, the choice of the most relevant preclinical models for toxicological evaluation has yet to be identified. Major toxicological issues commonly observed with cancer nanomedicines are development of immunological and hematological complications [73]. As an example, cationic dendrimers have recently been reported to induce disseminated intravascular coagulation in mice [74]. Anaphylactoid reactions are a primary concern for translational development of iron oxide nanoparticle MR contrast agents, which may be related to the polymer coatings used and has resulted in removal of many from the market [75, 76]. Additionally, endotoxin contamination and associated immunological complications, such as complement activation and pyrexia, is a common issue for nanomedicines [77]. For this reason, the use of immunologically sensitive species such rabbit, in addition to historic models (e.g., rodents and dog) which tend to be less sensitive, is warranted early in preclinical development to identify these possible concerns. A meta-analysis comparing study design issues of nanoparticle and small molecule anti-cancer agents in preclinical models and in phase I clinical trials was performed by Morgan and colleagues [78]. In this study, the degree of dose escalation from starting dose to MTD, number of dose levels and time to complete the phase I clinical studies were significantly greater for nanoparticle agents compared with small molecule drugs. These data suggest that the standard animal models and cross-species scaling paradigms employed to define the starting dose in the phase I clinical study for small molecule agents may not be optimal for nanoparticle agents.
In addition to the suggestions regarding animal model selection made above, proper study design is very important for evaluation of nanomedicines. Whereas the use of intraperitoneal administration in place of intravenous administration may have little consequence when evaluating preclinical efficacy or toxicity of small molecules in rodents, due to the high tissue permeability of these agents, this is not likely the case for many nanomedicines. Due to their size, nanomedicines do not freely diffuse across tissue barriers, and for this reason intraperitoneal administration cannot be thought of as a parenteral route equivalent to intravenous administration. Indeed, studies have shown substantial differences in distribution when comparing intraperitoneal and intravenous routes of administration [79]. Thus, it is important to use the intended clinical dosing route when evaluating nanomedicines. Another issue is the use of proper controls. There have been many examples of both nanoparticle-dependent toxicities and distribution-related shifts in drug toxicities [80, 81]. For this reason it is important to include both empty (drug-free) nanoparticles and a non-nanoparticle (small molecule) formulation of the drug as controls, in order to identify toxicities related to the nanoparticle platform and shifts in drug-related toxicity.
Role of Nanotech Characterization Laboratory
The Nanotechnology Characterization Laboratory is part of the Alliance for Nanotechnology in Cancer at NCI's federally funded research and development center (FFRDC; managed by SAIC Frederick, Inc.) and is a resource for preclinical development of nanomaterial based drug delivery and imaging agents that are beyond a proof-of-principle stage of development, with demonstrated biological efficacy. The facility has been established to accelerate clinical translation of promising nanotechnology derived formulations. This NCI funded resource, available to academic investigators, industry collaborators and government labs, is established by NCI in collaboration with the U.S. Food and Drug Administration (FDA), and the National Institute of Standards and Technology (NIST). Once a project is approved through a simple submission and material transfer agreement process, a large scale batch is obtained from the collaborator for testing at NCL facilities. The lab conducts preclinical assessment through an established assay cascade that includes thorough physico-chemical assessment, relevant in vitro studies to investigate biocompatibility and in vivo ADME/tox, efficacy and imaging studies in rodent models as appropriate. The outcome of the NCL studies is a client report that is provided to the collaborator to further their concept towards an investigational new drug (IND) submission or an investigational device exemption [82] with the FDA.
In addition to the pre-clinical assessment, NCL is actively engaged in standard protocol development and reference material standards development through collaborations. The NCL also plays an active role in educational and knowledge sharing efforts to advance the nanomedicine field. For further information, visit: http://ncl.cancer.gov
Scale-up and Manufacturing Issues
A critical element for successful development and commercialization of any pharmaceutical product is a scalable, reproducible manufacturing process. Besides typical considerations and challenges with scale up and commercial manufacture, there are additional challenges for nanotechnology-based products for treating cancer. Some of the most critical aspects of the manufacture of cancer nanomedicines include sterility (most will be administered intravenously), nanoparticle size and polydispersity, encapsulation efficiency, removal of free drug, and drug release rate. In addition, for actively-targeted cancer nanomedicines that employ receptor-mediated binding of nanoparticles to tumors, the amount and appropriate surface exposure of targeting ligand must be addressed.
The most important quality parameter for an injectable product is sterility. Achieving sterility may be quite difficult since terminal heat sterilization could disrupt nanoparticle size and polydispersity, negatively impacting trafficking and biodistribution. Also, if nanoparticle size is much greater than 100 nm and polydispersity is broad, sterile filtration may not work. This leaves few options, including use of sterile raw materials and aseptic processing, which is very costly, or gamma irradiation, which many nanoparticle products also may not withstand.
The removal of unencapsulated drug from nanoparticle drug products is often difficult, yet free or non-encapsulated drug contamination will compromise both efficacy and safety. Likewise, if the manufacturing process cannot control the drug release rate from nanoparticles, performance will be unreliable and potentially unsafe if there is a rapid or high burst of drug released from the carrier. Finally, actively-targeted nanomedicines require a well-controlled process providing consistent ligand exposure on nanoparticle surfaces to fully benefit from effective nanoparticle binding to yield higher tumor drug concentrations and/or cellular trafficking.
Interaction with Regulatory Agencies
Pathway to the Clinic: Pre-IND and IND Related Studies
Although the FDA and pharmaceutical industry have developed standards to assess drug and material biocompatibility, immune reactivity, purity and sterility, the unique properties of nanomaterials often hamper the execution of these standardized protocols and require special consideration. Thus, while FDA has criteria for the preclinical data that should be presented in an IND for small-molecule drugs, there is no standardized set of characterization methods for engineered nanomaterials. In consideration of the novel properties and often multi-component nature of nanoparticle-based therapeutics, a rational characterization strategy is comprised of three elements, namely physicochemical characterization, in vitro assays and in vivo studies. For the physico-chemical characterization, one needs to have reproducible synthesis and characterization assays for batch-to-batch consistency that are predictive of in vivo fate. Although the basic criteria for the chemistry, manufacture, and control (CMC) section of an IND filing is the same, the methodologies and instrumentation should be appropriate to the type of nanomaterial being assessed. Additional physicochemical properties that need to be considered include particle size, size distribution, polydispersity, surface ligand density, surface area, surface charge, surface functionality, shape and confirmation, composition, purity and stability.
While the number of properties one needs to assess seem to be daunting, once a set of characterization assays and tools have been identified that are predictive of components that cause variation in safety, efficacy and potency profiles, the methodologies can be standardized to qualify lots for batch-to-batch consistency. Nanomaterial size and surface characteristics are critical for predictive biodistribution and toxicity. For example, most nanomaterials have polyethylene glycol (PEG) coating. Differences in polydispersity of the PEG used and the density of the ligands on the surface of the nanoparticles, will result in significantly different toxicity profiles. For core-shell nanoparticles, impurities can come from the core and shell reagents that are used. They need to be appropriately assessed to see if residual free components are present in the drug product. If so, they need to be quantified using appropriate methods. Purity in the nanomedicine sense can be affected by the presence of residual solvents, bound and free components (such as unchelated Gadolinium or free drug) and finally homogeneity, inhomogeneity and heterogeneity in the ligand distribution that will have significant biological impact.
Apart from these parameters, one has to measure the stability of the formulations as a function of time, storage, temperature, pH, light (photo stability), diluent, vehicle, lyophilization, and centrifugation with appropriate methods that will be predictive of biological effects. These characterization methodologies become much more challenging for multifunctional nanomaterials intended for drug delivery and imaging. Some of these challenges can be addressed during the synthesis, purification and characterization steps in a multi-step synthetic methodology which would allow for purification of unreacted components and have controls in place to assure uniformity from multiple lots. For self-assembly methodologies, such as in the case of liposomes or emulsions, the characterization for a multi-component system is significantly more challenging. Thus, optimization of the methodology itself with appropriate controls is critical for their success in translation. In the case of targeted drug delivery systems, such as those with active targeting ligands that bind to over expressed receptors on tumor, a bioassay predictive of in vivo behavior is critical to have as part of the analytical techniques to assure activity.
In vitro characterization is principally performed to elucidate mechanisms of biological interaction and toxicity and not strictly to screen for biocompatibility. Nonetheless, these in vitro studies could be very useful to identify areas requiring attention during execution of in vivo animal studies. While a host of in vitro studies can be carried out with primary and transformed tissue culture cells to assess features such as nanoparticle uptake, subcellular localization, intracellular drug delivery, cytotoxic killing, ROS generation and pro-inflammatory effects, biocompatibility can also be evaluated ex vivo, using blood or blood cells to discern effects on coagulation, hemolysis, platelet aggregation, complement activation and phagocytosis. Many in vitro assays can be used to elucidate potential problems one might encounter during the in vivo assessment phase. While all in vitro assays are not predictive of in vivo outcome, a set of relevant in vitro assays may be used to characterize potential issues with the nanoparticle formulation. Since most rodent animal models are not predictive of human immunotoxicity, the use of human blood, albeit in an in vitro setting, would point to potential issues during clinical phase of development and can be used as a screening tool and to optimize the formulation. An important in vitro analysis is also checking sterility and endotoxin contamination. Preclinical pharmacology and toxicity studies in animals should be conducted in the most clinically relevant animal model, as discussed above.
Regulatory agencies are becoming increasingly stringent regarding characterization of the particle size distribution (PSD) of nanotechnology-based products. Since the PSD may have a significant influence on the biodistribution and biological efficacy of the formulation, this parameter is critical to measure and track. This is not a trivial task and needs to be well understood in order to generate meaningful and actionable data. A review of particle size analysis is beyond the scope of this article and the reader is referred to the literature [83].
Summary and Future Directions
Historically, treatment of patients with cancer using chemotherapeutic agents has been associated with debilitating and systemic toxicities, poor bioavailability, and unfavorable pharmacokinetics. Nanotechnology-based drug delivery systems which can specifically target cancer cells while avoiding their healthy neighbors, avoid rapid clearance from the body, and be administered without toxic solvents hold immense potential in addressing all of these issues. Such drug delivery systems will lead to cancer therapeutic agents which are not only less toxic to the patient but also significantly more efficacious.
With Doxil® and Abraxane [84, 85] approved by FDA and several new agents undergoing clinical trials, there is a growing confidence that nanotechnology-based therapeutics will become an important addition to currently available treatments. It is possible that initially these new formulations will have limited use due to their incremental improvement in performance and high cost. However, emerging research efforts indicate that nanotechnology can address uniquely serious cancer problems which do not have existing solutions. For example, effective systemic delivery of siRNA has been demonstrated to date only using nanoparticle delivery vehicles [86]. Additional host of examples include reduction or elimination of multi-drug resistance [16, 87, 88], broadening of therapeutic index of existing drug formulations [89-91], and development of anti-metastatic drugs [92-96]. Thus, persistent further development of nanoparticle drug delivery technologies will continue and thus these approaches will eventually become important part of contemporary cancer care.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure: Jeff Hkrach is employed by and acknowledges ownership interest in BIND Biosciences of Cambridge, MA. Robert Lee is a member of the advisory boards of both Savara Pharmaceuticals and NanoScan Imaging. William Zamboni has benefited from research funding and served on the advisory boards of Alza Pharmaceuticals, Mersana Therapeutics, Covidien, Yakult Pharmaceutical Industry, and Hana BioSciences. SciDose, LLC has contributed to his research and he has served as a consultant for Liquidia Technologies and Azaya Therapeutics.
References
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Abi 007. Drugs in R&D. 2004;5(3):155–9. doi: 10.2165/00126839-200405030-00003. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. DaunoXome Package Insert. 1998 [Google Scholar]
- 4.Anonymous. DepoCyt Package Insert. 1999 [Google Scholar]
- 5.Anonymous. Abraxane Package Insert. 2005 [Google Scholar]
- 6.Krown SE, Northfelt DW, Osoba D, Stewart JS. Use of liposomal anthracyclines in Kaposi's sarcoma. Semin Oncol. 2004;31(6 Suppl 13):36–52. doi: 10.1053/j.seminoncol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Gordon AN, McGuire WP, Muggia FM. Liposomal anthracycline treatment for ovarian cancer. Semin Oncol. 2004;31(6 Suppl 13):91–105. doi: 10.1053/j.seminoncol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Wesselinova D. Current major cancer targets for nanoparticle systems. Curr Cancer Drug Targets. 2010;11(2):164–83. doi: 10.2174/156800911794328484. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Tian S, Petros RA, Napier ME, Desimone JM. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J Am Chem Soc. 2010;132(32):11306–13. doi: 10.1021/ja1043177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estella-Hermoso de Mendoza A, Campanero MA, Mollinedo F, Blanco-Prieto MJ. Lipid nanomedicines for anticancer drug therapy. J Biomed Nanotechnol. 2009;5(4):323–43. doi: 10.1166/jbn.2009.1042. [DOI] [PubMed] [Google Scholar]
- 11.Akers WJ, Zhang Z, Berezin M, Ye Y, Agee A, Guo K, et al. Targeting of alpha(nu)beta(3)-integrins expressed on tumor tissue and neovasculature using fluorescent small molecules and nanoparticles. Nanomedicine (Lond) 2010;5(5):715–26. doi: 10.2217/nnm.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myc A, Douce TB, Ahuja N, Kotlyar A, Kukowska-Latallo J, Thomas TP, et al. Preclinical antitumor efficacy evaluation of dendrimer-based methotrexate conjugates. Anticancer Drugs. 2008;19(2):143–9. doi: 10.1097/CAD.0b013e3282f28842. [DOI] [PubMed] [Google Scholar]
- 13.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105(7):2586–91. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 107(3):1235–40. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert H, Azzariti A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann Oncol. 2011;22(1):i53–9. doi: 10.1093/annonc/mdq667. [DOI] [PubMed] [Google Scholar]
- 16.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, et al. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc. 2010;132(36):12690–7. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clinical Cancer Research. 2005;11(23):8230–4. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 18.Zamboni WCER, Mountz JM, et al., editors. The Development of Liposomal and Nanoparticle Anticancer Agents: Methods to Evaluate the Encapsulated and Released Drug in Plasma and Tumor and Phenotypic Probes for Pharmacokinetic (PK) and Pharmacodynamic (PD) Disposition. Proceedings of NSTI Nanotechnology Conf; Santa Clara, CA. 2007. [Google Scholar]
- 19.Yurkovetskiy AV, Hiller A, Syed S, Yin M, Lu XM, Fischman AJ, et al. Synthesis of a macromolecular camptothecin conjugate with dual phase drug release. Mol Pharm. 2004;1(5):375–82. doi: 10.1021/mp0499306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicity. Biochim Biophys Acta. 2005;1711(1):25–32. doi: 10.1016/j.bbamem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Zuhowski EG, Pluim D, et al. Systemic and tumor disposition of platinum after administration of cisplatin or STEALTH liposomal-cisplatin formulations (SPI-077 and SPI-077 B103) in a preclinical tumor model of melanoma. Cancer Chemother Pharmacol. 2004;53(4):329–36. doi: 10.1007/s00280-003-0719-4. [DOI] [PubMed] [Google Scholar]
- 22.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J Control Release. 2007;121(1–2):10–8. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743. [PubMed] [Google Scholar]
- 24.Litzinger DC, Buiting AM, van Rooijen N, Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim Biophys Acta. 1994;1190(1):99–107. doi: 10.1016/0005-2736(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 25.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113(2):171–99. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 26.Allen T, Stuart DD. Liposomal pharmacokinetics. Classical, sterically-stabilized, cationic liposomes and immunoliposomes. In: AS J, editor. Liposomes: Rational Design. New York: Marcel Dekker, Inc; pp. 2005pp. 63–87. [Google Scholar]
- 27.Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068(2):133–41. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- 28.Laverman P, Carstens MG, Boerman OC, Dams ET, Oyen WJ, van Rooijen N, et al. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298(2):607–12. [PubMed] [Google Scholar]
- 29.Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA. Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother Pharmacol. 1999;43(1):1–7. doi: 10.1007/s002800050855. [DOI] [PubMed] [Google Scholar]
- 30.Working PK, Newman MS, Huang SK, Mayhew E, Vaage J, Lasic DD. Pharmacokinetics, Biodistribution and Therapeutic Efficacy of Doxorubicin Encapsulated in Stealth® Liposomes (Doxil®) Journal of Liposome Research. 1994;4(1):667–87. [Google Scholar]
- 31.Northfelt DW. AIDS-related Kaposi's sarcoma: still a problem, still an opportunity. J Clin Oncol. 1994;12(6):1109–10. doi: 10.1200/JCO.1994.12.6.1109. [DOI] [PubMed] [Google Scholar]
- 32.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–4. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RK. Delivery of molecular medicine to solid tumors. Science. 1996;271(5252):1079–80. doi: 10.1126/science.271.5252.1079. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni WC, Houghton PJ, Hulstein JL, Kirstein M, Walsh J, Cheshire PJ, et al. Relationship between tumor extracellular fluid exposure to topotecan and tumor response in human neuroblastoma xenograft and cell lines. Cancer Chemotherapy and Pharmacology. 1999;43(4):269–76. doi: 10.1007/s002800050894. [DOI] [PubMed] [Google Scholar]
- 35.Maruca LJ, Ramanathan RK, Strychor S, Zamboni BA, Ramalingam S, Edwards RP, et al. Age-related effects on the pharmacodynamic (PD) relationship between STEALTH liposomal CKD-602 (S-CKD602) and monocytes in patients with refractory solid tumors. J Clin Oncol (Meeting Abstracts) 2007;25(18_suppl):2576. [Google Scholar]
- 36.Yu NY, Conway C, Pena RL, Chen JY. STEALTH liposomal CKD-602, a topoisomerase I inhibitor, improves the therapeutic index in human tumor xenograft models. Anticancer Res. 2007;27(4B):2541–5. [PubMed] [Google Scholar]
- 37.Zamboni W, Friedland DM, Ramalingam S, editors. ASCO 2006. 2006. Final results of a phase I and pharmacokinetic study of STEALTH liposomal CKD-602 (S-CKD602) in patients with advanced solid tumors. [Google Scholar]
- 38.Harrington KJ, Lewanski CR, Northcote AD, Whittaker J, Wellbank H, Vile RG, et al. Phase I-II study of pegylated liposomal cisplatin (SPI-077) in patients with inoperable head and neck cancer. Ann Oncol. 2001;12(4):493–6. doi: 10.1023/a:1011199028318. [DOI] [PubMed] [Google Scholar]
- 39.Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7(2):243–54. [PubMed] [Google Scholar]
- 40.Zamboni WC, Whitner H, Potter DM, Ramanathan RK, Strychor S, Tonda ME, et al. Allometric scaling of STEALTH(R) liposomal anticancer agents. AACR Meeting Abstracts. 2005;2005(1):326. [Google Scholar]
- 41.Sidone B, Edwards R, Zamboni B, Strychor S, Maruca L, Zamboni W. Evaluation of body surface area (BSA) based dosing, age, and body composition as factors affecting the pharmacokinetic (PK) variability of STEALTH liposomal doxorubicin (Doxil) AACR Meeting Abstracts. 2007;2007(3_Molecular_Targets_Meeting):C107. [Google Scholar]
- 42.Zamboni WCML, Strychor S, editors. Age and body composition related-effects on the pharmacokinetic disposition of STEALH liposomal CKD-602 (S-CKD602) in patients with advanced solid tumors. ASCO; 2007. [Google Scholar]
- 43.La-Beck NM, Zamboni BA, Gabizon A, Schmeeda H, Amantea M, Gehrig PA, et al. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother Pharmacol. doi: 10.1007/s00280-011-1664-2. Epub 2011/05/19. [DOI] [PubMed] [Google Scholar]
- 44.Zamboni WML, Strychor S, Zamboni B, Ramalingam S, Edwards R, Kim J, Bang Y, Lee H, Friedland D, Stoller R, Belani C, Ramanathan R. Bi-Directional Pharmacodynamic Interaction between Pegylated Liposomal CKD-602 (S-CKD602) and Monocytes in Patients with Refractory. Solid Tumor. 2009 doi: 10.3109/08982104.2010.496085. [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Ramanathan RK, Zamboni BA, Strychor S, Ramalingam S, Edwards RP, et al. Population Pharmacokinetics of Pegylated Liposomal CKD-602 (S-CKD602) in Patients With Advanced Malignancies. J Clin Pharmacol. 2011 doi: 10.1177/0091270010394851. [DOI] [PubMed] [Google Scholar]
- 46.Gabizon A, Isacson R, Rosengarten O, Tzemach D, Shmeeda H, Sapir R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol. 2008;61(4):695–702. doi: 10.1007/s00280-007-0525-5. [DOI] [PubMed] [Google Scholar]
- 47.La-Beck NM, BAZ, Tzemach D, Schmeeda H, Sapir R, Gabizon A, Zamboni WC University of North Carolina Eshelman School of Pharmacy, Chapel Hill, NC; Carlow University, Pittsburgh, PA; Shaare Zedek Medical Center, Jerusalem, Israel. Evaluation of the relationship between patient factors and the reduction in clearance of pegylated liposomal doxorubicin. ASCO Annual Meeting J Clin Oncol; 2008. [Google Scholar]
- 48.Charrois GJ, Allen TM. Multiple injections of pegylated liposomal Doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther. 2003;306(3):1058–67. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- 49.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, et al. Tumor targeting using anti-her2 immunoliposomes. Journal of Controlled Release. 2001;74(1–3):95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 50.Dark GG, Calvert AH, Grimshaw R, Poole C, Swenerton K, Kaye S, et al. Randomized trial of two intravenous schedules of the topoisomerase I inhibitor liposomal lurtotecan in women with relapsed epithelial ovarian cancer: a trial of the national cancer institute of Canada clinical trials group. J Clin Oncol. 2005;23(9):1859–66. doi: 10.1200/JCO.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 51.Giles FJ, Tallman MS, Garcia-Manero G, Cortes JE, Thomas DA, Wierda WG, et al. Phase I and pharmacokinetic study of a low-clearance, unilamellar liposomal formulation of lurtotecan, a topoisomerase 1 inhibitor, in patients with advanced leukemia. Cancer. 2004;100(7):1449–58. doi: 10.1002/cncr.20132. [DOI] [PubMed] [Google Scholar]
- 52.Hong K, Kirpotin DB, Park JW, Shao Y, Shalaby R, Colbern G, et al. Anti-HER2 immunoliposomes for targeted drug delivery. Ann N Y Acad Sci. 1999;886:293–6. doi: 10.1111/j.1749-6632.1999.tb09440.x. [DOI] [PubMed] [Google Scholar]
- 53.Kraut EH, Fishman MN, LoRusso PM, Gordon MS, Rubin EH, Haas A, Fetterly GJ, Cullinan P, Dul JL, Steinberg JL. Final results of a phase I study of liposome encapsulated SN-38 (LE-SN38): safety, pharmacogenomics, pharmacokinetics, and tumor response. Proc Am Soc Clin Oncol. 2005;23:139s. [Google Scholar]
- 54.Vizirianakis IS. Nanomedicine and personalized medicine toward the application of pharmacotyping in clinical practice to improve drug-delivery outcomes. Nanomedicine. 7(1):11–7. doi: 10.1016/j.nano.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Solomon M, Achilefu S. Perspectives and potential applications of nanomedicine in breast and prostate cancer. Med Res Rev. doi: 10.1002/med.20233. Epub 2010/11/11. [DOI] [PubMed] [Google Scholar]
- 56.Raffa V, Vittorio O, Riggio C, Cuschieri A. Progress in nanotechnology for healthcare. Minim Invasive Ther Allied Technol. 19(3):127–35. doi: 10.3109/13645706.2010.481095. [DOI] [PubMed] [Google Scholar]
- 57.Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, Ljubimova JY, et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? Neuroimage. 54(suppl 1):S106–24. doi: 10.1016/j.neuroimage.2010.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102(7):1247–52. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim Biophys Acta. 1810(3):317–29. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–95. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181(3):217–34. doi: 10.1002/aja.1001810302. [DOI] [PubMed] [Google Scholar]
- 62.Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. Am J Physiol. 1999;276(1 Pt 1):L146–54. doi: 10.1152/ajplung.1999.276.1.L146. [DOI] [PubMed] [Google Scholar]
- 63.Stern ST, Hall JB, Yu LL, Wood LJ, Paciotti GF, Tamarkin L, et al. Translational considerations for cancer nanomedicine. J Control Release. 2010;146(2):164–74. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caron WP, Clewell H, Dedrick R, Ramanathan RK, Davis WL, Yu N, et al. Allometric scaling of pegylated liposomal anticancer drugs. J Pharmacokinet Pharmacodyn. 2011;38(5):653–69. doi: 10.1007/s10928-011-9213-5. [DOI] [PubMed] [Google Scholar]
- 65.Tyner K, Sadrieh N. Considerations when submitting nanotherapeutics to FDA/CDER for regulatory review. Methods Mol Biol. 2010;697:17–31. doi: 10.1007/978-1-60327-198-1_3. [DOI] [PubMed] [Google Scholar]
- 66.Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6. [PubMed] [Google Scholar]
- 69.Sarin H, Kanevsky AS, Wu H, Sousa AA, Wilson CM, Aronova MA, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caruso RA, Speciale G, Inferrera A, Rigoli L, Inferrera C. Ultrastructural observations on the microvasculature in advanced gastric carcinomas. Histol Histopathol. 2001;16(3):785–92. doi: 10.14670/HH-16.785. [DOI] [PubMed] [Google Scholar]
- 71.Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989;78(6):561–71. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- 72.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424–31. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–78. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 74.Greish KHH, Thiagarajan G, Price R, Bauer H, Yu T, Anwar A, Ghandehari H, editors. In Vivo Evaluation of Nanomaterial Biosafety: Evidence of Disseminated Intravascular Coagulopathy (DIC) in Reponse to Cationic Dendrimer Administration. CRS annual meeting; Portland, Oregon. 2010. [Google Scholar]
- 75.Bernd H, De Kerviler E, Gaillard S, Bonnemain B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest Radiol. 2009;44(6):336–42. doi: 10.1097/RLI.0b013e3181a0068b. [DOI] [PubMed] [Google Scholar]
- 76.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004;19(6):1571–5. doi: 10.1093/ndt/gfh185. [DOI] [PubMed] [Google Scholar]
- 77.Dobrovolskaia MA, Neun BW, Clogston JD, Ding H, Ljubimova J, McNeil SE. Ambiguities in applying traditional Limulus amebocyte lysate tests to quantify endotoxin in nanoparticle formulations. Nanomedicine (Lond) 2010;5(4):555–62. doi: 10.2217/nnm.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgan K, Caron W, Walsh M, Zamboni B, Zamboni W, editors. Comparison of toxicity and study design issues of nanoparticle and small molecule anticancer agents in preclinical models and phase I clinical trials AACR. Orlando, Florida: 2011. [Google Scholar]
- 79.Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton's lymphoma tumor bearing mice. J Control Release. 2005;105(3):185–98. doi: 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 80.Manil L, Couvreur P, Mahieu P. Acute renal toxicity of doxorubicin (adriamycin)-loaded cyanoacrylate nanoparticles. Pharm Res. 1995;12(1):85–7. doi: 10.1023/a:1016290704772. [DOI] [PubMed] [Google Scholar]
- 81.Charrois GJ, Allen TM. Rate of biodistribution of STEALTH liposomes to tumor and skin: influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim Biophys Acta. 2003;1609(1):102–8. doi: 10.1016/s0005-2736(02)00661-2. [DOI] [PubMed] [Google Scholar]
- 82.Ramus SJ, Kartsonaki C, Gayther SA, Pharoah PD, Sinilnikova OM, Beesley J, et al. Genetic variation at 9p22.2 and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 103(2):105–16. doi: 10.1093/jnci/djq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shekunov BY, Chattopadhyay P, Tong HH, Chow AH. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–27. doi: 10.1007/s11095-006-9146-7. [DOI] [PubMed] [Google Scholar]
- 84.FDA Approval for Nanoparticle Paclitaxel. http://www.cancer.gov/cancertopics/druginfo/fda-nanoparticle-paclitaxel.
- 85.FDA Approval for Doxorubicin Hydrochloride Liposome. http://www.cancer.gov/cancertopics/druginfo/fda-doxorubicin-HCL-liposome.
- 86.Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, et al. A status report on RNAi therapeutics. Silence. 2010;1(1):14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong X, Mumper RJ. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond) 2010;5(4):597–615. doi: 10.2217/nnm.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schluep T, Gunawan P, Ma L, Jensen GS, Duringer J, Hinton S, et al. Polymeric tubulysin-peptide nanoparticles with potent antitumor activity. Clin Cancer Res. 2009;15(1):181–9. doi: 10.1158/1078-0432.CCR-08-1848. [DOI] [PubMed] [Google Scholar]
- 90.Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100(4):572–9. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108(5):1850–5. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68(21):9078–86. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008;105(27):9343–8. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scarberry KE, Mezencev R, McDonald JF. Targeted removal of migratory tumor cells by functionalized magnetic nanoparticles impedes metastasis and tumor progression. Nanomedicine (Lond) 2010;6(1):69–78. doi: 10.2217/nnm.10.103. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, Xiang J, Zhang W, Fan S, Wu M, Li X, et al. Nanoparticle delivery of anti-metastatic NM23-H1 gene improves chemotherapy in a mouse tumor model. Cancer Gene Ther. 2009;16(5):423–9. doi: 10.1038/cgt.2008.97. [DOI] [PubMed] [Google Scholar]
- 96.Lee GY, Park K, Nam JH, Kim SY, Byun Y. Anti-tumor and anti-metastatic effects of gelatin-doxorubicin and PEGylated gelatin-doxorubicin nanoparticles in SCC7 bearing mice. J Drug Target. 2006;14(10):707–16. doi: 10.1080/10611860600935701. [DOI] [PubMed] [Google Scholar]