Abstract
Oncolytic viruses are novel immunotherapeutics with increasingly promising outcomes in cancer patient clinical trials. Preclinical and clinical studies have uncovered the importance of virus-induced activation of antitumor immune responses for optimal therapeutic efficacy. Recently, several classes of chemotherapeutics have been shown to cause immunogenic cancer cell death characterized by the release of immunomodulatory molecules that activate antigen-presenting cells and thus trigger the induction of more potent anticancer adaptive immune responses. In preclinical models, several oncolytic viruses induce immunogenic cell death, which is associated with increased cross-priming of tumor-associated antigens. In this review, we discuss the recent advances in immunogenic cancer cell death as induced by chemotherapeutic treatments, including the roles of relevant danger-associated molecular patterns and signaling pathways, and highlighting the significance of the endoplasmic reticulum (ER) stress response. As virtually all viruses modulate both ER stress and cell death responses, we provide perspectives on future research directions that can be explored to optimize oncolytic viruses, alone or in combination with targeted drug therapies, as potent immunogenic cancer cell death–inducing agents. We propose that such optimized virus-drug synergistic strategies will improve the therapeutic outcomes for many currently intractable cancers.
Oncolytic Viruses for the Treatment of Cancer
Cancer arises from the accumulation of genetic and epigenetic alterations that drive the transformation of a normal cell towards increasingly malignant derivatives. This process shapes each tumor in such a dynamic and unique way that it is extremely difficult to determine the specific genetic alterations that cause, maintain, and spread the disease.1,2,3 Solid tumors have been treated by resection surgery for the past 4,000 years. After the discovery of X-rays, radiotherapy emerged as a novel therapeutic approach. Whereas localized tumors can be treated by focal therapy, extensive or metastatic tumors and hematological malignancies require systemic anticancer therapies. To date, surgery, radiotherapy, and standard systemic chemotherapy comprise the standard treatment options in the majority of cancer patients. Initial development of anticancer therapies relied on empirical observations; however, the current challenge is to develop novel targeted therapeutic paradigms exploiting the knowledge derived from molecular, cellular, and systems biology studies of tumor formation and progression.3,4,5
Oncolytic viruses are naturally occurring or engineered viruses that selectively replicate in tumor beds and kill cancer cells without harming healthy cells. Traditionally, oncolytic virus replication within tumors was assumed to cause direct oncolysis of all the resident cancer cells, leading to regression of the bulk of the tumor.6 However, it has become increasingly clear that oncolytic viruses also utilize other anticancer mechanisms to eradicate tumors. Oncolytic viruses such as vaccinia virus and vesicular stomatitis virus, for example, interfere with tumor vasculature, compromising the growth of tumors.7 However, the most relevant systemic mechanism of action of oncolytic viruses is likely the virus-induced engagement of the immune response to recognize and engage tumor antigens that were previously either unrecognized or had been subject to immune tolerance.6,8 Replicating virus within tumor tissues attracts immune cells into the tumor microenvironment, leading to cross-priming of tumor-associated antigens (TAAs) for activation of more effective anticancer immune responses. For example, Talimogene laherparepvec (T-VEC; herpes simplex virus-1 (HSV-1) recombinant expressing granulocyte macrophage colony-stimulating factor (GM-CSF)) showed a potent antimelanoma immune response after local intratumoral injection in patients with metastatic malignant melanoma in phase 2/3 clinical trials.9,10 There continues to be major challenges in designing the ideal oncolytic virus for systemic delivery to optimize both intratumoral virus spread as well as the cross-priming of anticancer immunity.11 Suppressing acquired immune responses may indeed increase intratumoral spread in the short term, but it also diminishes the cross-priming of tumor antigens needed for the most effective anticancer immune responses. Conversely, enhancing the early pathways needed for effective induction of antitumor immunity may improve cross-priming, but suppress the intratumoral virus spread that is thought to be needed for optimal oncolytic tumor debulking.
Recently, there is strong evidence in preclinical models that certain classes of chemotherapeutics, such as anthracylines and oxaliplatins, induce a type of immunogenic cell death (ICD) that precedes the release of immunomodulatory “eat-me” signals that increase effective antigen presentation and T-cell activation.12,13,14,15 Moreover, in the clinic, dendritic cells (DCs) that are loaded with cancer cells dying by ICD induce potent antitumor immune responses.16 Furthermore, there is emerging evidence that certain oncolytic viruses can also induce immunogenic cancer cell death when applied alone or in combination with certain chemotherapeutics.17,18,19,20,21
Here, we review the recent advances in ICD and oncolytic viruses that induce ICD to boost the functional immune responses against tumor antigens. We will present a perspective on ways of enhancing antitumor immunity by genetic manipulation of viral proteins as well as through combination of oncolytic viruses with targeted chemotherapeutic drugs that preferentially induce immunogenic cancer cell death.
Activation of Immune Response: Damps and Pamps
Antigen-presenting cells (APCs) recognize pathogens and become activated via pattern recognition receptor binding to specific classes of pathogen-associated molecular patterns (PAMPs).22 There are several classes of pattern recognition receptors, including the toll-like receptors, retinoic acid-inducible gene-1–like receptors, nucleotide oligodimerization domain–like receptors, and several C-type lectin receptors.6,23 DCs, professional APCs of the immune system, express a wide repertoire of pattern recognition receptors.23 Pattern recognition receptor signaling greatly augments the T cell stimulatory potential of DCs by potentiating antigen processing and presentation, inducing the expression of chemokine receptors that allow DC migration to secondary lymphoid tissues and promoting synthesis of costimulatory molecules and cytokines that act on T cells to promote their expansion and effector differentiation.6,8,24 Inducing these changes in DCs is the purpose of adding adjuvants to antigens in vaccination protocols and many microbial products are powerful adjuvants, consistent with their identity as PAMPs. However, some adaptive immune responses occur in the absence of infection or PAMP recognition. Matzinger originally formulated the “danger” theory of immune activation, suggesting that the role of the immune system is to react to cellular or tissue distress.25 According to this hypothesis, endogenous danger signals from stressed or dying cells activate APCs, providing the required stimulation to activate lymphocytes.6,8,25 Here, the key event in the initiation of adaptive immunity is the release or exposure of immunostimulatory intracellular molecules known as danger-associated molecular patterns (DAMPs).12,13,14,15 Much evidence now supports the concept that DAMPs contribute to adaptive immunity. For example, mouse models of immunization with dead cells bearing a foreign antigen often provoke an immune response including the generation of antitumor cytotoxic T lymphocytes.12
Immunogenic Cancer Cell Death: Lessons from Chemotherapeutics
A systematic classification of cell death has historically been based on conservative morphological rather than biochemical criteria, with a tendency to sort cell death into either one of two mutually exclusive groups.26 Thus, caspase-dependent, tolerogenic, programmed, and physiological cell death has been contrasted with caspase-independent, immunogenic, accidental, and pathological cell death.12,13,14,15,27 Although the Nomenclature Committee on Cell Death has set guidelines for classifying four typical cell death (apoptosis, autophagy, necrosis, and cornification) as well as eight atypical cell death types,26,28,29 most of the published data sorts cell death into the prototype autophagic, necrotic, or apoptotic cell death types.
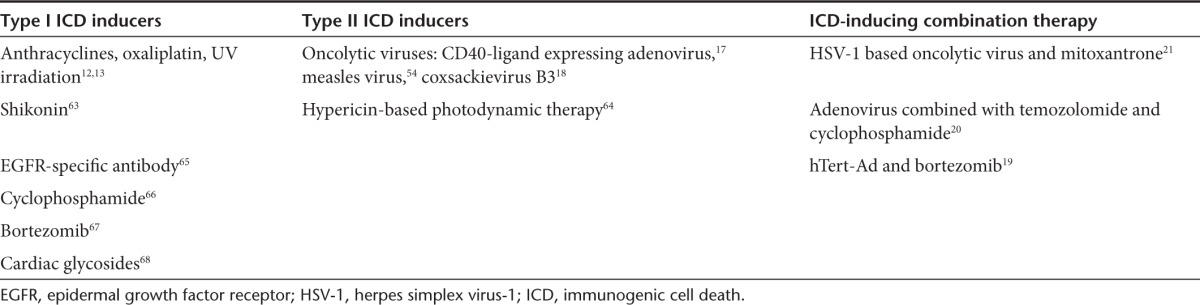
For decades, chemotherapy and radiotherapy have been considered to exert direct tumoricidal effects, including nonimmunogenic cell death and cell cycle arrest.30 The Kroemer group has recently described chemotherapeutics that cause ICD that elicits potent anticancer adaptive immune responses against antigens specific for tumor cells.12,13 ICD is a type of cell death that is characterized by the surface expression of calreticulin and active release of immunomodulatory molecules such as adenosine triphosphate (ATP) and high-mobility group box 1 (HMGB1) into the extracellular environment.12,13,14 To mount anticancer immunity, ICD requires an intact innate and adaptive immune system. ICD activation requires reactive oxygen species–induced endoplasmic reticulum (ER) stress or at least the concomitant activation of both ER stress and reactive oxygen species.31 Based on the direct involvement of ER stress, ICD inducers are systematically classified as type I or type II (Table 1).32 Type I ICD inducers activate apoptotic cell death through targets that are not associated with the ER but that stimulate ICD-associated immunogenicity through secondary or “collateral” ER stress effects. Most ICD inducers fall in the type I category as they target cytosolic proteins, plasma membrane channels or proteins, or DNA-replication proteins. Conversely, type II ICD inducers selectively target the ER and can induce immunogenic apoptosis by directly altering ER homeostasis and triggering ER stress. An extensive review of the individual ICD inducers is available.32
Table 1. Summary of ICD inducers and oncolytic virus–based combination therapies that induce ICD.
ER Stress and ICD
The ER is a membrane-enclosed organelle that serves as the site for folding of nascent membrane and secreted proteins, synthesis of lipids and sterols, and storage of free calcium. Physiologic stresses, such as increased secretory load, or pathological stresses, such as mutated proteins that cannot properly fold, can lead to an imbalance between the demand for protein folding and the capacity of the ER for protein folding, thereby causing ER stress.33 To sense and respond to ER stress, eukaryotic cells have evolved the unfolded protein response (UPR).34 The most ER-proximal regulators of the UPR consist of a set of transmembrane ER-resident proteins, including inositol-requiring protein 1 (IRE1), PKR-like endoplasmic reticulum kinase (PERK), and activating transcription factor (ATF)-6.35 These proteins bear domains protruding into the ER lumen, which sense ER stress, coupled to cytosolic effector domains. ER stress may be caused by factors that impair protein glycosylation or disulfide bond formation, by overexpression of or mutations in proteins entering the secretory pathway, or following certain viral infections.
In mammalian cells, the ER chaperone glucose–regulated protein 78 (GRP78) associates with the luminal domains of PERK, ATF6, and IRE1.34 Under stress conditions, GRP78 is sequestered to misfolded or unfolded proteins in the ER, where PERK, ATF6, and IRE1 are released. GRP78 release of PERK and IRE1 leads to their homodimerization through their luminal domains, which induces autophosphorylation and subsequent activation.35 Activation of PERK attenuates protein synthesis, whereas activation of IRE1 leads to the induction of a subset of genes encoding protein degradation enzymes. In parallel, GRP78 release of ATF6 leads to the translocation of ATF6 from the ER to the Golgi apparatus, where it is cleaved and activated. Activation of ATF6 stimulates the transcription of genes encoding chaperones that refold misfolded proteins. ATF6 also up regulates the expression of X-box–binding protein 1 mRNA, a substrate of IRE1.36 If the UPR fails to effectively counteract the ER stress, cell death ensues. ER stress induced cell death is diverse, involving both caspase-dependent apoptosis as well as caspase-independent necrosis.
Damp Components of ICD
The uptake of dead cell-derived antigens by DCs constitutes an early step in the sequence of events that leads to anticancer immune responses.15 The signaling pathways induced by distinct ICD inducers overlap but are not identical. One of the first and cardinal molecular events during ICD is the rapid exposure of molecules on the outer leaflet of the plasma membrane that act as “eat-me” signals for APCs such as DCs.37 All the ICD inducers were shown to mediate the surface exposure of the ER chaperone protein calreticulin.13,38 The translocation of calreticulin from the ER lumen to the outer leaflet of the plasma membrane often precedes the first morphologic signs of apoptosis and the translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane.39
Anthracyclines and oxaliplatin-induced calreticulin surface exposure requires the phosphorylation of PERK and eIF2α.40 Anthracyclines also cause the dissociation of the eIF2α-specific phosphatase protein phosphatase 1 (PP1) from its obligate cofactor GADD34, implying that PP1 is inhibited.41 However, ER stress alone is not sufficient to cause preapoptotic calreticulin exposure. ER Ca2+ levels contribute to the control of calreticulin exposure, which is inline with the direct effects of Ca2+ on the conformation of the calreticulin protein.42 The inhibition of caspases completely abolishes calreticulin exposure13 and subverts the immunogenicity of cell death induced by anthracyclines or oxaliplatin.12 Further analyses revealed that caspase-8 is activated downstream of ER stress by anthracyclines.40 Selective deletion, depletion, or inhibition of caspase-8 abolished calreticulin exposure induced by anthracyclines or oxaliplatin.40
Using genetic, biochemical and pharmacological tools, it was shown that the early surface exposure of calreticulin is essential for the phagocytosis of dying cancer cells by DCs.13,14 Induction of calreticulin surface exposure by hypericin–photodynamic therapy–induced ER stress is coordinated by a pathway that is different from the one induced by chemotherapeutics.32 In addition to this early eat-me signals expressed by dying tumor cells, there are additional DAMPs released by cells undergoing ICD. The two most common DAMPs are ATP and HMGB1.
Extracellular ATP is a well-known “find-me” signal that is released from apoptotic cells in response to ICD. Similar to calreticulin, the trafficking mechanism responsible for the secretion of ATP is strongly dependent on the apoptotic stage and the type of stress or cell death stimulus.32 Given that responses are different based on the cancer therapeutics used to induce ICD, there is little if any opportunity for generalization between the type of cancer therapy and the danger signaling pathways engaged. Extracellular ATP has the dual effect of stimulating monocyte attraction and inflammasome activation in DCs. ATP is sensed by P2Y2 receptors on monocytes and induces their recruitment to the site of apoptosis.43 Extracellular ATP can also activate purinergic P2X7 receptors on DCs, thereby initiating the NALP3/ASC/inflammasome and driving the secretion of IL-1β a cytokine, along with tumor antigen presentation, is required for the polarization of interferon-γ producing CD8+ T cells and for an adaptive immune response to cancer cells.43
HMGB1 is also an essential component of anticancer immunity after treatment with certain ICD inducers.31 Cells actively release HMGB1 at the late stage of apoptosis, during primary and secondary necrosis44 and during autophagic cell death.32 HMGB1 can also be released by immune cells such as macrophages and monocytes in response to inflammatory insult.32 HMGB1 has diverse immunomodulatory activities depending on its redox state. Redox changes switch the activity of HMGB1 between a chemoattractant DAMP (fully reduced HMGB1), proinflammatory cytokine-inducing DAMP (disulphide-bond possessing HMGB1) and inactivated DAMP (fully oxidized HMGB1).45 HMGB1 in the extracellular space acts as a chemoattractant for inflammatory leukocytes and stem cells,46,47 triggers the functional maturation of DCs and supports the clonal expansion of IFN-producing Th1 cells.48,49 It also influences the migration of DCs in secondary lymphoid organs.48,50
Viruses and ER Stress
Viruses can both induce and block many different cell death pathways. As viruses are dependent on host cellular machinery to replicate, many have evolved specific mechanisms to subvert or delay cell death until the impact on viral replication is minimal or until active induction of cell death is advantageous to facilitate release and spread of progeny virus.51,52 Enveloped viruses require membrane proteins and lipids for production of progeny virus, while many nonenveloped viruses depend upon pre-existing or modified intracellular membranes for their replication. Thus, it is not surprising that diverse viruses induce ER stress and the UPR, as well as manipulate the induced UPR.34 The interplay between each virus and the host UPR is complex and incompletely understood. ER stress caused by viruses modulates various signaling pathways leading to cell survival or cell death. Proteins from several viruses (influenza virus, simian virus, respiratory syncytial virus, and hepatitis C virus) stimulate the expression of GRP78, to assist the folding and assembly of viral proteins during the packaging stage of virus replication.36 Downstream of GRP78, PERK is activated in response to ER stress caused by virus infection. Activated PERK phosphorylates eIF-2α, leading to global shut down of protein synthesis while inducing the expression of ATF4, a transcription factor that stimulates the expression of DNA damage-inducible protein 34 (GADD34).36 GADD34 is expressed in conditions of DNA damage, growth arrest and cellular differentiation. GADD34 is a component of the PERK pathway that relieves translational repression and gene expression during ER stress.35,36 Viruses have been shown to activate as well as shutdown the PERK pathway at different stages of virus infection.36,52,53 HSV-1, cytomegalovirus, African swine fever, and bovine viral diarrhea virus are examples of viruses that manipulate the PERK pathway for their benefit.35,36 In HSV-1 infected cells, PERK is activated without eIF-2α phosphorylation. The γ134.5 protein of HSV-1 shows homology to the carboxyl-terminus of GADD34 and recruits PP1 to dephosphorylate eIF-2α. Compared to PERK, ATF6 and IRE1 are two components that function in the late stages of the UPR. Viruses such as hepatitis C virus and cytomegalovirus have evolved to manipulate the ATF6 and IRE1 components of the UPR for their own benefit.36
ICD Induced by Oncolytic Viruses
Several oncolytic viruses induce cell death with features similar to chemotherapeutic-induced ICD (Table 1). CD40-ligand expressing adenovirus,17 measles virus,54 and coxsackievirus B318 were shown to induce the typical features of ICD, including increased surface expression of calreticulin, higher levels of extracellular ATP and HMGB1, during infection in culture. In vivo, the antitumor activity of CD40-ligand expressing adenovirus depends on the adaptive immune system, in that immunocompromised (nude) mice failed to respond to the therapeutic intervention. The antitumor effects in immunocompetent mice were associated with immunogenic apoptosis in the tumor characterized by increased infiltration of CD45+ cells comprising macrophages and T cells and a systemic Th1 response.17 Similarly, coxsackievirus B3 treatment of xenograft tumors of human nonsmall cell lung cancer in nude mice provided circumstantial evidence that innate immune cells are recruited in the tumor for cross-priming of tumor antigens.18 Oncolytic reovirus induced the expression of genes involved in ER stress and increased intracellular levels of Ca2+, and the in vivo efficacy of treatment correlated with increased expression of GRP78 and cleaved caspase 3.55
There are additional studies that combined oncolytic viruses, typically oncolytic HSV-1 and adenoviruses expressing transgenes, with chemotherapeutic drugs for induction of ICD in preclinical models and human patients.19,20,21 In vitro combination therapy generates the salient features of ICD such as calreticulin surface exposure, HMGB1 and ATP release to the extracellular environment.20 A study using hTert-Ad (human telomerase reverse transcriptase promoter-regulated adenovirus) in combination with the proteasome inhibitor bortezomib had in vitro features of enhanced ER stress, activation of the UPR and associated apoptotic cell death translated into in vivo antitumor activity.19 When dying cells were used to vaccinate animals, they generated TAA specific CD8+ T-cell responses only if they died by apoptotic cell death.19 Moreover, CD8+ T-cell depletion abolished the therapeutic benefit of the combination treatment. We have used a preclinical murine breast cancer model to apply combinatorial therapy using oncolytic HSV-1 and the immunogenic cancer cell death–inducing drug mitoxantrone to enhance therapeutic efficacy in a nontolerized TAA model and break immunological tolerance established in a tolerized TAA model.21 Characterization of the immune cells involved showed that the combinatorial therapy requires CD8+, CD4+ T cells and Ly6G+ neutrophils.21
A very exciting clinical observation was reported when oncolytic Adenovirus was administered in combination with temozolomide and cyclophosphamide to chemotherapy refractory patients.20 Evaluation of ascites samples of pancreatic carcinoma patients treated with the combination therapy had evidence of autophagy with an increase in LC3 punctate pattern-positive cells. There was a significant correlation between HMGB1 response and survival, with the highest increase in serum HMGB1 seen in a patient who had prolonged disease control and the longest ongoing survival. Notably, in 8/9 evaluable cases, there was a correlation between serum HMGB1 levels and antitumor T-cell responses, suggesting that HMGB1 is a candidate predictive marker for antitumor immune responses.20 Median survival in combination-treated patients treated was 269 days versus 170 days in virus-only treated patients.20 This study highlights the immunotherapeutic potential of utilizing combination therapies including oncolytic viruses for enhancing anticancer efficacy.
Perspectives on Exploiting the Immunotherapeutic Potential of Oncolytic Virus–Induced ICD
The therapeutic benefits of ICD induced by chemotherapeutics have been widely shown in preclinical murine models. In the context of anthracyclines and oxaliplatin, there are biochemical and genetic studies that detail the signaling pathways and immune cells required for the effective translation of pre- and postmortem cellular processes into anticancer immune responses.12,13,14,15 While there is now clinical verification that how a cancer cell dies affects its immunogenicity,16 there is also a need to perform comparison and dose escalation studies to identify the specific ICD inducers that are effective in inducing immunogenicity of TAAs.32
Oncolytic viruses are novel immunotherapeutics that engage the immune system while they replicate within solid tumors. A few studies have reported the immunogenicity of virus-induced oncolysis, and showed that at least some oncolytic viruses activate similar DAMPs as ICD inducers, leading to potent adaptive immunity that translates into increased therapeutic efficacy.17,18 What remains to be investigated are the detailed signaling pathways and immune cells participating in the context of oncolytic virus–induced ICD. Moreover, in the context of oncolytic virus therapy, there will be local expression and release of virus-derived PAMPs in addition to host-derived DAMPs, underscoring that utilizing viruses to induce ICD may enhance recruitment of immune cells to the tumor microenvironment, causing efficient cross-priming of TAAs. On the other hand, as an evolutionary survival mechanism, viruses may interfere with ICD to avoid the antiviral immune response.52 Therefore, oncolytic viruses may require genetic modification to inactivate ICD interfering viral proteins and rewire ER stress or reactive oxygen species signaling pathways for optimal induction of ICD. For example, viruses can be genetically engineered to express calreticulin or immunomodulatory molecules on the surface of virus infected cancer cells to recruit APCs to the tumor microenvironment.
Cancer progression is a process of biological evolution56 that consists of a series of genetic changes and adaptations.57,58 During this microevolution, different mutations confer a selective advantage depending on the changes in the tumor microenvironment.59 During tumor initiation, the immune system exerts a strong selection pressure on the tumor cells as a part of tumor immunosurvelliance.60 Radiotherapy and/or chemotherapy exert a strong selection pressure on tumor cells, which might lead to therapy-resistant microevolution.57 On a similar line, ICD depends on DAMPs and the signaling pathways that mediate their release.32 In the new “pan-omics” era, it is appreciated that patients have widely varying genetic loss or gain of function mutations within the main signaling pathways of ICD. For example, most ICD inducers will likely have no useful therapeutic benefit in cancer cells deficient for caspase 8.59,61,62 Thus, the development of genetically engineered viruses that complement mutations within the signaling pathways of ICD will have potential clinical benefits. For example, oncolytic viruses can be engineered to express transgenes such as caspase-8 to sensitize ICD resistant cancer types. Moreover, oncolytic viruses can be combined with existing chemotherapeutics or alternative immunotherapies for enhancing their antitumor efficacy. Since many patients with end stage metastatic cancer also suffer from aborted antitumor immune responses, for example as mediated by excessive levels of suppressor myeloid cells or inhibitory Tregs, such ICD-inducing strategies may also help break the immune tolerance barriers that have contributed to tumor progression in the first place.
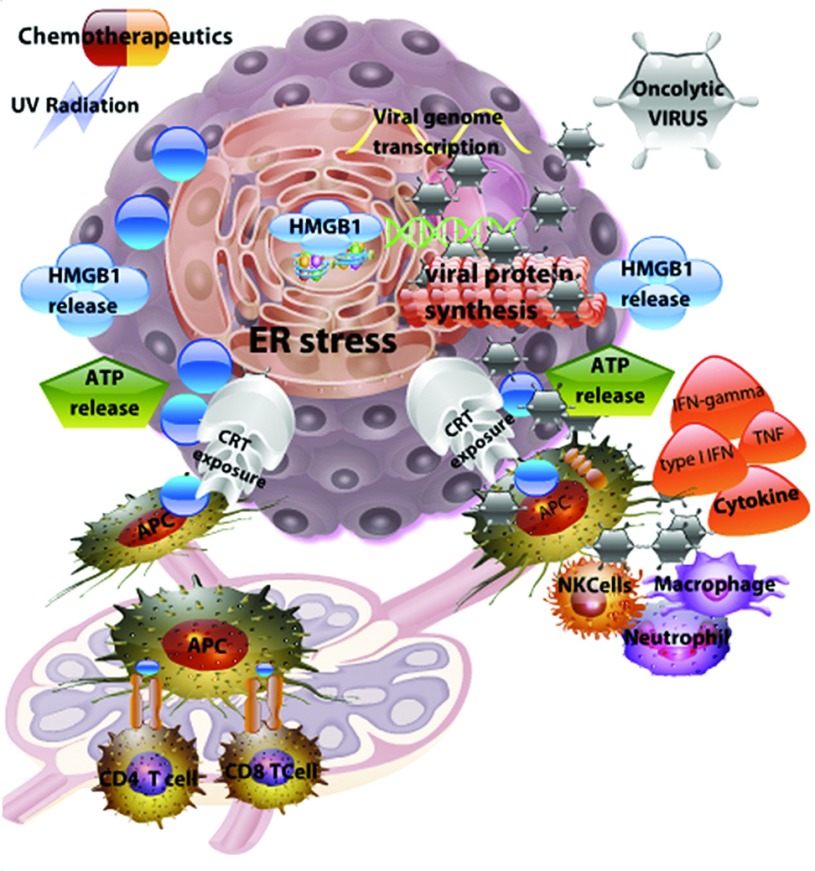
Figure 1.
Conventional immunogenic cell death (ICD) inducers (chemotherapeutics and UV radiation) and oncolytic viruses induce a similar danger response, leading to anticancer immunity. Immunogenic cell death induced by UV radiation and specific chemotherapeutic agents results in reactive oxygen species (ROS) production and an endoplasmic reticulum (ER) stress response (left side of figure). Active infection of tumor cells by oncolytic viruses overwhelms the cellular machinery, resulting in ER stress and tumor cell death (right side of figure). During these sequences of events, tumor cells express calreticulin (CRT) on the cell surface that attracts antigen-presenting cells (APCs). In addition, dying cells release immunomodulatory molecules such as high-mobility group box 1 (HMGB1) and adenosine triphosphate (ATP) into the extracellular tumor microenvironment, leading to potent antigen presentation. APCs that take up tumor-associated antigens migrate to the lymph nodes to present antigens to T cells for establishment of anticancer immunity. In addition to danger-associated molecular patterns (DAMPs), oncolytic virus infected tumor cells release pathogen-associated molecular patterns (PAMPs) (foreign viral proteins and viral dsRNA) that are potent activators of innate immune cells to secrete cytokines, such as the type I IFN. These cytokines help orchestrate the anticancer adaptive immune response.
Acknowledgments
We thank Grant McFadden and Guido Kroemer for critical reading of this manuscript and their valuable suggestions. Research in the laboratory of KLM is supported by the Canadian Cancer Society (formerly the Canadian Breast Cancer Research Alliance) and the Cancer Research Society. The authors declare no conflict of interest.
References
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Pavet V, Portal MM, Moulin JC, Herbrecht R, Gronemeyer H. Towards novel paradigms for cancer therapy. Oncogene. 2011;30:1–20. doi: 10.1038/onc.2010.460. [DOI] [PubMed] [Google Scholar]
- Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37:649–658. doi: 10.1016/j.exphem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Zhenchuk A, Lotfi K, Juliusson G, Albertioni F. Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol. 2009;78:1351–1359. doi: 10.1016/j.bcp.2009.06.094. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angarita FA, Acuna SA, Ottolino-Perry K, Zerhouni S, McCart JA. Mounting a strategic offense: fighting tumor vasculature with oncolytic viruses. Trends Mol Med. 2013;19:378–392. doi: 10.1016/j.molmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Donahue JM, Mullen JT, Tanabe KK. Viral oncolysis. Surg Oncol Clin N Am. 2002;11:661–680. doi: 10.1016/s1055-3207(02)00025-x. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- Sivendran S, Pan M, Kaufman HL, Saenger Y. Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin Biol Ther. 2010;10:1145–1153. doi: 10.1517/14712598.2010.495383. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Zappasodi R, Pupa SM, Ghedini GC, Bongarzone I, Magni M, Cabras AD, et al. Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res. 2010;70:9062–9072. doi: 10.1158/0008-5472.CAN-10-1825. [DOI] [PubMed] [Google Scholar]
- Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72:2609–2621. doi: 10.1158/0008-5472.CAN-11-3185. [DOI] [PubMed] [Google Scholar]
- Boozari B, Mundt B, Woller N, Strüver N, Gürlevik E, Schache P, et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut. 2010;59:1416–1426. doi: 10.1136/gut.2009.196519. [DOI] [PubMed] [Google Scholar]
- Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workenhe ST, Simmons G, Pol JG, Lichty BD, Halford WP, Mossman KL. Immunogenic HSV-mediated oncolysis shapes the antitumor immune response and contributes to therapeutic efficacy. Mol Ther. 2013. [DOI] [PMC free article] [PubMed]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Di Paolo NC, Mossman KL. Recognition of virus infection and innate host responses to viral gene therapy vectors. Mol Ther. 2010;18:1422–1429. doi: 10.1038/mt.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G. The Sirens' song. Nature. 2001;412:23. doi: 10.1038/35083653. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Nomenclature Committee on Cell Death 2009 Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, et al. Nomenclature Committee on Cell Death Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12 suppl. 2:1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24:319–333. doi: 10.1016/j.cytogfr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141:38–41, 41.e1. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Lee J, Sugden B. The unfolded protein response and autophagy: herpesviruses rule! J Virol. 2009;83:1168–1172. doi: 10.1128/JVI.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- Ladoire S, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, et al. Cell-Death-Associated Molecular Patterns As Determinants of Cancer Immunogenicity. Antioxid Redox Signal. 2013. [DOI] [PubMed]
- Martins I, Kepp O, Galluzzi L, Senovilla L, Schlemmer F, Adjemian S, et al. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann N Y Acad Sci. 2010;1209:77–82. doi: 10.1111/j.1749-6632.2010.05740.x. [DOI] [PubMed] [Google Scholar]
- Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Giordanetto F, Tesniere A, Vitale I, Martins I, et al. Disruption of the PP1/GADD34 complex induces calreticulin exposure. Cell Cycle. 2009;8:3971–3977. doi: 10.4161/cc.8.23.10191. [DOI] [PubMed] [Google Scholar]
- Villamil Giraldo AM, Lopez Medus M, Gonzalez Lebrero M, Pagano RS, Labriola CA, Landolfo L, et al. The structure of calreticulin C-terminal domain is modulated by physiological variations of calcium concentration. J Biol Chem. 2010;285:4544–4553. doi: 10.1074/jbc.M109.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, et al. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J Immunol. 2007;179:8051–8058. doi: 10.4049/jimmunol.179.12.8051. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A, et al. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol. 2008;180:2270–2275. doi: 10.4049/jimmunol.180.4.2270. [DOI] [PubMed] [Google Scholar]
- Kaminskyy V, Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. J Intern Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Morselli E, Vitale I, Senovilla L, Pinti M, et al. Viral strategies for the evasion of immunogenic cell death. J Intern Med. 2010;267:526–542. doi: 10.1111/j.1365-2796.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Kepp O, Senovilla L, Galluzzi L, Panaretakis T, Tesniere A, Schlemmer F, et al. Viral subversion of immunogenic cell death. Cell Cycle. 2009;8:860–869. doi: 10.4161/cc.8.6.7939. [DOI] [PubMed] [Google Scholar]
- Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Espitia CM, Mahalingam D, Oyajobi BO, Coffey M, Giles FJ, et al. Reovirus therapy stimulates endoplasmic reticular stress, NOXA induction, and augments bortezomib-mediated apoptosis in multiple myeloma. Oncogene. 2012;31:3023–3038. doi: 10.1038/onc.2011.478. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Frank SA, Rosner MR. Nonheritable cellular variability accelerates the evolutionary processes of cancer. PLoS Biol. 2012;10:e1001296. doi: 10.1371/journal.pbio.1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Slöetjes AW, de Witte T, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- Chen HM, Wang PH, Chen SS, Wen CC, Chen YH, Yang WC, et al. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immunother. 2012;61:1989–2002. doi: 10.1007/s00262-012-1258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AD, Krysko DV, Vandenabeele P, Agostinis P. DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci. 2011;10:670–680. doi: 10.1039/c0pp00294a. [DOI] [PubMed] [Google Scholar]
- Garrido G, Rabasa A, Sánchez B, López MV, Blanco R, López A, et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol. 2011;187:4954–4966. doi: 10.4049/jimmunol.1003477. [DOI] [PubMed] [Google Scholar]
- Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lara PN, Jr, Mack PC, Gandara DR. Incorporating bortezomib into the treatment of lung cancer. Clin Cancer Res. 2007;13 15 Pt 2:s4647–s4651. doi: 10.1158/1078-0432.CCR-07-0334. [DOI] [PubMed] [Google Scholar]
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra99. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]