Abstract
The cure of a human immunodeficiency virus (HIV)-1–infected patient following allogeneic transplantation from a CCR5-null donor and potential cure of two patients transplanted with CCR5 wild-type hematopoietic stem cells (HSC) have provided renewed optimism that a potential alternative to conventional antiretroviral therapy (ART) is forthcoming. While allogeneic grafts have thus far suggested complete eradication of viral reservoirs, it has yet to be observed following autologous HSC transplantation. Development of curative autologous transplantation strategies would significantly increase the number of treatable patients, eliminating the need for matched donors and reducing the risks of adverse events. Recent studies suggest gene therapy may provide a mechanism for developing curative therapies. Expression of cellular/artificial restriction factors or disruption of CCR5 has been shown to limit viral replication and provide protection of genetically modified cells. However, significant obstacles remain with regards to the depletion of established viral reservoirs in an autologous transplantation setting devoid of the “allo-effect”. Here, we discuss results from early-stage clinical trials and recent findings in animal models of gene modified HSC transplantation. Finally, we propose innovative combination therapies that may aid in the reduction and/or elimination of viral reservoirs in HIV-1–infected patients and promote the artificial development of a natural controller phenotype.
Gene therapy approaches have recently come to the forefront of alternative methods in the quest for the development of curative strategies for the treatment of human immunodeficiency virus (HIV)-1–infected patients. Nearly all of these studies have been aimed at developing infection-resistant immune cell populations highlighting the findings published by Hütter et al., in which a single HIV-1–infected patient, known as the “Berlin patient”, was effectively cured of HIV following allogeneic hematopoietic stem cell transplantation (HSCT) from a CCR5-null donor.1 While several questions remain concerning what aspects of treatment or if multiple interventions led to the cure of this patient, a new role for gene therapy in the pursuit of a cure for HIV/acquired immunodeficiency syndrome has emerged.
Although current antiretroviral therapy (ART) is perhaps one of the most vital breakthroughs in the modern era of medicine, this therapy does not equate a cure. Strategies to genetically modify target cells have evolved over the last two decades; however, for the most part, the underlying theme of preventing the expression of cellular factors needed for viral infection and/or the expression of restrictive factors has remained relatively unchanged (see ref. 2 for review of previously characterized methods for developing infection-resistant cells). Few approaches aimed at targeting proviral DNA have been developed; however, recent breakthroughs in genetic engineering of sequence specific nucleases may pave the way for future targeted therapies aimed at reducing viral reservoirs (reviewed in ref. 3). This review discusses the early-stage clinical findings, the current progress made in preclinical animal models, the ambitious goals needed to achieve a functional cure with relation to findings in elite controllers, and the innovative therapies that may be needed to eliminate or reduce viral reservoirs in combination with autologous, gene modified HSCT. Here, a “functional cure” is defined as conditions that lead to the complete and sustainable control of plasma viremia at levels that are currently achievable by conventional highly active ART (HAART). We refer to “eradication” as a sterilizing cure in which no replication competent forms of HIV exist.
The Berlin Patient Revisited
In a recent study published by Yukl et al.4, the most sensitive detection assays currently available were employed to determine whether Timothy Brown, known as the Berlin patient, was indeed functionally cured of HIV. Viral DNA and RNA were undetectable in peripheral blood mononuclear cells, lymphoid tissue and in all other tissues examined, and there is evidence of the eradication of viral reservoirs demonstrated by the nearly complete absence of immunological responses against HIV.4 Based on their findings, the authors concluded that the therapy undergone by Timothy Brown led to the first clinical cure of an HIV-1–infected patient.
Five years later, there continues to be an intense debate as to what specific components of Timothy Brown's treatment led to his functional cure. Could an intense conditioning regimen that includes total body irradiation, immune suppression with cyclosporine A, and CD3+ T-cell depletion with antithymocyte globulin eliminate viral reservoirs? Did graft-versus-host disease effectively lead to the eradication of all HIV-infected cells? Can the cure be attributed to CCR5−/− HSC transplant or would a similar outcome be attainable following allogeneic transplantation of wild-type HSCs? This latter possibility is currently being examined in two patients heterozygous for the CCR5 delta 32 deletion by Henrich et al.5 Nearly 4 years after allogeneic transplant from wild-type donors, both patients maintain undetectable levels of viral DNA or RNA in peripheral blood mononuclear cells.5 Although these patients have remained on HAART following transplantation, the authors suggest that by performing allogeneic, wild-type HSC transplants on patients with fully suppressed HIV replication, viral reservoirs may be reduced and/or eliminated with the onset of full donor chimerism. Allogeneic transplantation may prove effective at reducing viral reservoirs and even providing cure; however, its associated risks suggest that this approach should only be considered for HIV-1–infected patients being treated for malignancies.6
Evidence of Therapeutic Efficacy and Current Limitations of Autologous HSCT
Multiple clinical trials aimed at developing infection resistance following genetic modification of CD4+ or HSC have thus far been conducted or are currently ongoing worldwide.7 A summary of recent genetically modified CD4+ T-cell and CD34+ HSCs clinical trials in HIV-1–infected patients is outlined in Table 1. Significantly, only minor complications have been reported following the infusion of genetically modified cells suggesting that this therapeutic approach, while complex in with regards to administration, is not detrimental in terms of patient safety.8,9,10,11 Studies by Simonelli et al.10 have shown that viral replication is not increased, and HIV reservoirs do not rise above pretransplant levels. In addition, immune recovery following autologous HSCT of HIV-1–infected patients was reported to be identical in comparison to HIV-negative lymphoma patients.10
Table 1. Select recent autologous gene therapy clinical trials.
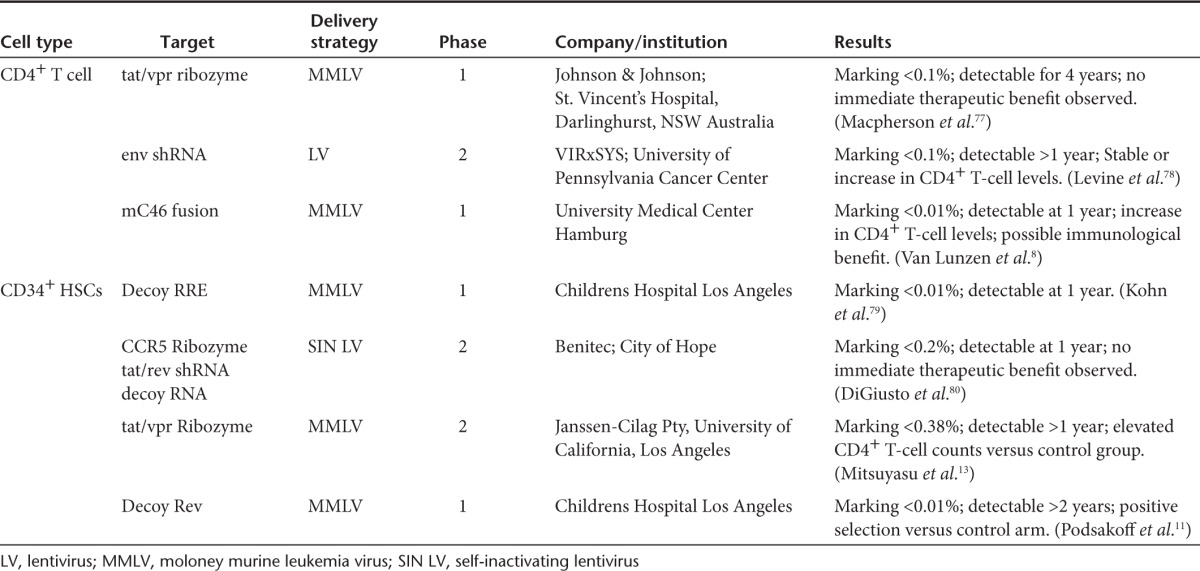
Mitsuyasu et al.12 have reported findings from a phase 1/2 clinical trial involving autologous transplantation of CD34+ HSCs genetically modified to express OZ1 (ribozyme against vpr and tat overlapping reading frames of HIV-1), in which marking levels of 0.38% in peripheral blood resulted in a therapeutically beneficial outcome. In comparing experimental and control groups, viral load was decreased with a lower rate of plasma viremia rebound resulting in an extended duration before reinitiating HAART treatment. Most importantly, CD4+ T cells increased in the experimental cohort, demonstrating the therapeutic value of transplanting genetically modified HSCs.12,13 It is difficult to assign an exact correlation between the clinical benefits following the infusion of genetically modified HSCs versus that typically seen in any transplant setting following infusion of nonmodified HSCs. Myeloblation and reconstitution following engraftment of HSCs will result in an initial decrease in peripheral CD4+ T cells harboring proviral DNA; however, proviral DNA content in peripheral blood has been shown to gradually increase despite continued ART.14,15
As observed in the aforementioned clinical trial, and in addition to all published clinical trials to date in which gene modified cells are infused into HIV-1 patients, an exceeding low percentage of genetically modified cells remains detectable in patients infused with either genetically modified HSCs or CD4+ T cells. Clinical studies have yet to progress to an efficacy stage and determining the threshold of genetically modified HSCs that are needed to effectively lead to a clinically beneficial outcome will be a significant obstacle based on the levels currently attainable.12,13,14,15,16 The possibility for in vivo selection through inclusion of a selection cassette in gammaretroviral and lentiviral vectors has been shown to be extremely effective in the nonhuman primate (NHP) model following treatment with chemotherapeutic agents.17,18 This possibility is currently under investigation in an ongoing clinical trial for HIV-1–infected patients with lymphoma (NCT01769911), though the prospect of utilizing chemotherapeutic agents for nonlymphoma HIV-1 patients is a less than appealing option.
Despite its limitations, positive selection of genetically modified CD4+ T cells has been observed in clinical trials following infusion of gene modified CD34+ cells and following direct infusion of genetically modified CD4+ T cells.11,18 In a pediatric clinical trial, Podsakoff et al.11 reported that following infusion of CD34+ HSCs genetically modified with the hum10 gene, a dominant negative Rev protein, gene modified CD4+ T cells increased from undetectable to 1/10,000 following treatment interruption. However, the increase in gene marking was not sustained long term and this study demonstrated the selective advantage of infection-resistant CD4+ T cells in vivo.11 Direct genetic modification and infusion of CD4+ T cells has also provided similar results in conferring a selective advantage to CD4+ T cells expressing anti-HIV genes. When compared to CD4+ T cells expressing a control gene, anti-HIV gene containing CD4+ T cells exhibited an increase in both long-term and short-term positive selection. Similar to the results reported by the Podsakoff group, during a period of higher replication of the virus, there was a peak of anti-HIV gene containing cells.18 In contrast, van Lunzen et al.8 reported no observations of a selective advantage for genetically modified CD4+ T cells expressing the mC46 fusion inhibiting peptide. Reasons cited for this result include a possible immune response, downregulation of mC46 expression, and complications arising from ex vivo expansion that, in turn, could create a survival disadvantage for proliferation of the gene modified cell population.8
Continued efforts are needed to increase the overall percentage of genetically modified cells in vivo. It has been shown that despite the low levels observed following the initial infusion of HSCs, genetically modified cells remain detectable for several years following autologous HSC transplantation.8,11,12,13 HIV itself could be used as a selective agent in vivo following cessation of HAART; however, it is important to note in our NHP studies, nonmodified CD4+ T cells persist despite a clear selective advantage following the acute phase of infection.17 The recovery of nonmodified cells poses a significant challenge in maintaining reduced viral reservoirs following the cessation of HAART.
In the following section, we propose that in order for autologous transplantation to succeed as an alternative therapy for HIV-1–infected patients, a phenotype comparable to elite or natural controllers must be attainable following the infusion and engraftment of infection-resistant, genetically modified HSCs. As previously indicated, autologous transplantation in and of itself does not result in the elimination of viral reservoirs but rather results in a significant reduction in the number of provirus-containing cells.15 The ability of engrafted, infection-resistant immune cell populations to control viral replication in the absence of ART will by and large determine the efficacy of autologous transplantation as a curative therapy for HIV-1–infected patients.
Gene Therapy: Can We Achieve Elite or Natural Controller Status?
The loss of immunological control of viral replication, at least in part, has been attributed to the loss of HIV-specific CD4+ T-cell responders shortly after infection.2,3,19,20 Due to their central role in the development and maintenance of an adaptive immune response, CD4+ T-cell depletion causes widespread immune dysregulation.21 In addition to playing a central role in the initial acute phase of viral infection, CD4+ T cells are also critical for maintaining functionality and diversity of cytotoxic T-cell responses during chronic stages of disease.22 Studies of long-term nonprogressing HIV-1–infected patients, herein referred to as “elite controllers” (ECs), have indicated the virus-specific CD4+ T-cell responses in ECs are suggestive of a more functional, long-lived memory T-cell population that maintains a heightened capacity to respond to small antigen load.19,23,24,25,26 ECs also have a higher frequency of mucosal HIV-specific polyfunctional CD4+ T cells than patients with progressive disease.27 Overall, a subpopulation of HIV-specific CD4+ T cells in ECs exhibit higher functional avidity compared to noncontrollers; however, the association of specific HLA class II alleles with EC status remains to be confirmed, as no HLA class II association reached genome wide significance.22,28 Therefore, genomic determinants may not be a necessary component for the development of an EC-phenotype in an otherwise disease progressor background, suggesting that if HIV-specific CD4+ T cells are artificially rendered infection resistant, an EC-phenotype may be achieved.
Studies in animal models and data in humans provide strong evidence that CD4+ T-cell helper function is critical for the generation of effective cytotoxic T-cell and B-cell responses and for the mobilization of cytotoxic T cells to infected mucosa.29,30 Thus, the maintenance and establishment of an HIV-specific CD4+ T-cell response will be an integral component of any immunotherapy-based approach aimed at either preventing HIV infection (e.g., vaccine) or for controlling viral replication in HIV-1–infected patients in the absence of conventional HAART.19,31,32 While CD4+ T cells are critically important for the coordinated immune response against HIV, equally important is the role played by other HIV-target cells. Macrophages and dendritic cells are both antigen-presenting cell types that have been shown to be susceptible to HIV-1 infection both in vivo and in vitro.33,34,35,36 Macrophages are thought to be key viral reservoirs; hence, unless these cells are effectively depleted, they will continue to pose the greatest obstacle to viral eradication strategies.37,38,39 Both macrophages and dendritic cells are central to dissemination of HIV and subsequent transfer of HIV to CD4+ T cells.40,41,42 Although these cells may be rendered resistant to direct infection, it is currently unknown whether macrophages and/or dendritic cells derived from genetically modified HSCs will continue to support trans infection of CD4+ T cells.
The manipulation of the immunological networks typically lost during HIV infection through the broad creation of infection-resistant immunological cell types may restore immunological control over viral replication in the absence of ART. In the following section, we will examine recent findings in murine and NHP studies that provide intriguing, albeit inconclusive, results in the ability of genetically modified cells to reduce plasma viremia and aid in the development of an artificially induced natural controller phenotype.
Developing Infection-Resistant Immune Cell Populations Following Infusion of Ex Vivo Genetically Modified HSCS or CD4+ T Cells
Multiple in vivo studies have demonstrated that ex vivo genetic modification of either mature T cells or CD34+ HSCs confer a selective advantage of genetically modified cells, but plasma viremia levels, despite significant reductions, remain well above those observed following ART.19,20,22,23,24,25,43 Studies by Holt et al.19 have emulated the “Berlin patient” approach in humanized mouse models by utilizing zinc finger nucleases to disrupt the CCR5 locus in human CD34+ cells and the subsequent HIV-1 infection following NOD scid gamma mouse xenotransplantation. Post-HIV-1 BaL infection, the population of CCR5-negative T cells increased clearly indicating a selective advantage, while plasma viremia levels were significantly reduced. In parallel to the success of CCR5 knockdown, and in an attempt to develop a broader clinical therapeutic approach capable of preventing infection independent of viral tropism, several groups have examined the efficacy of expressing cellular or artificial restriction factors (reviewed in ref. 44).17,18,45,46,47,48 One such alternative strategy is to incorporate the expression of a 46 amino acid peptide that correlates to the fusion domain of gp41. When fused to a membrane anchor (mC46) and expressed on the surface of target cells, a potent inhibition of viral entry is observed due to an inability of HIV Env to undergo a conformational change necessary for membrane fusion.17,18,45,46,47,48 Expression of the mC46 peptide has been experimentally shown to have an inhibitory effect on CCR5, CXCR4, and dual-tropic strains of HIV and simian-human immunodeficiency virus (SHIV), therefore, eliminating any selective advantage of CXCR4-tropic strains. Using a xenotransplantation murine model, Kimpel et al.45 demonstrated that gene modified human CD4+ T cells expressing mC46 underwent positive selection following HIV infection. An eventual decline of protected cells was observed, though this may have been due to graft-versus-host disease. Other obstacles to the success of T-cell therapies were indicated in a clinical trial involving the modification of mC46-expressing CD4+ T cells in HIV-1–infected patients.8 Widespread increases and proliferation were not observed, resulting in a gradual decline of genetically modified cells. A potential benefit of genetically modifying HSCs as opposed to CD4+ T cells lies with the ability of HSCs to provide long-term, infection-resistant immune cell populations that include all known HIV-1 target cells.49,50,51
Our recent studies in NHPs demonstrated the potential for immune response modulation due to the maintenance of genetically modified cells following SHIV challenge.17 In the pigtailed macaque transplant model, we demonstrated that in addition to maintaining CD4+ T cell at or near normal levels, experimental macaques receiving autologous transplantation following ex vivo modification with lentiviral vectors encoding the mC46-fusion inhibitor maintained high levels of gene modified, SHIV-specific, CD4+ T cells (representing >85% of total responders). Corresponding with the maintenance of these SHIV-specific CD4+ T cells, a significantly enhanced immune response was observed as determined by increased and broader SHIV-specific CD8+ T-cell responders and improved neutralizing antibody responses. Supporting the above-mentioned contributions of other protected immunological cells, we noted that interleukin (IL)-12 levels were maintained at high levels shortly following the acute phase of infection. These results are noteworthy, as IL-12 is generally produced by dendritic cells following interactions with CD4+ T cells.52,53 While analytically complex, studies in NHP models are of utmost importance in determining the effectiveness of such approaches.
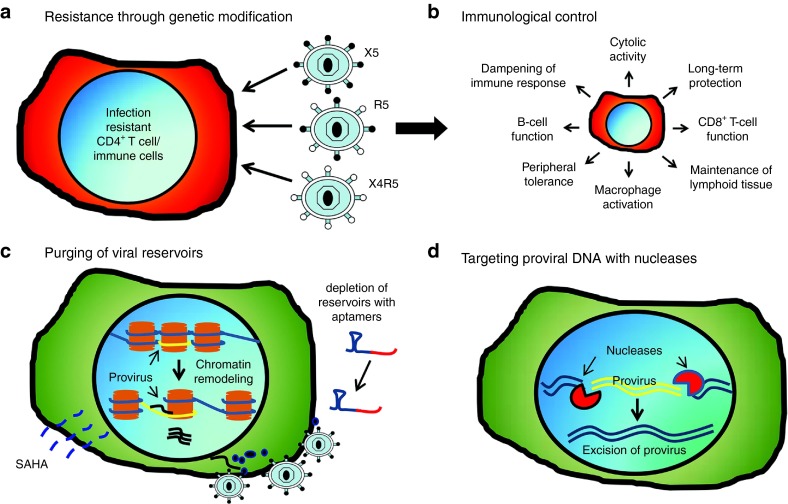
An unexpected, yet novel finding following the acute phase of infection was observed in which nonmodified CD4+ T cells exhibited a gradual yet robust recovery, indicating the possibility of a positive bystander effect. In addition to the CD4+ T-cell recovery, a 102–103 log decrease in plasma viremia and an enhanced immune response against the challenge virus were detected in the mC46 macaques. These studies provide initial evidence indicating that not only are genetically modified cells protected against infection in vivo, but that these cells, in turn, contribute to the development of a heightened immune response against the challenge virus (Figure 1a,b).
Figure 1.
Achieving immunological control in human immunodeficiency virus (HIV)-1–infected patients. (a) Genetic modification of target cells (hematopoietic stem cells or CD4+ T cells) has been shown to significantly reduce viral infectivity in vitro and in vivo. Disruption of CCR5 prevents infection from R5-tropic strains while expression of the mC46-fusion inhibitor prevents infection of HIV regardless of viral tropism. (b) The development of infection-resistant immune cell populations following infusion of genetically modified hematopoietic stem cells (HSCs) or CD4+ T cells promotes an enhanced immune response against HIV (e.g., antibody response) and HIV-infected cells (e.g., cytotoxic T-cell response). Protected cells maintain functional activity improving both innate and adaptive immune responses against the virus, in addition to reducing the number of potential target cells, which serve to further reduce the latent reservoir. (c) Elimination of viral reservoirs will require the identification of latently infected cells in order to promote targeted deletion of infected cells through immunological responses or through targeted therapies. Reagents including Prostratin or suberoylanilide hydroxamic acid have previously been shown to promote viral reactivation in latently infected cells. Following the induced expression and transport of viral antigens to the plasma membrane of infected cells, targeted therapies such as aptamer-mediated delivery of toxic siRNAs can be utilized to diminish and/or eliminate viral reservoirs. (d) Highly conserved sequences within the viral genome can be targeted with sequence specific nucleases to disrupt or delete conserved regions fundamentally required for viral replication (e.g., transactivation response element).
Promising results have been observed in both murine and NHP models, but it is clearly evident that viral reservoirs are seeded regardless of the infusion of protected cells prior to HIV/SHIV infection. Taking into consideration that the goal of this therapy is to perform HSCT in HIV-1–infected patients, who maintain viral reservoirs despite being on suppressive HAART, it is unlikely that HSCT on its own will effectively lead to a stable long-term reduction in viral reservoirs. As previously indicated, multiple studies have shown that autologous transplantation in HIV-1–infected patients does not effectively eliminate viral reservoirs.15 Thus, innovative therapeutic approaches will likely be needed in combination with the benefits attainable following autologous HSCT. The following section provides an overview of novel therapeutic approaches that may prove beneficial in reducing and/or eliminating viral reservoirs.
Combinatorial Therapies: Novel Approaches for Targeting Viral Reservoirs
Targeting latently infected cells in vivo
Infected memory CD4+ T cells, macrophages, and other long-lived cells that contribute to the persistent maintenance of viral reservoirs in spite of intense ART represents a significant obstacle to curing an HIV-infected patient.54 Although patients can maintain very low plasma viral loads for years while on ART, virus rebound is rapidly observed following cessation of antiretroviral therapies. A major challenge is how to purge these latent viral reservoirs.55 The ideal strategy for targeting the HIV-infected cells that contribute to the latent reservoir is to develop a method for marking these cells followed by the targeted deletion of this specific population using a directed therapeutic approach (Figure 1c). In the allogeneic transplant setting, targeted elimination of virus-harboring cells was accomplished by the graft-versus-host effect associated with allogeneic transplantation. In an autologous transplantation setting, however, viral reactivation will be necessary for therapeutic approaches aimed at targeting latently infected cells in order to facilitate directed targeting and gene delivery to viral reservoirs.
Following provirus integration into the host chromosomal DNA, multiple mechanisms regulate viral transcription. Expression of the viral transcriptional activator and subsequent binding to the transactivation response (TAR) element in the viral long terminal repeat promotes the recruitment of RNA polymerase II, thereby leading to viral gene expression.56 Depending on the integration site and cellular activation status, gene silencing may occur due to modification in chromatin structure. Gene expression and heterochromatin assembly are regulated by the addition or removal of acetyl moieties from histone tails by histone acetyltransferase and histone deacetylases, respectively.57,58,59 In addition to chromatin remodeling, the cellular activation status plays a central role in controlling viral replication. Resting CD4+ maintain low levels of nuclear factor κ-light-chain enhancer and nuclear factor of activated T cells, two transcription factors that are essential for efficient viral gene expression.60,61 The absence of these factors results in a cellular environment that silences gene expression and promotes the development of latently infected cells.
In both instances, strategies to purge viral reservoirs by promoting histone deacetylation and activation of resting CD4+ T cells has yielded inconclusive results. In vivo studies aimed at inducing viral gene expression by promoting activation of latent reservoirs with the proinflammatory cytokines (IL-2 and interferon-γ) or by infusion of anti-CD3 antibody resulted in long-term depletion of all CD4+ T-cell subsets and failed to measurably reduce latent reservoirs.62,63,64 Prostratin was previously shown to induce viral expression by activating nuclear factor κ-light-chain enhancer and protein kinase C (PKC) pathways in a portion of latently infected cells,65 but latency was not reversed in latent cells harboring chromatin silenced provirus.64 Similarly, studies using the histone deacetylase inhibitor, suberoylanilide hydroxamic acid or Vorinostat, have shown impressive results in terms of activation of viral transcription ex vivo,31,66 however, the clinical benefits indicating reduced viral reservoir pools are yet to be determined.67
Due to the complex nature of latency, multiple strategies to reverse the pathways involved in the development of latent pools will be needed for clinical purging therapies. Although it is anticipated that cell death will ensue following reactivation of transcription from proviral DNA in latent cells, either due to direct cytopathic effect or immune recognition following viral gene expression, the results obtained to date, for the most part, suggest that multiple rounds of activation may be required to effectively reduce viral reservoirs.58 To this end, combining strategies designed to target cells expressing viral antigens may prove to be an invaluable tool for reducing and/or eliminating viral reservoirs. One such strategy that has shown promise is the development of RNA aptamers that, similar to antibody recognition, can be used to target specific epitopes. Using this approach, Neff et al.68 demonstrated that Env-binding aptamers can be used to deliver anti-HIV siRNAs, leading to a significant reduction in viral loads in vivo following injection into HIV-infected mice. By conjugating Env-binding aptamers to cytotoxic siRNAs (e.g., targeting Bcl-2), it may be possible to induce apoptosis of HIV-infected cells. Therefore, combining targeted depletion strategies with viral reactivation methods may prove to be a powerful approach for reducing latent reservoirs in vivo.
Targeting proviral DNA with genetically engineered nucleases
The inability of current ART to eliminate cells that harbor integrated HIV proviral DNA represents a major obstacle for the clearance of viral reservoirs.69 Developing strategies that target proviral DNA is perhaps one of the most daunting challenges for curing HIV-1–infected patients; to date, few if any promising methods have been developed to meet this challenge.70 Unless integrated virus is rendered replication deficient, viral replication will once again emerge the moment antiretroviral treatment is interrupted (Figure 1d). Furthermore, it has been well documented that persistent viral replication occurs regardless of the apparent complete control of viral replication in patients receiving ART.71 The specific targeting of proviral DNA in latently infected cells will require genetically engineered nucleases with both a sequence specific binding domain and a catalytically active cleavage domain. While there are a number of highly conserved sequences within the viral genome, including the TAR region72 and fusion region within Gag,73 a foremost challenge will be the delivery of these nucleases to viral reservoirs in vivo. Preventing off-target cleavage events is of paramount importance to establish the safety of this approach, yet there are limited options when one considers aggressive measures to neutralize provirus.
Studies previously published by Sarkar et al.70 have shown that modification of a recombinase could effectively be used to excise integrated viral genomes. However, a major obstacle is the delivery of functional nucleases to latently infected cells. Several approaches are currently under investigation, including conjugation of mRNAs to the aforementioned aptamers that specifically recognize cell surface receptors (e.g., CD4 or HIV-Env) and liposome-mediated targeting.74 Significant limitations remain, although these approaches may prove beneficial and contribute to a reduction in the overall pool of latently infected cells. In particular, the systemic delivery of proviral targeting nucleases will remain suboptimal using the aforementioned methods to target latently infected cells. Novel methods are needed to deliver nucleases to the broad range and anatomical locations to target viral reservoirs.
Combining gene therapy and conventional vaccination
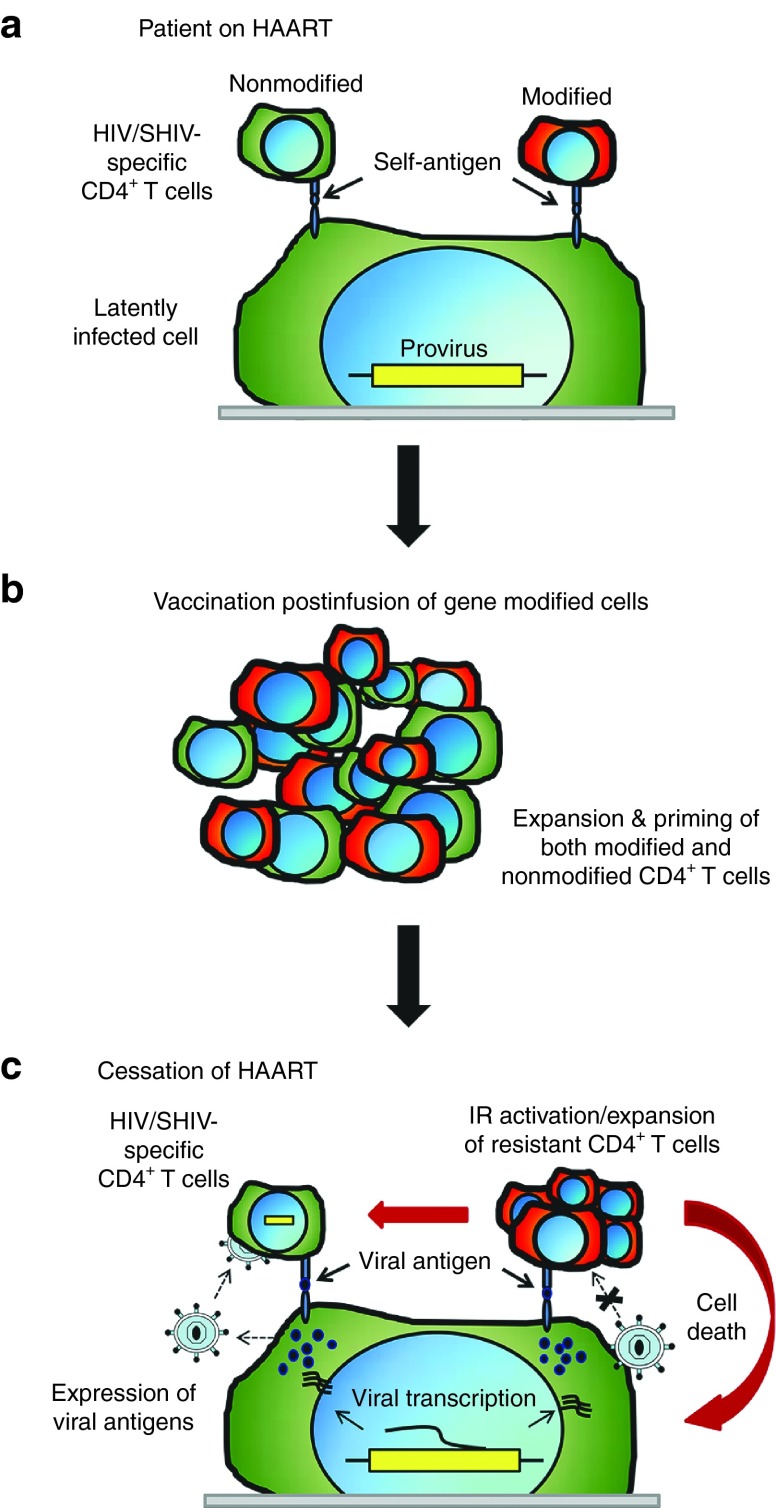
Genetic determinants likely play an important role in the maintenance of CD4+ T cells in elite/natural controllers despite gene therapy having previously been shown to render HIV/SHIV-specific CD4+ T cells resistant to infection.17 As both CD4+ and CD8+ T-cell responses are critical for the development of an effective immune response during chronic HIV infection,75 it is conceivable that posttransplantation vaccination of patients may lead to a heightened immunological control. For instance, it may be possible to “spike” the immune response following engraftment of genetically modified cells in preparation for exposure to viral antigens prior to cessation of ART (Figure 2). Such postinfection vaccination strategies have been examined in the absence of genetically modified cells in an SIV/acquired immunodeficiency syndrome NHP model.76 The results from these studies are highly encouraging, as a long-term reduction in plasma viremia was observed. In support of the dual vaccination concept, in which patients are vaccinated post-HSCT while on ART, the reduction in plasma viremia was attributed to an increase in SIV-specific CD8+ and CD4+ T-cell responders. Therefore, by developing and priming infection-resistant CD4+ T cell specific for HIV, the balance between host-and-pathogen may be tilted in favor of the immune response.
Figure 2.
Postinfusion vaccination and priming of immune response prior to cessation of highly active antiretroviral therapy (HAART). (a) Infected patients receiving HAART maintain nonmodified, human immunodeficiency virus (HIV)-specific CD4+ T cells; however, following infusion and engraftment of hematopoietic stem cells (HSCs), genetically modified, infection-resistant CD4+ T-cells specific for SHIV-antigens have yet to be exposed to viral antigens. (b) Following conventional vaccination, both nonmodified and genetically modified, HIV-specific CD4+ T-cell responders are expanded and primed for exposure to replicating virus upon cessation of HAART. (c) Following end of HAART treatment, latently infected cells express viral antigens and release nascent virions. Nonmodified CD4+ T-cells are targets for infection and will undergo apoptosis due to direct viral cytopathic effects or due to immunological recognition. Gene modified CD4+ T-cells that are resistant to infection, will detect viral antigens as peptide-MHCII complexes and elicit an immune response. By pre-exposing genetically modified cells to viral antigens via post-engraftment vaccination (and while on ART), SHIV-specific, infection-resistant CD4+ T cells can be generated prior to cessation of ART, which in turn, will activate and maintain a functional immune response upon activation of latent reservoirs. IR, immune response.
The Future
The methods for artificially modifying would-be target cells or their progenitors have by and large, already been developed. The utility of the gene therapy approach as a curative therapy is currently under intense investigation with promising, yet incomplete, evidence supporting the potential of this approach. The elimination of viral reservoirs will continue to pose the principal obstacle to an effective cure because, although the development of a natural controller phenotype may be a realistic goal, a functional cure in which plasma viremia is maintained at levels that are near the undetectable limit currently attainable by conventional HAART will remain a formidable task. Nonetheless, gene therapy is at the forefront of alternative therapies for the treatment of HIV-1–infected patients and may likely be part of future curative treatments, although a combination of therapeutics interventions will be needed to achieve the ultimate goal of developing ART-free therapies for HIV-1–infected patients.
Acknowledgments
This effort was supported in part by NIH-supported R01 AI080326 and Martin Delaney Collaboratory grant U19 AI 096111. H.P.K. is a Markey Molecular Medicine Investigator and is the recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research. The authors thank Grace Choi, Helen Crawford, and Bonnie Larson for their help in preparing this manuscript. The authors declare no conflict of interest.
References
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Heeney JL. The critical role of CD4(+) T-cell help in immunity to HIV. Vaccine. 2002;20:1961–1963. doi: 10.1016/s0264-410x(02)00078-6. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207:1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10:137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta C, Porcellini S, Alberici L. Therapeutic genes for Anti-HIV/AIDS Gene Therapy. Curr Pharm Biotechnol. 2012;13:488–500. doi: 10.2174/138920101405131111104009. [DOI] [PubMed] [Google Scholar]
- van Lunzen J, Glaunsinger T, Stahmer I, von Baehr V, Baum C, Schilz A, et al. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol Ther. 2007;15:1024–1033. doi: 10.1038/mt.sj.6300124. [DOI] [PubMed] [Google Scholar]
- Resino S, Pérez A, Seoane E, Serrano D, Berenguer J, Balsalobre P, et al. Short communication: Immune reconstitution after autologous peripheral blood stem cell transplantation in HIV-infected patients: might be better than expected. AIDS Res Hum Retroviruses. 2007;23:543–548. doi: 10.1089/aid.2006.0071. [DOI] [PubMed] [Google Scholar]
- Simonelli C, Zanussi S, Pratesi C, Rupolo M, Talamini R, Caffau C, et al. Immune recovery after autologous stem cell transplantation is not different for HIV-infected versus HIV-uninfected patients with relapsed or refractory lymphoma. Clin Infect Dis. 2010;50:1672–1679. doi: 10.1086/652866. [DOI] [PubMed] [Google Scholar]
- Podsakoff GM, Engel BC, Carbonaro DA, Choi C, Smogorzewska EM, Bauer G, et al. Selective survival of peripheral blood lymphocytes in children with HIV-1 following delivery of an anti-HIV gene to bone marrow CD34(+) cells. Mol Ther. 2005;12:77–86. doi: 10.1016/j.ymthe.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu RT, Zack JA, Macpherson JL, Symonds GP. Phase I/II Clinical Trials Using Gene-Modified Adult Hematopoietic Stem Cells for HIV: Lessons Learnt. Stem Cells Int. 2011;2011:393698. doi: 10.4061/2011/393698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15:285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Zaia JA. Allogeneic haematopoietic stem cell transplantation in patients with human immunodeficiency virus: the experiences of more than 25 years. Clin Exp Immunol. 2011;163:284–295. doi: 10.1111/j.1365-2249.2010.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo AR, Krishnan A, Mitsuyasu RT, McMahon DK, Li S, Rossi JJ, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr. 2013;63:438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statham S, Morgan RA. Gene therapy clinical trials for HIV. Curr Opin Mol Ther. 1999;1:430–436. [PubMed] [Google Scholar]
- Younan PM, Polacino P, Kowalski JP, Peterson CW, Maurice NJ, Williams NP, et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood. 2013;122:179–187. doi: 10.1182/blood-2013-01-482224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D, et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS ONE. 2009;4:e7693. doi: 10.1371/journal.pone.0007693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichis F, Kaufmann DE. HIV-specific CD4 T cells and immune control of viral replication (Review). Current Opinion in HIV & AIDS. 2011;6:174–180. doi: 10.1097/COH.0b013e3283454058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4:123ra25. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, et al. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103:2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- Fonseca SG, Procopio FA, Goulet JP, Yassine-Diab B, Ancuta P, Sekaly RP. Unique features of memory T cells in HIV elite controllers: a systems biology perspective (Review). Current Opinion in HIV & AIDS. 2011;6:188–196. doi: 10.1097/COH.0b013e32834589a1. [DOI] [PubMed] [Google Scholar]
- Imami N, Westrop SJ, Grageda N, Herasimtschuk AA. Long-term non-progression and broad HIV-1-specific proliferative T-cell responses. Front Immunol. 2013;4:58. doi: 10.3389/fimmu.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre AL, Hunt PW, McConnell DH, Morris MM, Garcia JC, Pollard RB, et al. HIV controllers with HLA-DRB1*13 and HLA-DQB1*06 alleles have strong, polyfunctional mucosal CD4+ T-cell responses. J Virol. 2010;84:11020–11029. doi: 10.1128/JVI.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HIV Controllers Study Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI.et al.2010The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 3301551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut LH, Ertl HC. Obstacles to the successful development of an efficacious T cell-inducing HIV-1 vaccine. J Leukoc Biol. 2009;86:779–793. doi: 10.1189/jlb.0209094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surman SL, Sealy R, Jones BG, Hurwitz JL. HIV-1 vaccine design: harnessing diverse lymphocytes to conquer a diverse pathogen. Hum Vaccin. 2009;5:268–271. doi: 10.4161/hv.5.4.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CA, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–443. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- Nobile C, Petit C, Moris A, Skrabal K, Abastado JP, Mammano F, et al. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol. 2005;79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, et al. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perno CF, Svicher V, Schols D, Pollicita M, Balzarini J, Aquaro S. Therapeutic strategies towards HIV-1 infection in macrophages. Antiviral Res. 2006;71:293–300. doi: 10.1016/j.antiviral.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Cobos-Jimenez V, Booiman T, Hamann J, Kootstra NA. Macrophages and HIV-1 (Review). Current Opinion in HIV & AIDS. 2011;6:385–390. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Janas AM, Wang JH, Olson WJ, Wu L. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol. 2007;81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Kirschner DE, De Boer R. Dynamics of HIV infection of CD4+ T cells. Math Biosci. 1993;114:81–125. doi: 10.1016/0025-5564(93)90043-a. [DOI] [PubMed] [Google Scholar]
- Peterson CW, Younan P, Jerome KR, Kiem HP. Combinatorial anti-HIV gene therapy: using a multipronged approach to reach beyond HAART. Gene Ther. 2013;20:695–702. doi: 10.1038/gt.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J, Braun SE, Qiu G, Wong FE, Conolle M, Schmitz JE, et al. Survival of the fittest: positive selection of CD4+ T cells expressing a membrane-bound fusion inhibitor following HIV-1 infection. PLoS ONE. 2010;5:e12357. doi: 10.1371/journal.pone.0012357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhofer M, Brandenburg G, Martinius H, Schult-Dietrich P, Melikyan G, Kunert R, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78:568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiem HP, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010;17:37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Voss G, Lekutis C, Tenner-Racz K, Racz P, et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Gilboa E, Smith C. Gene therapy for infectious diseases: the AIDS model. Trends Genet. 1994;10:139–144. doi: 10.1016/0168-9525(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Yu M, Poeschla E, Wong-Staal F. Progress towards gene therapy for HIV infection. Gene Ther. 1994;1:13–26. [PubMed] [Google Scholar]
- Miro F, Nobile C, Blanchard N, Lind M, Filipe-Santos O, Fieschi C, et al. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J Immunol. 2006;177:3625–3634. doi: 10.4049/jimmunol.177.6.3625. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- Lewinski MK, Bisgrove D, Shinn P, Chen H, Hoffmann C, Hannenhalli S, et al. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J Virol. 2005;79:6610–6619. doi: 10.1128/JVI.79.11.6610-6619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusic M, Marcello A, Cereseto A, Giacca M. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 2003;22:6550–6561. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, Klomp LW, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- Jeeninga RE, Westerhout EM, van Gerven ML, Berkhout B. HIV-1 latency in actively dividing human T cell lines. Retrovirology. 2008;5:37. doi: 10.1186/1742-4690-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–74. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, et al. Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci Transl Med. 2011;3:66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeuillade A. Potential strategies for an HIV infection cure. HIV Clin Trials. 2011;12:121–130. doi: 10.1310/hct1203-121. [DOI] [PubMed] [Google Scholar]
- Sarkar I, Hauber I, Hauber J, Buchholz F. HIV-1 proviral DNA excision using an evolved recombinase. Science. 2007;316:1912–1915. doi: 10.1126/science.1141453. [DOI] [PubMed] [Google Scholar]
- Cohen J. HIV/AIDS research. Tissue says blood is misleading, confusing HIV cure efforts. Science. 2011;334:1614. doi: 10.1126/science.334.6063.1614. [DOI] [PubMed] [Google Scholar]
- Qu X, Wang P, Ding D, Li L, Wang H, Ma L, et al. Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res. 2013;41:7771–7781. doi: 10.1093/nar/gkt571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singwi S, Joshi S. Potential nuclease-based strategies for HIV gene therapy (Review). Frontiers in Bioscience. 2000;5:D556–D579. doi: 10.2741/singwi. [DOI] [PubMed] [Google Scholar]
- Zhou J, Bobbin ML, Burnett JC, Rossi JJ. Current progress of RNA aptamer-based therapeutics. Front Genet. 2012;3:234. doi: 10.3389/fgene.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi RT, Walker BD. Immunologic control of HIV-1. Annu Rev Med. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- Fuller DH, Rajakumar P, Che JW, Narendran A, Nyaundi J, Michael H, et al. Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PLoS ONE. 2012;7:e33715. doi: 10.1371/journal.pone.0033715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson JL, Boyd MP, Arndt AJ, Todd AV, Fanning GC, Ely JA, et al. Long-term survival and concomitant gene expression of ribozyme-transduced CD4+ T-lymphocytes in HIV-infected patients. J Gene Med. 2005;7:552–564. doi: 10.1002/jgm.705. [DOI] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn DB, Bauer G, Rice CR, Rothschild JC, Carbonaro DA, Valdez P, et al. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34(+) cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood. 1999;94:368–371. [PubMed] [Google Scholar]
- DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]