Abstract
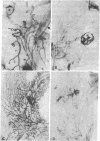
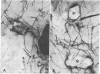
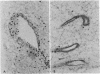
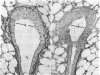
In culture, cAMP is known to be mitogenic for mammary cells and several other epithelia, but evidence for a similar role in vivo has been only correlative. We have used plastic implants to cause slow release of cholera toxin and other cAMP-active agents to local areas of mammary glands in ovariectomized mice. Elevated levels of intracellular cAMP around the implants promoted vigorous growth and normal ductal morphogenesis, while distant sites were unaffected. Local effects of cAMP included restoration of normal ductal caliber, formation of new end buds, and reinitiation of DNA synthesis in both epithelium and surrounding stroma. Thus, cAMP is both a mitogenic and a morphogenetic factor in this tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armato U., Draghi E., Andreis P. G. Effects of purine cyclic nucleotides on the growth of neonatal rat hepatocytes in primary tissue culture. Exp Cell Res. 1977 Mar 15;105(2):337–347. doi: 10.1016/0014-4827(77)90132-x. [DOI] [PubMed] [Google Scholar]
- Berger J. J., Daniel C. W. Diurnal rhythms in developing ducts of the mouse mammary gland. J Exp Zool. 1982 Nov 20;224(1):115–118. doi: 10.1002/jez.1402240113. [DOI] [PubMed] [Google Scholar]
- Berger J. J., Daniel C. W. Stromal DNA synthesis is stimulated by young, but not serially aged, mouse mammary epithelium. Mech Ageing Dev. 1983 Nov-Dec;23(3-4):277–284. doi: 10.1016/0047-6374(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ethier S. P., Ullrich R. L. Detection of ductal dysplasia in mammary outgrowths derived from carcinogen-treated virgin female BALB/c mice. Cancer Res. 1982 May;42(5):1753–1760. [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Greene R. M., MacAndrew V. I., Lloyd M. R. Stimulation of palatal glycosaminoglycan synthesis by cyclic AMP. Biochem Biophys Res Commun. 1982 Jul 16;107(1):232–238. doi: 10.1016/0006-291x(82)91694-1. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Weiss B., Costa E. Adenosine 3',5'-monophosphate concentrations and isoproterenol-induced synthesis of deoxyribonucleic acid in mouse parotid gland. Mol Pharmacol. 1972 Sep;8(5):521–530. [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Küng W., Bechtel E., Geyer E., Salokangas A., Preisz J., Huber P., Torhorst J., Jungmann R. A., Talmadge K., Eppenberger U. Altered levels of cyclic nucleotides, cyclic AMP phosphodiesterase and adenylyl cyclase activities in normal, dysplastic and neoplastic human mammary tissue. FEBS Lett. 1977 Oct 1;82(1):102–106. doi: 10.1016/0014-5793(77)80895-8. [DOI] [PubMed] [Google Scholar]
- Minton J. P., Matthews R. H., Wisenbaugh T. W. Elevated adenosine 3',5'-cyclic monophosphate levels in human and animal tumors in vivo. J Natl Cancer Inst. 1976 Jul;57(1):39–41. doi: 10.1093/jnci/57.1.39. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Murray J. B., Brown L., Langer R., Klagsburn M. A micro sustained release system for epidermal growth factor. In Vitro. 1983 Oct;19(10):743–748. doi: 10.1007/BF02618093. [DOI] [PubMed] [Google Scholar]
- NANDI S. Endocrine control of mammarygland development and function in the C3H/ He Crgl mouse. J Natl Cancer Inst. 1958 Dec;21(6):1039–1063. [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Pasco D., Quan A., Smith S., Nandi S. Effect of hormones and EGF on proliferation of rat mammary epithelium enriched for alveoli. An in vitro study. Exp Cell Res. 1982 Oct;141(2):313–324. doi: 10.1016/0014-4827(82)90219-1. [DOI] [PubMed] [Google Scholar]
- Rhine W. D., Hsieh D. S., Langer R. Polymers for sustained macromolecule release: procedures to fabricate reproducible delivery systems and control release kinetics. J Pharm Sci. 1980 May;69(3):265–270. doi: 10.1002/jps.2600690305. [DOI] [PubMed] [Google Scholar]
- Rillema J. A. Cyclic nucleotides, adenylate cyclase, and cyclic AMP phosphodiesterase in mammary glands from pregnant and lactating mice. Proc Soc Exp Biol Med. 1976 Apr;151(4):748–751. doi: 10.3181/00379727-151-39299. [DOI] [PubMed] [Google Scholar]
- Sakakura T., Nishizuka Y., Dawe C. J. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976 Dec 24;194(4272):1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L. The role of cyclic nucleotides in the development and function of rat mammary tissue. FEBS Lett. 1974 Sep 15;46(1):180–183. doi: 10.1016/0014-5793(74)80363-7. [DOI] [PubMed] [Google Scholar]
- Schaefer F. V., Custer R. P., Sorof S. Induction of abnormal development and differentiation in cultured mammary glands by cyclic adenine nucleotide and prostaglandins. Nature. 1980 Aug 21;286(5775):807–810. doi: 10.1038/286807a0. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Elvax 40P implants: sustained, local release of bioactive molecules influencing mammary ductal development. Dev Biol. 1982 Sep;93(1):272–278. doi: 10.1016/0012-1606(82)90259-7. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982 Mar;90(1):215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Stack M. T., Brandt K. D. Dibutyryl cyclic AMP affects hyaluronate synthesis and macromolecular organization in normal adult articular cartilage in vitro. Biochim Biophys Acta. 1980 Aug 13;631(2):264–277. doi: 10.1016/0304-4165(80)90301-3. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro. 1982 Jun;18(6):531–537. doi: 10.1007/BF02810076. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Fentiman I. S. Cholera toxin and analogues of cyclic AMP stimulate the growth of cultured human mammary epithelial cells. J Cell Physiol. 1980 Mar;102(3):317–321. doi: 10.1002/jcp.1041020306. [DOI] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Effects of adenosine 3':5'-cyclic monophosphate and serum on synthesis of hyaluronic acid in confluent rat fibroblasts. Biochem J. 1977 Mar 15;162(3):539–543. doi: 10.1042/bj1620539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Daniel C. W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983 Jun;97(2):274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Imagawa W., McCormick K., Nandi S. Growth factor- and cyclic nucleotide-induced proliferation of normal and malignant mammary epithelial cells in primary culture. Endocrinology. 1980 Jul;107(1):35–41. doi: 10.1210/endo-107-1-35. [DOI] [PubMed] [Google Scholar]