Abstract
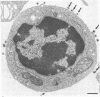
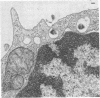
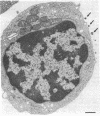
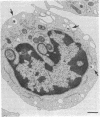
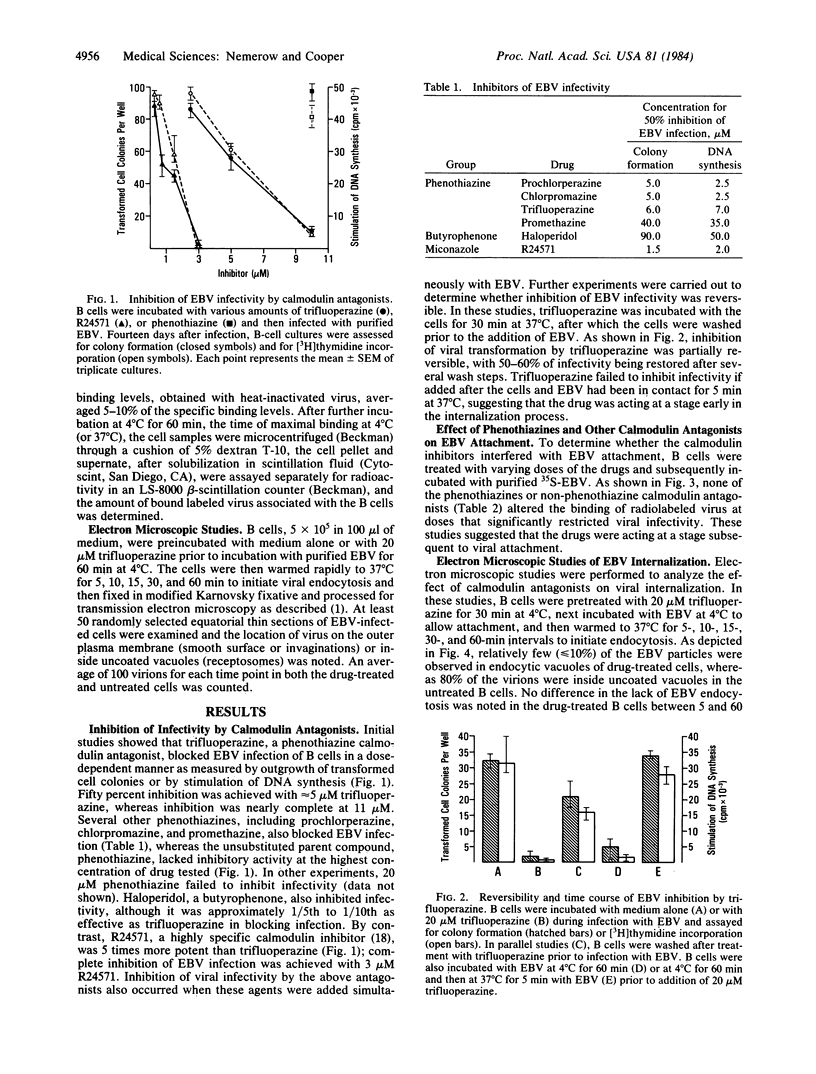
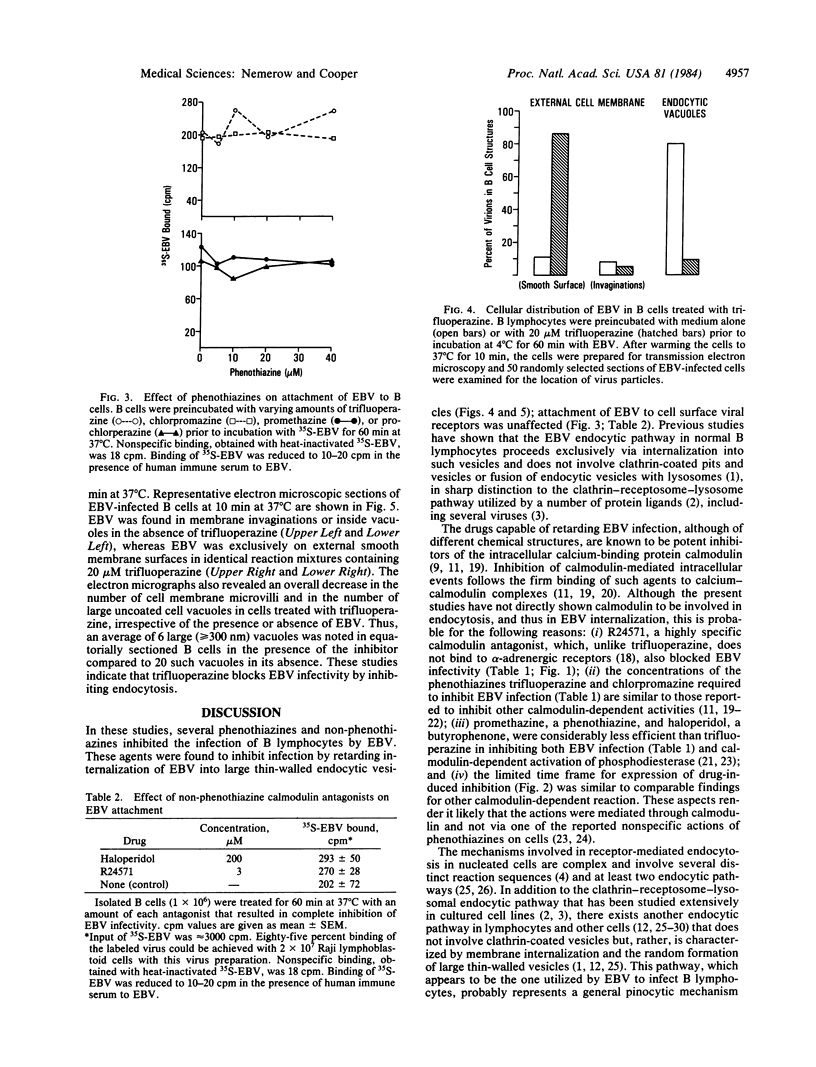
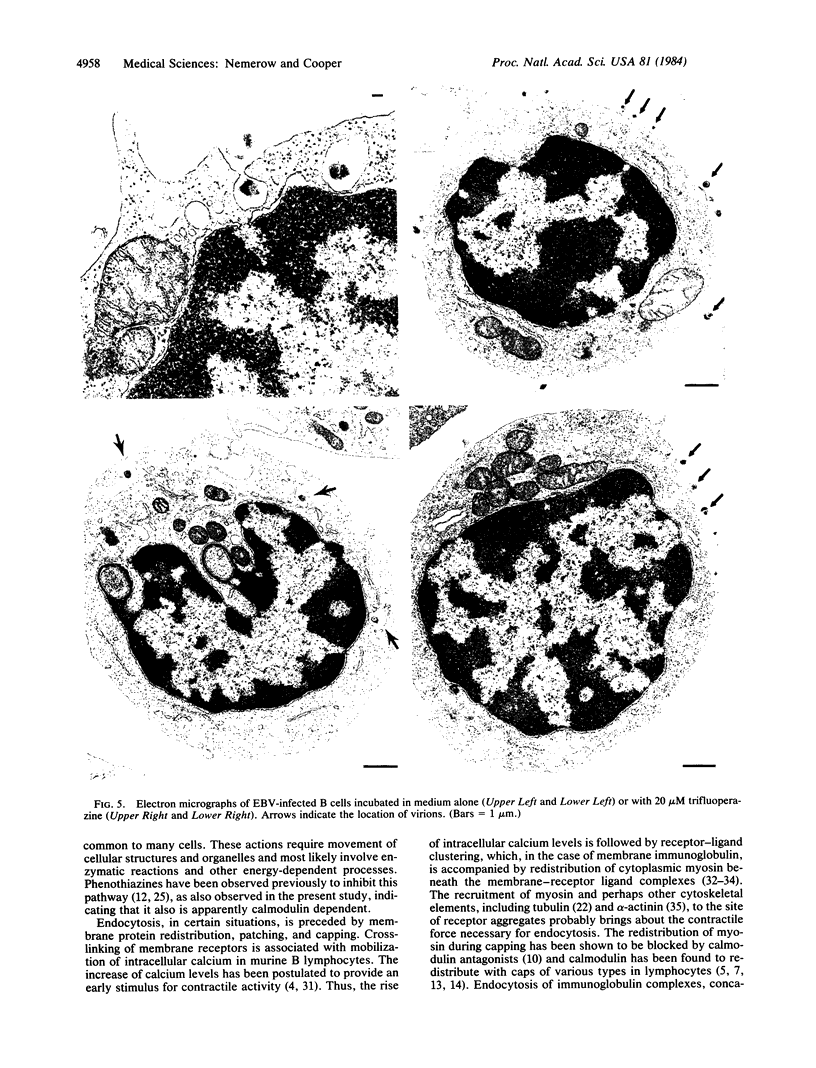
Epstein-Barr virus (EBV) is a human herpesvirus that selectively binds to and infects human B lymphocytes (B cells). In the studies presented here, we found that several phenothiazines, including trifluoperazine, chlorpromazine, prochlorpromazine, and promethazine, blocked EBV infectivity of isolated adult human B cells as measured either by outgrowth of transformed cell colonies or by [3H]thymidine incorporation. Trifluoperazine, chlorpromazine, and prochlorpromazine were equally effective with 20 microM fully inhibiting infectivity, whereas 100 microM promethazine was required for a comparable effect. Inhibition by trifluoperazine was partially reversible. Studies with radiolabeled EBV demonstrated that the inhibitors did not impair virus binding to B cells. Electron microscopic examination of B lymphocytes revealed that trifluoperazine reduced the number of large uncoated cell vacuoles and the number of membrane microvilli, indicating that this agent interfered with cell pinocytosis. This process was accompanied by inhibition of EBV endocytosis into B cells. Phenothiazines bind to and inhibit calmodulin, an intracellular calcium-binding protein that regulates several key enzymes, some of which directly affect cytoskeletal elements, although they also may interact nonspecifically with other cellular constituents. In this regard, haloperidol, a non-phenothiazine calmodulin antagonist, and R24571, a derivative of the antimycotic miconazole, which is a potent and highly specific calmodulin inhibitor, also blocked EBV infection. These studies suggest that calmodulin or a calmodulin-regulated cellular enzyme(s) is involved in normal cellular endocytic processes in B lymphocytes and thereby in the early stages of EBV infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Adelstein R. S. Regulation of contractile proteins by phosphorylation. J Clin Invest. 1983 Dec;72(6):1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Balazovich K. Effect of the antidepressant drug-Stelazine on lymphocyte capping. Cell Biol Int Rep. 1980 Oct;4(10):947–952. doi: 10.1016/0309-1651(80)90197-6. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Kerrick W. G. Receptor capping in mouse T-lymphoma cells: a Ca2+ and calmodulin-stimulated ATP-dependent process. J Membr Biol. 1983;75(1):65–72. doi: 10.1007/BF01870800. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Tokuyasu K. T., Singer S. J. The capping of lymphocytes and other cells, studied by an improved method for immunofluorescence staining of frozen sections. J Cell Physiol. 1978 Jun;95(3):239–257. doi: 10.1002/jcp.1040950302. [DOI] [PubMed] [Google Scholar]
- Braun J., Fujiwara K., Pollard T. D., Unanue E. R. Two distinct mechanisms for redistribution of lymphocyte surface macromolecules. I. Relationship to cytoplasmic myosin. J Cell Biol. 1978 Nov;79(2 Pt 1):409–418. doi: 10.1083/jcb.79.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Fujiwara K., Pollard T. D., Unanue E. R. Two distinct mechanisms for redistribution of lymphocyte surface macromolecules. II. Contrasting effects of local anesthetics and a calcium ionophore. J Cell Biol. 1978 Nov;79(2 Pt 1):419–426. doi: 10.1083/jcb.79.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Sha'afi R. I., Unanue E. R. Crosslinking by ligands to surface immunoglobulin triggers mobilization of intracellular 45Ca2+ in B lymphocytes. J Cell Biol. 1979 Sep;82(3):755–766. doi: 10.1083/jcb.82.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R. K., Grinstein S., Gelfand E. W. Permissive role of calcium in the inhibition of T cell mitogenesis by calmodulin antagonists. J Immunol. 1983 Nov;131(5):2291–2295. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Corps A. N., Hesketh T. R., Metcalfe J. C. Limitations on the use of phenothiazines and local anaesthetics as indicators of calmodulin function in intact cells. FEBS Lett. 1982 Feb 22;138(2):280–284. doi: 10.1016/0014-5793(82)80461-4. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981 Jul;39(1):172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Griffith G. R., Consigli R. A. Isolation and characterization of monopinocytotic vesicles containing polyomavirus from the cytoplasm of infected mouse kidney cells. J Virol. 1984 Apr;50(1):77–85. doi: 10.1128/jvi.50.1.77-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marsh M. Endocytosis of enveloped animal viruses. Ciba Found Symp. 1982;(92):59–76. doi: 10.1002/9780470720745.ch4. [DOI] [PubMed] [Google Scholar]
- Huet C., Ash J. F., Singer S. J. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980 Sep;21(2):429–438. doi: 10.1016/0092-8674(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Bourguignon L. Y. Regulation of receptor capping in mouse lymphoma T cells by Ca2+-activated myosin light chain kinase. Proc Natl Acad Sci U S A. 1984 Jan;81(1):165–169. doi: 10.1073/pnas.81.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Bhalla D. K., Dragsten P., Hoover R. L., Karnovsky M. J. Model for capping derived from inhibition of surface receptor capping by free fatty acids. Proc Natl Acad Sci U S A. 1980 Jan;77(1):437–441. doi: 10.1073/pnas.77.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Nelson G. A., Andrews M. L., Karnovsky M. J. Participation of calmodulin in immunoglobulin capping. J Cell Biol. 1982 Dec;95(3):771–780. doi: 10.1083/jcb.95.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984 Jan 15;132(1):186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Isolation of Epstein Barr-virus and studies of its neutralization by human IgG and complement. J Immunol. 1981 Jul;127(1):272–278. [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufogalis B. D. Phenothiazine antagonism of calmodulin: a structurally-nonspecific interaction. Biochem Biophys Res Commun. 1981 Feb 12;98(3):607–613. doi: 10.1016/0006-291x(81)91157-8. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Maihle N. J., Satir P. Calmodulin localization during capping and receptor-mediated endocytosis. Nature. 1981 Nov 12;294(5837):163–166. doi: 10.1038/294163a0. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Evidence for the involvement of calmodulin in endocytosis. Ann N Y Acad Sci. 1980;356:429–432. doi: 10.1111/j.1749-6632.1980.tb29659.x. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980 Oct;87(1):132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Yakara I., Kakimoto-Sameshima F. Microtubule organization of lymphocytes and its modulation by patch and cap formation. Cell. 1978 Sep;15(1):251–259. doi: 10.1016/0092-8674(78)90100-9. [DOI] [PubMed] [Google Scholar]