Abstract
We report here a synthetic route to oxime, azide and nitrone-bearing copolymers via reversible addition-fragmentation chain transfer copolymerization of 4-vinylbenzaldehyde and 1-(chloromethyl)-4-vinylbenzene with styrene. The azide and nitrone moieties could be employed in strain-promoted 1,3-dipolar cycloadditions with various functionalized dibenzocyclooctynols (DIBO) for metal-free post-functionalization of the polymers. In situ oxidation of the oximes with hypervalent iodine gave nitrile oxides, which could also be employed as 1,3-dipoles for facile cycloadditions with DIBO derivatives. Kinetic measurements demonstrated that the pendant nitrile oxides reacted approximately twenty times faster compared to similar cycloadditions with azides. A block copolymer, containing azide and oxime groups in segregated blocks, served as a scaffold for attachment of hydrophobic and hydrophilic moieties by sequential strain-promoted alkyne-azide and strain-promoted alkyne-nitrile oxide cycloadditions. This sequential bi-functionalization approach made it possible to prepare in a controlled manner multi-functional polymers that could self-assemble into well-defined nanostructures.
Keywords: grafting, click chemistry, SPAAC, SPANOC, SPANC
INTRODUCTION
Progress in biomedical sciences and nanotechnology relies on soft materials with tunable properties and complex yet well-defined polymeric architectures.1 Functional polymers can be prepared either by direct polymerization of monomers bearing a desired functional group or by post-polymerization modification.2 The latter grafting onto approach has gained popularity due to the development of efficient and chemoselective coupling reactions and polymerization methods that have high functional group tolerance.3 The grafting onto approach circumvents problems associated with the slow polymerization of complex and bulky monomers that may result in heterogeneity.4 In addition, the post-polymerization modification approach makes it possible to readily synthesize libraries of functionalized polymers from common polymeric precursors thereby facilitating investigations of structure-property relationships.5
Polymers containing several pendant reactive groups offer versatile scaffolds for the preparation of new materials with controlled properties.6 In particular, hetero-bi-functional block copolymers with distinct reactive groups in each block can be used to prepare materials that self-assemble in solution or be used in drug delivery, nanolithography and the preparation of nanomembranes.7 Several groups have reported the synthesis of such bi-functional block copolymers. For example, Maynard and coworkers employed reversible addition-fragmentation chain transfer (RAFT) polymerization to prepare a bi-functional block copolymer with pendant activated ester and aldehyde groups for modification via amide coupling and Schiff base formation.8 Tunca and coworkers used ring opening metathesis polymerization to prepare bi-functional block copolymers for sequential bi-functionalization by copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and Diels-Alder reactions.9 Nilles and Theato employed activated esters of different reactivity for sequential modification of a polymeric backbone with various amines.10 Recently, research groups of Hawker and Zhang designed biodegradable block copolymers for orthogonal CuAAC and thiol-ene modifications.11 Despite the attractive features of these functional scaffolds, the lack of chemoselectivity of amide coupling poses limitations on the types of moieties that can be attached to polymeric backbones. Furthermore, CuAAC exhibits high chemoselectivity but the use of copper species causes toxicity issues and may lead to the degradation of biopolymers.12
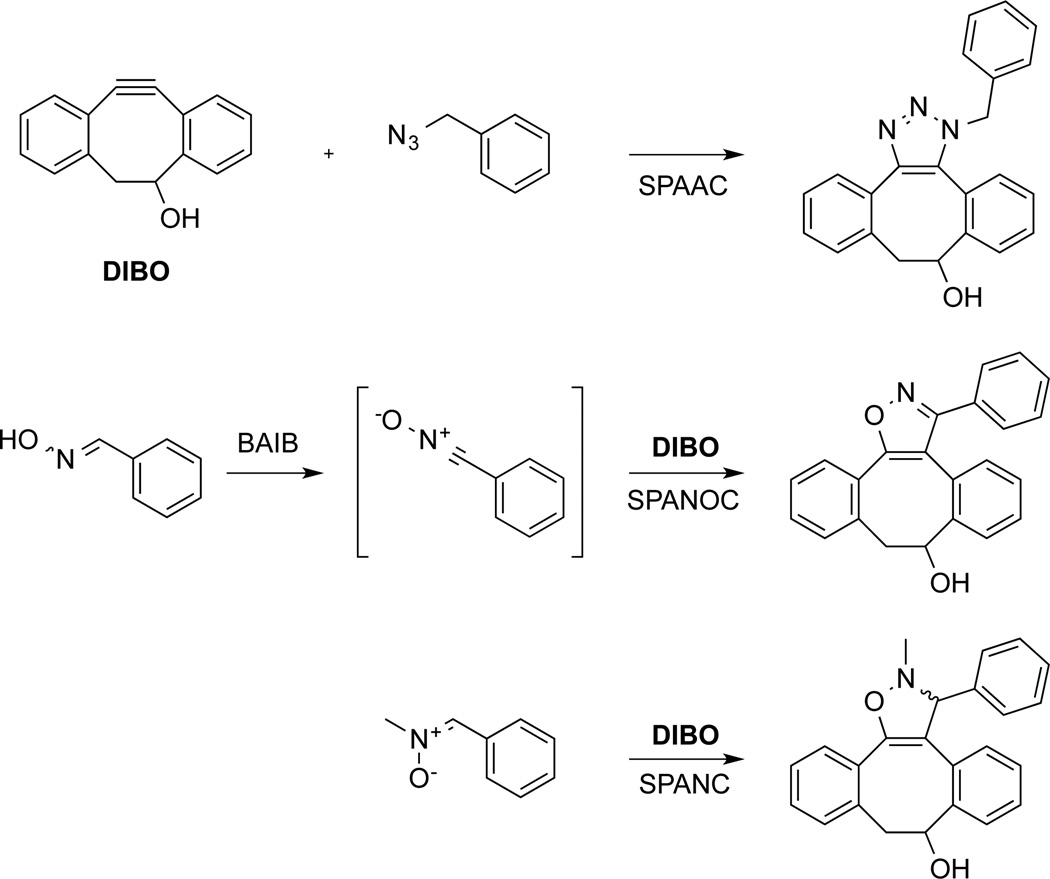
Strain-promoted alkyne-azide cycloadditions (SPAAC),13 which have high functional group tolerance, are relatively fast and do not require toxic metal catalysts. These reactions have been employed for side-14 and end-functionalization15 of polymers, creation of functional surfaces,16 polymeric networks,17 and derivatization of dendrimers.18 We have reported that derivatives of 4-dibenzocyclooctynol19 (DIBO, Scheme 1) react fast with azido-containing compounds and have attractive features such as easy access to the compound by a simple synthetic approach, nontoxicity and the possibility of straightforward attachment of a variety of probes.20 Furthermore, the structure of DIBO is amenable to analog synthesis and derivatives have been introduced that exhibit even higher rates of reaction than the parent compound.21 In our quest to expand the scope of strain-promoted cycloadditions, we have explored the use of 1,3-dipoles such as nitrile oxides22 and nitrones.23 It was found that strain-promoted alkyne-nitrone cycloadditions (SPANC) proceed with rates similar to that of SPAAC, whereas strain-promoted alkyne-nitrile oxide cycloadditions (SPANOC)24 are sixty times faster. Nitrile oxides can easily be prepared by direct oxidation of the corresponding oximes using hypervalent iodine reagents such as (diacetoxyiodo)benzene (BAIB) (Scheme 1).25 Furthermore, oximes and azides provide a pair of functional groups for sequential metal-free click reactions.
Scheme 1.
Schematic representation of SPAAC, SPANOC and SPANC reactions with DIBO.
We envisage that polymer functionalization by SPANOC will be attractive when high rates of reaction are required.14c Furthermore, it was expected that the use of nitrile oxide and nitrone bearing polymers would expand the range of reactive polymeric scaffolds that are amenable to modification by strain-promoted cycloadditions. It may also alleviate challenges associated with the synthesis of azido-containing polymers.26 Oximes can also serve as latent dipoles during SPAAC22 thereby offering possibilities for sequential SPAAC and SPANOC modifications to provide bi-functional polymers in a controlled manner. These cycloadditions have high functional group tolerance and therefore their use should widen the scope of polymer bi-functionalization.8–12
RESULTS AND DISCUSSION
Synthesis of oxime-containing copolymers
First, attention was focused on the development of a procedure for the preparation of nitrone and oxime-bearing polymers. Reactive nitrile oxides can be formed by oxidation of oximes with a hypervalent iodine reagent such as (diacetoxyiodo)benzene (BAIB).24c Furthermore, oximes and nitrones can be prepared by reaction of a corresponding aldehyde with hydroxylamine or N-methylhydroxylamine, respectively. Thus, it was anticipated that copolymers based on 4-vinylbenzaldehyde (VBA),27 which can be prepared by free radical polymerization without the need for protection of the aldehyde moieties, would be ideal for the preparation of nitrone and nitrile oxide bearing polymers. The VBA-co-styrene polymers 1a–c were prepared using 2-cyano-2-propyl dodecyl trithiocarbonate as a chain transfer agent and azobisisobutyronitrile (AIBN) as an initiator (Scheme 2a) via RAFT polymerization. The VBA monomer was obtained in one step from 1-(chloromethyl)-4-vinylbenzene (VBC) using a Sommelet reaction.28 Three polymers with varying VBA to styrene ratios were synthesized to establish the proper ratio between functional group density and solubility. The molar ratio of [AIBN]:[CTA]:[monomer] in the feed was 0.001:0.01:1. Monomer feed ratios of 1:30, 1:20 and 1:5 gave polymers with VBA to styrene ratios of 1:13.5 (1a), 1:9.4 (1b) and 1:2.7 (1c), respectively.
Scheme 2.
Schematic representation of the synthetic route towards mono-functionalized copolymers. a) Preparation of isoxazole bearing polymers 4a–c. b) Preparation of N-methyl isoxazole bearing polymer 6. c) Preparation of triazole bearing polymer 10.
The discrepancy between the feed and observed monomer ratios was expected due to a higher reactivity of VBA compared to styrene.27c The polymerizations were carried out in 1,4-dioxane at 70 °C for 20 h to achieve 37, 40 and 49 % conversions for polymers 1a, 1b and 1c, respectively. The resulting polymers had monomodal molecular weight distributions and low molar-mass dispersity (ĐM), characteristic for RAFT polymerization. The molecular weights were determined by gel permeation chromatography (GPC) and NMR spectroscopy and the results are summarized in Table 1. The discrepancies in molecular weights determined by GPC and NMR are probably due to partial loss of the trithiocarbonate end group during polymerization or isolation of polymers. Since the loss of the RAFT chain end is a well known issue,29 we employed the GPC-based molecular weights to calculate the number of repeating units (m for VBA, n for styrene) per polymer chain and degree of polymerization (DP, DP = m+n) (Table 1). The ratio of the repeating units of the copolymers was determined by integration of 1H NMR spectra: n/m = ([Iar-ICHO×4]/5)/ICHO, in which ICHO is the integral area of aldehyde protons at 9.9 ppm and Iar corresponds to the integration of aromatic CH protons of styrene and VBA at 6.2–7.6 ppm. The calculations of m and DP were carried out using the formulas indicated in Table 1.
Table 1.
Characterization of random copolymers 1–10
| Entry | Mn(GPC) g/mola |
Mw(GPC) g/mola |
ĐM (GPC)a |
Mn(NMR) g/molb |
mc | DPd |
|---|---|---|---|---|---|---|
| 1a | 3,300 | 3,650 | 1.10 | 4,950 | 1.9 | 28 |
| 1b | 3,400 | 3,700 | 1.09 | 5,200 | 2.6 | 29 |
| 1c | 4,200 | 4,700 | 1.12 | 6,400 | 9.4 | 34 |
| 3a | 3,250 | 3,600 | 1.11 | |||
| 3b | 3,350 | 3,700 | 1.10 | |||
| 3c | 4,550 | 5,050 | 1.11 | |||
| 4a | 3,500 | 3,900 | 1.11 | |||
| 4b | 3,800 | 4,150 | 1.10 | |||
| 4c | 4,900 | 5,450 | 1.11 | |||
| 5 | 2,800 | 3,150 | 1.12 | |||
| 6 | 3,500 | 3,800 | 1.11 | |||
| 7 | 3,550 | 3,900 | 1.10 | 5,250 | 3.4 | 29 |
| 9 | 3,350 | 3,700 | 1.11 | |||
| 10 | 3,850 | 4,200 | 1.10 |
Determined against narrow polystyrene standards at 40 °C using tetrahydrofuran as the mobile phase.
NMR-based Mn of dodecyl trithiocarbonate-terminated polymers was calculated by comparing the integral areas under CHar, CHO or CH2Cl peaks of repeating units with integrals of CH2-S and CH-S signals of ω-chain end. The molecular weight of CTA (345 g/mol) was then added to the sum of weights of repeating units.
The number of functional groups per chain was obtained based on GPC Mn and ratio of monomers from NMR analysis. The formula used for calculation of number of VBA units per polymer chain: mVBA=(Mn-345)/(132+(n/m)×104), n/m (St/VBA) was obtained by from NMR integration and equals to 9.4 for polymer 1b.
Degree of polymerization was calculated based on Mn from GPC and relative ratio of monomers (n/m) obtained from NMR. DP = m+m×(n/m).
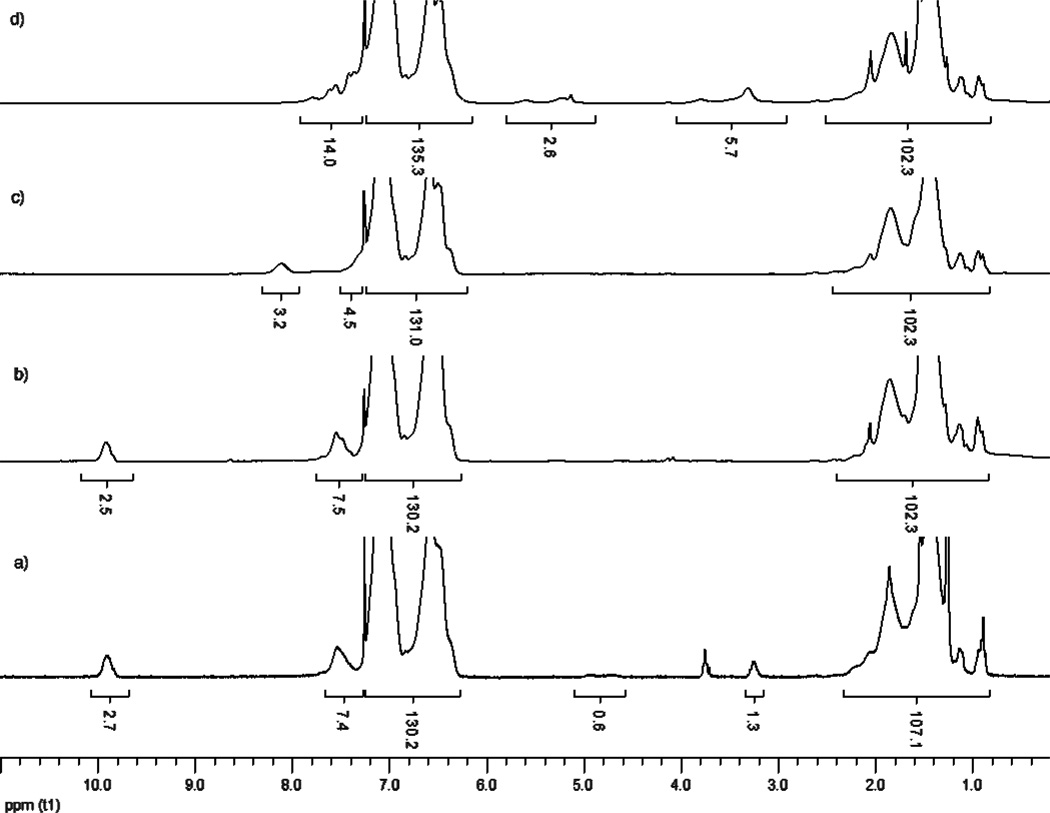
The trithiocarbonate RAFT moiety of polymers 1a–c was cleaved to give 2a–c using benzoyl peroxide (BPO) and AIBN at 80 °C for 10 h.30 The use of AIBN alone gave incomplete cleavage. In presence of BPO, however, the dodecyl trithiocarbonate cleavage was complete as confirmed by the disappearance of CH2-S and CH-S proton resonances at 3.2 and 4.8 ppm, respectively in the 1H NMR spectra (Figure 1b). The removal of the trithiocarbonate was also confirmed by the disappearance of the absorption band at 317 nm in the UV-Vis spectra (Figure S1). A small decrease in molecular weight as determined by GPC was also observed for polymers 2a–c, which is consistent with the removal of a dodecyl trithiocarbonate.
Figure 1.
1H NMR spectra of polymers 1b–4b. The integral values are based on the GPC-based molecular weight and ratio of repeating units in polymer. a) 1H NMR spectrum of polymer 1b. b) 1H NMR spectrum of polymer 2b. Note the disappearance of CH-S and CH2-S signals at 4.8 and 3.2 ppm respectively. The integral area of aromatic signals was matched to 1b. c) 1H NMR spectrum of polymer 3b. Note the disappearance of an aldehyde signal at 9.9 ppm and appearance of oxime signal at 8 ppm. The integral area of backbone aliphatic signals was matched to 2b. d) 1H NMR spectrum of polymer 4b. The grafting efficiency was estimated comparing the integral areas of isoxazole at 2.8–4.0 (CH2CH) and 4.8–5.8 (CHOH) ppm to aldehyde proton signal of 1b. In each case, the CDCl3 signal has been excluded in the integration for n/m, DP, and Mn(NMR) calculations.
Removal of the trithiocarbonate end groups of polymers 1a–c was essential because treatment with hydroxylamine to introduce oximes resulted in aminolysis of the RAFT moiety and formation of a free thiol, which dimerized resulting in an increase in molecular weight and ĐM. Also, it is known that thiols can react with nitrile oxides thereby compromising the SPANOC reaction.31 Furthermore, aldehydes are incompatible with aminolysis conditions, and these conditions could not be employed for modification of the trithiocarbonate end group.32 We also prepared a polymer similar to 1 using 4-vinylbenzaldehyde diethyl acetal as a monomer and employed aminolysis/thio-Michael addition for removal of the RAFT moiety.33 This route was, however, lengthier due to the need of acetal protection-deprotection steps. Also, diethyl acetal moieties were rather labile and polymers bearing this functional group could not be stored for prolonged periods of time.
Polymers 2a–c were treated with hydroxylamine and triethylamine in dichloromethane (DCM) to give oxime containing polymers 3a–c. The quantitative conversion of the aldehyde moieties to oximes was confirmed by the disappearance of the aldehyde (CHO) signal at 9.9 ppm and appearance of oxime (CHN) singlet at 8 ppm in the 1H NMR spectra. Also, disappearance of a carbonyl stretch in the IR spectra at ca. 1,700 cm−1 confirmed the completion of the reaction (Figure S14). Although the conversion was quantitative for all three polymers, it was found that the solubility of the oxime-rich polymer 3c in organic solvents was inferior to polymers with lower oxime content. Therefore, for the subsequent experiments polymer 3b was used having a VBA:styrene ratio of 1:9.4.
Nitrone-bearing polymer 5 was conveniently prepared by treatment of 2b with N-methylhydroxylamine hydrochloride and triethylamine in DCM (Scheme 2b).34 Quantitative nitrone formation was evident from the disappearance of the aldehyde (CHO) signal in the 1H NMR spectrum at 9.90 ppm and the appearance of distinct (CHar) and CH3N signals at 7.9 and 3.8 ppm, respectively and also by the disappearance of a carbonyl stretch signal in the IR spectrum (Figure S14).
Finally, copolymerization of styrene and 1-(chloromethyl)-4-vinylbenzene (VBC) gave polymer 7 that served as the precursor for the preparation of azido-functionalized polymer 9 (Scheme 2c). The polymerization was carried out in 1,4-dioxane at 70 °C using AIBN as the initiator and 2-cyano-2-propyl dodecyl trithiocarbonate as the chain transfer agent to achieve a conversion of 40% in 20 h. The VBC monomer had a slightly higher reactivity than styrene and therefore, a feed ratio of 1:10 VBC to styrene resulted in polymer 7 having 1:7.6 VBC to styrene ratio and 3.4 functional groups per chain according to GPC and NMR. These calculations were carried out in a fashion similar as for polymers 1a–c using the CH2Cl signal of VBC at 4.5 ppm as a reference. The VBC-co-styrene polymer 7 had a monomodal molecular weight distribution and a narrow ĐM (GPC) (Table 1). Next, the RAFT moiety of polymer 7 was cleaved by treatment with BPO and AIBN at 80 °C in dioxane for 10 h. The RAFT cleavage was complete as confirmed by NMR and UV-Vis spectroscopy. Comparison of the GPC chromatograms showed a slight reduction of molecular weight of polymer 8 (Mn = 3,400 g/mol, GPC) compared to 7 (Mn = 3,550 g/mol, GPC) corroborating cleavage of the dodecyl trithiocarbonate moiety. The chlorides of polymer 8 were quantitatively displaced by NaN3 in DMF to give polymer 9 as shown by IR and NMR spectroscopy (Figures S14 and S41). An attempt to directly polymerize an azido-containing monomer 1-(azidomethyl)-4-vinylbenzene (AzMVB) at 70 °C resulted in a polymer having a high molecular weight shoulder probably due to nitrene formation and subsequent crosslinking or cycloaddition of azide to the double bond of styrenic monomers.26 Also, it is note worthy that treatment of the polymer 7 with sodium azide resulted in partial cleavage of the RAFT moiety resulting in an increase in ĐM, probably due to the formation of disulfides.
Optimization of reaction conditions for polymer modification by SPANOC
Nitrile oxides are reactive species that can dimerize to form furoxans.35 For example, benzohydroxamoyl chloride has been reported to dimerize to give bis-phenyl-3,4-furoxan when treated with triethylamine at room temperature.36 Also, nitrile oxides formed by BAIB-mediated oxidation of the corresponding oximes, can react with acetic acid to form N-acetoxy amides and for example, oxidation of benzaldoxime with BAIB in absence of dipolarophiles did not give the dimerization adduct but provided N-acetoxy benzamide.37 When polymer 3a was treated with BAIB in absence of DIBO, a high molecular weight shoulder was observed in the GPC chromatogram of the resulting product (Figure S2).
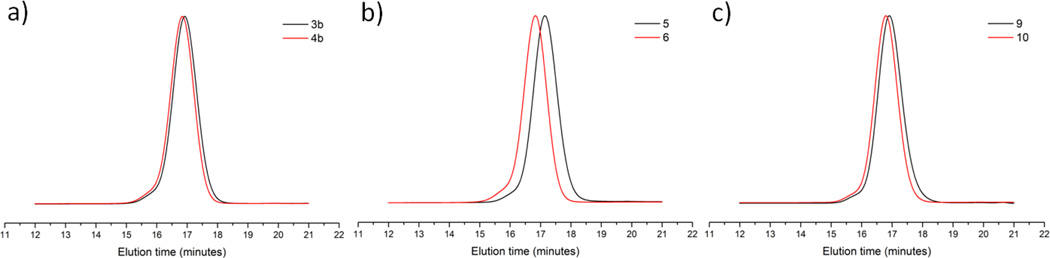
This observation indicates that in absence of dipolarophiles, the polymeric nitrile oxides can undergo intermolecular dimerization. The crosslinking was observed during conversion of 3a even at low concentrations (2 mM) in DCM. However, no crosslinking was observed when DIBO (3 eq) was premixed with polymer 3a in a mixture of methanol (MeOH) and DCM (4/1, v/v) followed by the dropwise addition of a solution of BAIB. The formation of isoxazole 4a was confirmed by the disappearance of the 1H NMR CHN signal of the oxime at 8.0 ppm and the appearance of new broad signals of regioisomeric isoxazoles at 2.8–4.0 (CH2CH) and 4.8–5.8 (CHOH) ppm (Figure S35). The modification was quantitative based on a comparison of integrations (relative to aromatic protons) of these peaks and aldehyde signal of polymer 1a (Figure S29). The degree of modification of polymer 1 a was calculated in the following manner: % of functionalization = 100×(m/n)4a/(m/n)1a. The m/n values were obtained by NMR integration using the following formulas: (m/n)1a = ICHO/([Iar-ICHO×4]/5), (m/n)4a = ([ICHOH+ICH2]/3)/([Iar-([ICHOH+ICH2]/3)×12]/5), in which ICHOH and ICH2 are the integrals of isoxazole signals at 4.5–5.8 and 2.8–4.0 ppm, respectively. Similarly, treatment of polymers 3b and 3c with DIBO and BAIB gave, after purification by precipitation from cold methanol, monodisperse polymers without a sign of intermolecular crosslinking. The level of modification of the resulting polymers 4b and 4c was near quantitative (Figures 1 and S36). GPC traces of all three isoxazole bearing polymers 4a–c showed a slight decrease in elution time indicating an increase of molecular weight as a result of successful pendant modification (Figures 2 and S6–S8). Similarly, treatment of polymer 5 with small excess of DIBO in DCM gave N-methyl isoxazole bearing polymer 6. The near quantitative conversion of nitrone groups was evident from the 1H NMR spectrum, which showed a disappearance of CHar and CH3N signals of the nitrone at 8.0 and 3.9 ppm, respectively and appearance of new broad signals of N-methyl isoxazole at 3.0–3.8 (CH2CH, CH3N), 4.9–5.2 (CHOH, CHN). The degree of modification for polymer 6 was calculated in the following manner: % of functionalization = 100×(m/n)6/(m/n)5. The m/n values were obtained by NMR integration using the following formulas: (m/n)5 = ([ICH3]/3)/([Iar-(ICH3×5)/3]/5), (m/n)6 = ([ICHOH+ICH2+ICH3+ICH]/7)/([Iar-([ICHOH+ICH2+ICH3+ICH]/7)×12]/5). The calculation gave a 97% functionalization after SPANC. Interestingly, GPC of the polymer 6 showed a significant increase in hydrodynamic volume compared to precursor polymer 5. We hypothesize that this observation is due to the conversion of polar nitrones into less polar isoxazoles that are better solvated by tetrahydrofuran resulting in an extended conformation. Finally, triazole-bearing polymer 10 was obtained by treatment of azido-containing polymer 9 with DIBO in DCM. The complete consumption of the azide groups was confirmed by IR and 1H NMR spectroscopy (Figures S14 and S42). The conversion from azido- to ‘triazole-bearing polymer 10 was quantitative based on a comparison of integral areas of the CH2N3 signal at 4.2 ppm and triazole signals at 2.8–3.7 (CH2) and 4.6–5.8 (CHOH, CH2N) ppm (Figures S41 and S42). GPC of polymer 10 showed a small increase in molecular weight due to the pendant modification (Figure 2).
Figure 2.
GPC traces of polymers before and after reaction with DIBO. a) 3b and respective isoxazole–bearing polymer 4b. b) 5 and respective N-methyl isoxazole–bearing polymer 6. c) 9 and respective triazole–bearing polymer 10.
Kinetic measurements of SPAAC, SPANC and SPANOC
Having established that the 1,3-dipoles of polymers 3a–c, 5 and 9 can react readily with DIBO, attention was focused on determining the second order rate constants of the cycloadditions. For this purpose, polymers 3b, 5 and 9 were chosen because they have similar molecular weights and functional group densities. The rate constants were determined by monitoring the consumption of DIBO by 1H NMR spectrometry using CDCl3 as the solvent (Supporting Information). The concentration of DIBO was equal to the concentration of the respective 1,3-dipole, which was calculated from the number of functional groups per polymer chain. The starting DIBO concentrations were chosen to be 0.0067 M for benzyl azide, polymers 9, and 3b, and 0.01 M for polymer 5. The concentration of DIBO at any given time during the reaction was determined by integration of aliphatic CH and CH2 signals of DIBO and CH signals of triazole or isoxazoles. The second order rate constants (Table 2) were determined from the slope of the plot of 1/[DIBO] vs. time (Figure S3), according to the integrated second order reaction rate law (1/[DIBO]=1/[DIBO]0+kt). It was found that nitrile oxides are the most reactive 1,3-dipoles, which is in agreement with our previous observations using low molecular weight compounds.22 The second order rate constant for the SPANOC cycloaddition of polymer 3b with DIBO was found to be 0.77±0.2 M−1s−1. The rate constant for reaction of azido-containing polymer 9 with DIBO was found to be 0.039±0.012 M−1s−1 and nitrone bearing polymer 5 exhibited a second order rate constant of 0.016±0.001 M−1s−1. The data shows that that SPANOC is approximately twenty times faster than the SPAAC. Interestingly, the rate constant of cycloaddition of azido-containing polymer 9 with DIBO was slightly smaller than that of benzyl azide, which is probably a consequence of steric hindrance caused by the polymeric nature of the 1,3-dipoles.
Table 2.
Second order rate constants of cycloadditions of DIBO with benzyl azide and polymers 3b, 5 and 9
| Dipole | Second Order Rate constants (M−1s−1)a |
|---|---|
| Benzyl azide | 0.058±0.004 |
| 3b | 0.77±0.2 |
| 5 | 0.016±0.001 |
| 9 | 0.039±0.012 |
Measurements were performed in CDCl3 at 25 °C. Errors are calculated from standard deviation of three independent measurements.
Synthesis of bi-functional block copolymers containing azide and oxime moieties
Previously, we demonstrated that oximes can serve as latent 1,3-dipoles making it possible to perform sequential SPAAC/SPANOC reactions.22 Therefore, it was expected that copolymers having azide and oxime groups would allow bi-functionalization in a controlled manner. Also, due to the living nature of RAFT polymerization, VBC-containing polymer 7 can be employed as a macro chain transfer agent for copolymerizing styrene and 4-vinylbenzaldehyde to give block copolymers bearing chloride and aldehyde moieties in segregated blocks (Scheme 3). The chlorides of the resulting polymers can then be displaced with NaN3 and the aldehydes can be reacted with hydroxylamine to give oximes. The azides can then be reacted with functionalized DIBO, followed by oxidation of the oximes to nitrile oxides, which in turn can be reacted with another DIBO derivative bearing a second moiety of interest.
Scheme 3.
Schematic representation of the synthetic route towards bi-functional polymer 14.
The synthesis of bi-functional block copolymer 11 was achieved by copolymerizing VBA and styrene in 1,4-dioxane for 20 h at 70 °C using AIBN as the initiator and polymer 7 as the macro-CTA. The molar ratio [AIBN][CTA][VBA][Styrene] in the feed was 0.001:0.01:0.05:1 to achieve a block length and functional group density similar to that of polymer 1b. According to GPC, the molecular weight of the resulting polymer 11 (7,700 g/mol) was twice as large as that of precursor polymer 7, demonstrating successful chain extension (Table 3).
Table 3.
Characterization data of block copolymers 11, 14, 19, 20, 21 a–c and 22 a–c
| Entry | Mn(GPC)a g/mol |
Mw(GPC)a g/mol |
ĐM (GPC)a |
|---|---|---|---|
| 11 | 7,700 | 8,800 | 1.15 |
| 14 | 7,100 | 7,950 | 1.12 |
| 19 | 7,850 | 8,850 | 1.13 |
| 20 | 10,900 | 13,450 | 1.23 |
| 21a | 9,450 | 10,800 | 1.14 |
| 21b | 12,150 | 13,550 | 1.12 |
| 21c | 6,850 | 7,950 | 1.16 |
| 22a | 10,550 | 12,200 | 1.16 |
| 22b | 15,200 | 17,700 | 1.16 |
| 22c | 7,250 | 8,600 | 1.18 |
Determined against narrow dispersity polystyrene standards at 40 °C using THF as the mobile phase
Polymer 11 has approximately an equal number of chloride and aldehyde groups as determined by integral areas of CH2Cl (4.5 ppm) and CHO (9.9 ppm) signals in the 1H NMR spectrum (Figure S43). The number of VBA and VBC repeating units was approximately 3.6 and 3.3 per polymer chain, respectively. Based on the ratio of repeating units and end group analysis, the NMR Mn was calculated to be 11,300 g/mol, which is larger than the value obtained by GPC (7,700 g/mol). We also observed a slight increase in ĐM of polymer 11 compared to that of 7 and a small high molecular weight shoulder in the GPC chromatogram. These observations could arise either from partial uncontrolled propagation of polymer chains or from homo-coupling termination of propagating polymeric radicals due to inefficient radical transfer by bulky macro-CTA. The degree of polymerization of polymer 11 was 68 as determined by GPC Mn and the ratio of repeating units obtained by NMR. The RAFT moiety was cleaved using the conditions described for compounds 2 and 8 to give block copolymer 12. The chlorides were quantitatively converted into azides by using NaN3 in DMF as confirmed by IR and 1H NMR (Figures S15 and S44). Finally, treatment of polymer 13 with hydroxylamine afforded the target bi-functional block copolymer 14, bearing azide and oxime groups in distinct blocks.
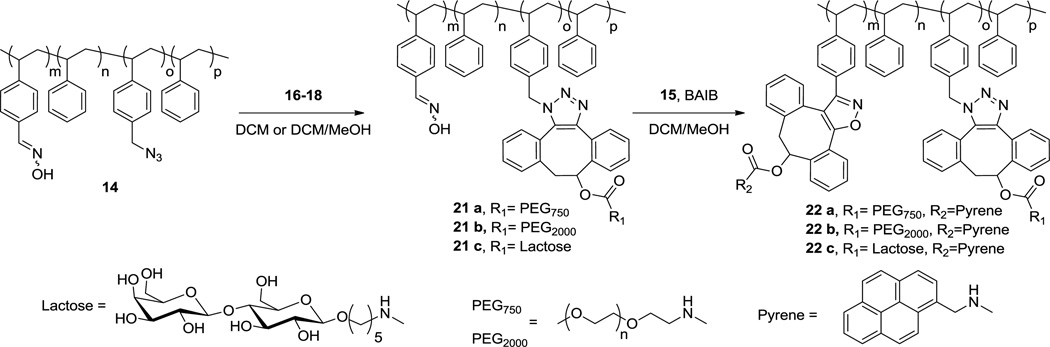
Polymers such as 14 can be reacted with DIBO derivatized with a wide variety of functional groups. Furthermore, the block copolymeric architecture and the possibility to sequentially attach hydrophilic and hydrophobic compounds, offers opportunities to prepare materials that can self-assemble in aqueous medium. To test this hypothesis, four different DIBO derivatives were prepared having hydrophobic pyrene (15), hydrophilic polyethyleneglycol (PEG) moieties of different molecular weights (16 and 17 derived from PEG with MW of 750 and 2,000 g/mol, respectively) and the disaccharide lactose (18) (Scheme 4).
Scheme 4.
DIBO derivatives 15–18.
SPAAC coupling of block copolymer 14 with hydrophobic DIBO-pyrene 15 at room temperature in DCM for 18 h afforded polymer 19 (Scheme 5). The calculation of the degree of modification for polymer 14 was achieved as follows: % of functionalization = 100×(m/n)19/(m/n)14. The m/n values were obtained from NMR integrations using the following formulas: (m/n)14 = (ICH2N3/2)/([Iar-ICH2N3×2]/5), (m/n)19 = ([ICHOH+ICH2+ICH2N]/7)/([Iar-([ICHOH+ICH2+ICH2N]/7)×12]/5) in which ICHOH, ICH2N and ICH2 are the integrals of triazole signals at 4.6–5.8 and 2.8–3.8 ppm, respectively. The extent of modification for SPAAC reaction was found to be 94%. Also, near quantitative formation of triazoles was confirmed by the disappearance of methylene signals at 4.21 ppm in the 1H NMR spectra and the disappearance of azide signal in the IR spectra (Figure S47). Next, a SPANOC reaction was carried out between polymer 19 and DIBO-PEG750 (16) in the presence of BAIB in a mixture of MeOH:DCM (1:5 v/v) for 4 h.
Scheme 5.
Schematic representation of the synthetic route towards amphiphilic polymer 20.
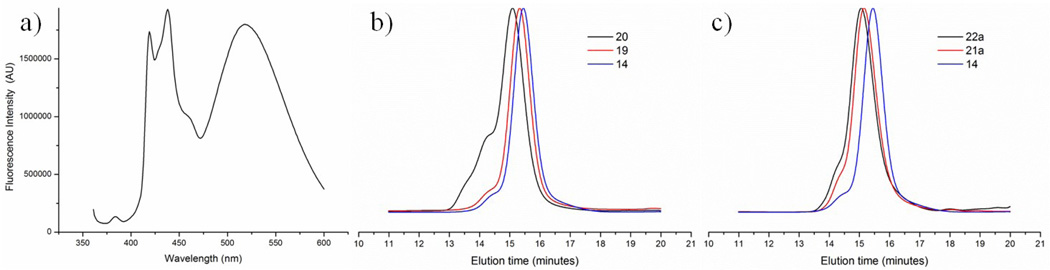
Polymer 20 was isolated by precipitation with cold methanol and the resulting product was characterized by 1H NMR spectroscopy, IR spectroscopy and GPC. Due to overlap of isoxazole and triazole signals in the NMR spectrum, the accurate degree of functionalization could not be determined. The approximate degree of functionalization was estimated by assuming an equal integral area of polymeric backbone signals at 0.9–2.4 ppm of polymers 19 and 20 (Figures S47 and S48) and considering that total number of hydrogens of 16 is approximately 80. The subtracted integral area of 19 from 20 at 2.8–4.0, 4.8–5.8 and 6–7.8 ppm suggests that approximately 3.2 DIBO-PEG750 moieties were attached to the polymeric backbone through SPANOC reaction to give a coupling efficiency of 90% (based on aldehyde content). Although the SPANOC reaction led to efficient grafting of hydrophilic PEG750 moieties onto the polymer backbone, the resulting construct had a relatively high ĐM and exhibited a high molecular weight shoulder in the GPC chromatograms (Figure 3b). Due to the fact that no apparent crosslinking of nitrile oxides was observed for polymers 4a–c, having a similar or higher functional density as polymer 19, we hypothesized that stacking of the pyrene moieties during SPANOC-mediated modification resulted in polymer aggregation, which in turn induced crosslinking as observed by GPC. Indeed, the fluorescence emission spectrum of polymer 19 in CHCl3 showed a strong band with a maxima at 525 nm (Figure 3a), supporting stacking of pyrene moieties and formation of excimers. The excimer emission band persisted even in highly diluted solutions of polymer 19 (up to 0.01 µg/mL in CHCl3, data not shown).
Figure 3.
a) Fluorescence spectra of polymer 19 in CHCl3 (1 µg/mL) excitation at 310 nm. b) GPC traces of polymers 14, 19 and 20. c) GPC traces of polymers 14, 21a and 22a.
To avoid crosslinking, the order of attachment of the hydrophilic and hydrophobic moieties was changed (Scheme 6). Thus, polymer 14 was first derivatized with PEG750 (16) via SPAAC to give polymer 21a. The modification was quantitative according to 1H NMR (calculations were performed similarly to 19). Polymer 21a was then reacted with DIBO-Pyrene 15 in presence of BAIB to provide bi-functional polymer 22a. The intensity of the pyrene signals at 7.8–8.3 ppm in the 1H NMR spectrum suggest that approximately three pyrene moieties were grafted onto each polymer chain resulting in a conversion of 82% (based on comparison to aldehyde signals of 11). Fortunately, the high molecular weight shoulder in the GPC chromatogram of polymer 22a was significantly reduced (Figure 3c). Molar-mass dispersity values obtained from GPC further supported a better control over the functionalization; polymer 22a had Đm=1.16 (Mn=10,550, Mw=12,200 g/mol) whereas polymer 20 had ĐM=1.23. In a similar fashion, graft copolymers with hydrophilic PEG2000 and lactose side chains were prepared from polymer 14 and DIBO-derivatives 17 and 18. The solvent of choice for SPAAC-mediated coupling of polymer 14 and PEG derivative 17 was DCM. The excess of DIBO-PEG 17 was removed by treatment with an azide resin for 16 h to give pure polymer 21b with quantitative conversion. Grafting of lactose onto polymer 14 required a solvent mixture of MeOH/DCM (1:1 v/v). The resulting polymer 21c was purified by precipitation in cold MeOH, and a grafting efficiency of 75% was determined from 1H NMR signals of the lactose anomeric protons at 4.2–4.4 ppm. Interestingly, the hydrodynamic radius of a lactose-modified polymer was significantly reduced according to GPC, probably due to a collapsed conformation of the lactose-containing polymer in THF. Finally, oxime bearing polymers 21b and 21c were reacted with DIBO-Pyrene conjugate 15 in presence of BAIB in MeOH/DCM (1:5 v/v) mixture for 4 h to provide bi-functional polymers 22b and 22c. The extent of the functionalization was approximately 98% for polymer 21b and 94% for polymer 21c, based on comparison of pyrene signals at 7.8–8.3 ppm to aldehyde signals of 11 in 1H NMR. Polymers 22a–c were isolated by precipitation in a cold Et2O/hexanes (1:2 v/v) mixture to remove excess of reagents. DIBO derivatives 15–18 exhibited a strong absorbance at 310 nm, which was not observed in the UV spectra of polymers 22 a–c indicating complete removal of the excess of the DIBO derivatives (Figure S4).
Scheme 6.
Schematic representation of the synthetic route towards amphiphilic polymers 22a–22c
Dynamic light scattering and transmission electron microscopy study of polymeric self-assembies
Following the successful synthesis of comb shaped block copolymers with hydrophilic and hydrophobic pendant groups, the self-assembly properties of polymers 22a–c were studied. The polymers were dissolved in THF (10 mg/mL) and the resulting solution was slowly added to distilled water while stirring to reach a final concentration of 1 mg/mL. After stirring for 2 h to allow the organic solvent to evaporate, the resulting solutions were filtered through 0.8 µm filter. Dynamic light scattering (DLS) of the resulting aqueous solutions showed formation of self-assembled materials only for polymers 22a and 22c. According to DLS, polymer 22a formed nanoparticles with mean diameter of 78 nm and polydispersity of 0.112 (Figure S5a). Polymer 22c self-assembled into larger particles with mean diameter of 122 nm with polydispersity of 0.147 (Figure S5b).
To confirm the DLS results, the self-assembled polymers 22 a–c were examined by transmission electron microscopy (TEM). In support of the DLS data, polymer 22a having pendant PEG750 moieties assembled into polydisperse spherical structures having a size range of 30 to 150 nm (Figure 4a).
Figure 4.
TEM images of drop casted aqueous solutions stained with uranyl acetate: a) polymer 22a, b) polymer 22c. Scale bar is 100 nm.
Polymer 22b did not form well-defined nanoparticles, instead rod and sheet-like structures were observed by TEM. We hypothesize that the hydrophilic segment of this polymer is too large to form stable nanoparticles. On the other hand, polymer 22a has a suitable hydrophilic-hydrophobic balance to form stable assemblies in aqueous solutions. Polymer 22c with grafted lactose moieties also demonstrated robust self-assembly forming larger nanoparticles with sizes of 50 – 300 nm according to DLS and TEM (Figure 4b).
CONCLUSION
Polymers containing orthogonal pendant reactive groups for post-polymerization modification offer versatile scaffolds for the creating multi-functional materials with controlled properties. In this paper, we describe the synthesis of polymers bearing pendant azide, oxime and nitrone groups amenable for functionalization with cyclooctyne derivatives via SPAAC, SPANOC and SPANC. The kinetic study revealed that the rate of SPANOC cycloaddition was approximately 20 times faster than that of SPAAC. For the first time, it is demonstrated that and SPANC and SPANOC can be employed for post-polymerization modification of polymers. Attractive features of SPANOC for polymer functionalization include: i) the oxime is easily introduced via an aldehyde precursor; ii) oximes are stable during SPAAC and, therefore two sequential cycloadditions can be performed for bi-functionalization; and iii) both nitrile oxides and azides react with cyclooctynes such as DIBO, thereby reducing the number of derivatives required for post-polymerization multi-functionalization. The controlled nature of RAFT polymerization made it possible to prepare block copolymer containing both azide and oxime moieties in segregated blocks. This bi-functional polymeric scaffold was derivatized with hydrophilic and hydrophobic moieties using sequential SPAAC/SPANOC reactions to give a library of amphiphilic bottle-brush copolymers. The modular nature of the synthetic approach makes it possible to determine in a facile manner the optimal hydrophilic-hydrophobic balance for self-assembly of the resulting polymers in aqueous solutions. The expansion of sequential SPAAC/SPANOC reactions in the area of polymeric scaffolds will facilitate the design and assembly of intricate polymeric structures for applications in material science and as biomaterials.
EXPERIMENTAL SECTION
Materials
All reagents were purchased from Sigma-Aldrich® and used as received unless stated otherwise. Dichloromethane was distilled over calcium hydride. Styrene was washed with 1N NaOH, followed by water to remove inhibitors, dried over MgSO4 and then purified by vacuum distillation over calcium hydride. AIBN was recrystallized from MeOH twice prior to use. Azido resin was synthesized by reacting Merrifields resin with sodium azide in DMF.
Measurements
The NMR spectra were recorded on a Varian Mercury 300 MHz or Varian Inova 500 MHz spectrometers using CDCl3 as a solvent unless stated otherwise. 1H NMR-based Mn of dodecyl trithiocarbonate-terminated polymers was calculated by comparing the integral areas under CHar, CHO and CH2Cl peaks of repeating units with integrals of CH2-S signal of ω-chain end. The weight of CTA (345 g/mol) was then added to the sum of weights of repeating units. NMR-based Mn was used to calculate quantities of reagents for dodecyl trithiocarbonate cleavage. For side chain transformations the quantities of reagents were calculated based on ratio of repeating units obtained from 1H NMR. GPC analyses were performed in on Shimadzu LC-20AD liquid chromatography instrument, equipped with RI detector. Two Waters Styragel columns (HR3 and HR4) were placed in series. THF was used as eluent at 1 mL/min flow rate; the column oven was set to 40 °C. Molecular weights were calculated against polystyrene standards. IR spectra were acquired on Thermo-Nicolet FTIR Spectrometer in KBr pellets. TEM images were obtained on Philips/FEI Technai 20 instrument using copper TEM grids and uranyl acetate staining.
Representative random copolymerization procedure. Synthesis of poly(4-vinylbenzaldehyde-co-styrene) 1b via RAFT polymerization
A dry Shlenk flask was charged with styrene (2.00 g, 19.20 mmol), VBA (127 mg, 0.96 mmol), AIBN (3.3 mg, 0.02 mmol), 2-cyano-2-propyl dodecyl trithiocarbonate (76 mg, 0.22 mmol) and 1,4-dioxane (3 mL). The mixture was subjected to three freeze-pump-thaw cycles and then stirred for 20 h at 70 °C. The polymerization was terminated by submersion into liquid nitrogen. Then the reaction mixture was diluted with THF (3 mL) and the polymer was purified by precipitation in cold MeOH (250 mL) twice to give polymer 1b (0.82g, 40%) as yellowish solid. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.6 Hz, CH3CH2), 1.13–2.6 (m, CH2CH2, CHCH2, CCH3), 3.24 (brs, CH2S), 4.67– 4.95 (m, CHS), 6.37–7.62 (m, CHar), 9.89 (brs, CHO); Mn (g/mol) = 3,400 (GPC), 5,200 (NMR). ĐM=1.09(GPC).
Representative dodecyl trithiocarbonate cleavage procedure. Synthesis of poly(4-vinylbenzaldehyde-co-styrene) 2b
A dry Shlenk flask was charged with AIBN (650 mg, 4 mmol, 20 eq per end group), benzoyl peroxide (97 mg, 0.4 mmol, 2 eq per end group), poly(4-vinylbenzaldehyde-co-styrene) polymer 1b (800 mg) and 1,4-dioxane (3 mL). The mixture was subjected to three freeze-pump-thaw cycles and then stirred for 10 h at 80°C. The reaction mixture was diluted with THF (5 mL) and the polymer was purified by precipitation into cold MeOH (250 mL) twice to give a polymer 2b (683 mg, 85%) as a white solid. 1H NMR (500 MHz, CDCl3) δ 0.88–2.37 (m, CHCH2, CCH3), 6.37–7.52 (m, CHar), 9.89 (brs, CHO); Mn (g/mol) = 3,250 (GPC, ĐM=1.11).
Representative oximation procedure. Synthesis of poly(4-vinylbenzaldoxime-co-styrene) 3b
A solution of poly(4-vinylbenzaldehyde-co-styrene) 2b (300 mg), hydroxylamine hydrochloride (52 mg, 0.75 mmol) and triethylamine (210 µL, 1.50 mmol) in DCM (5 mL) was stirred for 18 h at room temperature. The reaction mixture was diluted with DCM (50 mL), washed with water 3×10 mL, dried over MgSO4 and concentrated. The polymer was purified by precipitation into cold hexanes 100 mL to yield 3b (230 mg, 77%) as white solid. 1H NMR (500 MHz, CDCl3) δ 0.88–2.30 (m, CHCH2, CCH3), 6.36–7.34 (m, CHar), 8.08 (brs, CHNOH); Mn (g/mol) = 3,350 (GPC, ĐM=1.10).
Representative procedure for preparation of azido-polymers. Synthesis of poly(1-(azidomethyl)-4-vinylbenzene-co-styrene) 9
A solution of sodium azide (117 mg, 1.8 mmol) and poly(1-(chloromethyl)-4-vinylbenzene-co-styrene) 8 (600 mg) in DMF (10 mL) was stirred overnight at room temperature. The resulting polymer was purified by precipitation into MeOH/water mixture (250 mL, 1/1, v/v) twice to give polymer 9 (482 mg, 80%) as white solid. 1H NMR (500 MHz, CDCl3) δ 0.88–2.42 (m, CHCH2, CCH3), 4.21 (brs, CH2N3), 6.37–7.51 (m, CHar); Mn (g/mol) = 3,350 (GPC, ĐM=1.11).
Representative procedure for SPAAC reaction with DIBO. Synthesis of polymer 10
A solution of poly((1-azidomethyl)-4-vinylbenzene-co-styrene) 9 (40 mg) and DIBO (17 mg, 0.08 mmol) in DCM (5 mL) was stirred for 18 h at room temperature. The polymer was purified by precipitation into cold MeOH (50 mL) to yield polymer 10 as white solid (30 mg, 75%). 1H NMR (500 MHz, CDCl3) δ 0.86–2.15 (m, CHCH2, CCH3), 2.80–3.70 (m, CH2CHO), 4.60–5.55 (m, CH2N, CH2CHO), 6.05–7.80 (m, CHar); Mn (g/mol) = 3,850 (GPC, ĐM=1.10).
Representative procedure for SPANOC reaction with DIBO. Synthesis of polymer 4b
A solution of BAIB (19 mg, 0.06 mmol) in DCM/MeOH mixture (1.5 mL, 5/1 v/v) was added dropwise to a stirred solution of polymer 3b (30 mg) and DIBO (20 mg, 0.09 mmol) in DCM/MeOH (5 mL, 5/1 v/v) the resulting mixture was stirred for 4 h at room temperature. The polymer was purified by precipitation into cold MeOH (50 mL) to yield polymer 4b as white solid (22 mg, 73 %). 1H NMR (500 MHz, CDCl3) δ 0.93–2.15 (m, CHCH2, CCH3), 3.12–3.85 (m, CH2CHO), 5.14–5.58 (m, CH2CHO), 6.27–7.60 (m, CHar); Mn (g/mol) =3,800 (GPC, ĐM=1.10).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Sean Marrache and Dr. Shanta Dhar for assistance with GPC and Rachelle Arnold and Dr. Jason Locklin for assistance with IR measurements. This research was supported by the National Cancer Institute of the US National Institutes of Health (R01CA088986).
Footnotes
Supporting Information. Detailed experimental procedures, synthesis of VBA, polymers 1–22 and DIBO derivatives 15–18. Figures S1–S54: NMR spectra of all compounds, GPC traces and IR spectra of polymers. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Matyjaszewski K, Tsarevsky NV. Nat. Chem. 2009;1:276–288. doi: 10.1038/nchem.257. [DOI] [PubMed] [Google Scholar]; (b) Moad G, Chen M, Haussler M, Postma A, Rizzardo E, Thang SH. Polym. Chem. 2011;2:492–519. [Google Scholar]
- 2.(a) Bhattacharya A, Misra BN. Prog. Polym. Sci. 2004;29:767–814. [Google Scholar]; (b) Nagesh K, Ramakrishnan S. Synthetic Met. 2005;155:320–323. [Google Scholar]; (c) Kolishetti N, Ramakrishnan S. J. Chem. Sci. 2007;119:185–193. [Google Scholar]; (d) Gunay KA, Theato P, Klok HA. J. Polym. Sci. Pol. Chem. 2013;51:1–28. [Google Scholar]
- 3.(a) Campos LM, Killops KL, Sakai R, Paulusse JMJ, Damiron D, Drockenmuller E, Messmore BW, Hawker CJ. Macromolecules. 2008;41:7063–7070. [Google Scholar]; (b) Gauthier MA, Gibson MI, Klok HA. Angew. Chem. Int. Ed. 2009;48:48–58. doi: 10.1002/anie.200801951. [DOI] [PubMed] [Google Scholar]; (c) Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Chem Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becer CR. Macromol. Rapid. Commun. 2012;33:742–752. doi: 10.1002/marc.201200055. [DOI] [PubMed] [Google Scholar]
- 5.Gibson MI, Frohlich E, Klok HA. J. Polym. Sci. Pol. Chem. 2009;47:4332–4345. [Google Scholar]
- 6.(a) Goldmann AS, Glassner M, Inglis AJ, Barner-Kowollik C. Macromol. Rapid Commun. 2013;34: 810–849. doi: 10.1002/marc.201300017. [DOI] [PubMed] [Google Scholar]; (b) Durmaz H, Sanyal A, Hizal G, Tunca U. Polym. Chem. 2012;3: 825–835. [Google Scholar]; (c) Malkoch M, Thibault RJ, Drockenmuller E, Messerschmidt M, Voit B, Russell TP, Hawker CJ. J. Am. Chem. Soc. 2005;127:14942–14949. doi: 10.1021/ja0549751. [DOI] [PubMed] [Google Scholar]; (d) Hwang JY, Li RC, Maynard HD. J. Control. Release. 2007;122:279–286. doi: 10.1016/j.jconrel.2007.04.010. [DOI] [PubMed] [Google Scholar]; (e) Yang SK, Weck M. Macromolecules. 2008;41:346–351. [Google Scholar]; (f) Durmaz H, Dag A, Hizal G, Tunca U. J. Polym. Sci. Pol. Chem. 2010;48:5083–5091. [Google Scholar]; (g) Schaefer M, Hanik N, Kilbinger AFM. Macromolecules. 2012;45:6807–6818. [Google Scholar]
- 7.(a) Hamley IW. Soft Matter. 2005;1:36–43. doi: 10.1039/b418226j. [DOI] [PubMed] [Google Scholar]; (b) Segalman RA. Mater. Sci. Eng. R-Rep. 2005;48:191–226. [Google Scholar]; (c) Bockstaller MR, Mickiewicz RA, Thomas EL. Adv. Mater. 2005;17:1331–1349. doi: 10.1002/adma.200500167. [DOI] [PubMed] [Google Scholar]; (d) Sheiko SS, Sumerlin BS, Matyjaszewski K. Prog. Polym. Sci. 2008;33:759–785. [Google Scholar]
- 8.Li RC, Hwang J, Maynard HD. Chem. Comm. 2007:3631–3633. doi: 10.1039/b709304g. [DOI] [PubMed] [Google Scholar]
- 9.Dag A, Sahin H, Durmaz H, Hizal G, Tunca U. J. Polym. Sci. Pol. Chem. 2011;49:886–892. [Google Scholar]
- 10.Nilles K, Theato P. J. Polym. Sci. Pol. Chem. 2010;48:3683–3692. [Google Scholar]
- 11.(a) Robb MJ, Connal LA, Lee BF, Lynd NA, Hawker CJ. Polym. Chem. 2012;3:1618–1628. doi: 10.1039/C2PY20131C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tang H, Zhang D. Polym Chem. 2011;2:1542–1551. [Google Scholar]
- 12.(a) Hegg EL, Deal KA, Kiessling LL, Burstyn JN. Inorg. Chem. 1997;36:1715–1718. doi: 10.1021/ic960955b. [DOI] [PubMed] [Google Scholar]; (b) Martin ME, Parameswarappa SG, O’Dorisio MS, Pigge FC, Schultz MK. Bioorganic & medicinal chemistry letters. 2010;20:4805–4807. doi: 10.1016/j.bmcl.2010.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qian YC, Zheng Y, Abraham L, Ramos KS, Tiffany-Castiglioni E. Mol Brain. Res. 2005;134:323–332. doi: 10.1016/j.molbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]; (d) Stohs SJ, Bagchi D. Free Radical Bio. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 13.(a) Baskin JM, Bertozzi CR. Qsar Comb. Sci. 2007;26:1211–1219. [Google Scholar]; (b) Debets MF, Van Berkel SS, Dommerholt J, Dirks AJ, Rutjes FPJT, Van Delft FL. Acc. Chem. Res. 2011;44:805–815. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]; (c) Sletten EM, Bertozzi CR. Acc. Chem. Res. 2011;44:666–676. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 14.(a) Lallana E, Fernandez-Megia E, Riguera R. J. Am. Chem. Soc. 2009;131:5748–5750. doi: 10.1021/ja8100243. [DOI] [PubMed] [Google Scholar]; (b) Xu JW, Prifti F, Song J. Macromolecules. 2011;44:2660–2667. doi: 10.1021/ma200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Canalle LA, van der Knaap M, Overhand M, van Hest JCM. Macromol. Rapid. Commun. 2011;32:203–208. doi: 10.1002/marc.201000507. [DOI] [PubMed] [Google Scholar]; (d) Zeng DX, Lee NS, Liu YJ, Zhou D, Dence CS, Wooley KL, Katzenellenbogen JA, Welch MJ. ACS Nano. 2012;6:5209–5219. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Guo J, Chen GJ, Ning XH, Wolfert MA, Li XR, Xu BQ, Boons GJ. Chem.-Eur. J. 2010;16:13360–13366. doi: 10.1002/chem.201002532. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kempe K, Hoogenboom R, Jaeger M, Schubert US. Macromolecules. 2011;44:6424–6432. [Google Scholar]; (c) Zheng JK, Liu KY, Reneker DH, Becker ML. J. Am. Chem. Soc. 2012;134:17274–17277. doi: 10.1021/ja307647x. [DOI] [PubMed] [Google Scholar]; (d) Zheng JK, Xie SB, Lin F, Hua G, Yu TY, Reneker DH, Becker ML. Polym. Chem. 2013;4:2215–2218. [Google Scholar]
- 16.(a) Canalle LA, van Berkel SS, de Haan LT, van Hest JCM. Adv. Funct. Mater. 2009;19:3464–3470. [Google Scholar]; (b) Kuzmin A, Poloukhtine A, Wolfert MA, Popik VV. Bioconj. Chem. 2010;21:2076–2085. doi: 10.1021/bc100306u. [DOI] [PubMed] [Google Scholar]; (c) Orski SV, Poloukhtine AA, Arumugam S, Mao LD, Popik VV, Locklin J. J. Am. Chem. Soc. 2010;132:11024–11026. doi: 10.1021/ja105066t. [DOI] [PubMed] [Google Scholar]; (d) Orski SV, Sheppard GR, Arumugam S, Arnold RM, Popik VV, Locklin J. Langmuir. 2012;28:14693–14702. doi: 10.1021/la3032418. [DOI] [PubMed] [Google Scholar]
- 17.(a) Johnson JA, Baskin JM, Bertozzi CR, Koberstein JT, Turro NJ. Chem. Comm. 2008:3064–3066. doi: 10.1039/b803043j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu JW, Filion TM, Prifti F, Song J. Chem.-Asian J. 2011;6:2730–2737. doi: 10.1002/asia.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) DeForest CA, Anseth KS. Angew. Chem. Int. Ed. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Ornelas C, Broichhagen J, Weck M. J. Am. Chem. Soc. 2010;132:3923–3931. doi: 10.1021/ja910581d. [DOI] [PubMed] [Google Scholar]; (b) Ledin PA, Friscourt F, Guo J, Boons GJ. Chem.-Eur. J. 2011;17:839–846. doi: 10.1002/chem.201002052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huang BH, Desai A, Zong H, Tang SZ, Leroueil P, Baker JR. Tetrahedron Lett. 2011;52:1411–1414. doi: 10.1016/j.tetlet.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ning X, Guo J, Wolfert MA, Boons GJ. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Mbua NE, Guo J, Wolfert MA, Steet R, Boons GJ. Chembiochem. 2011;12:1911–1920. doi: 10.1002/cbic.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guo J, Chen GJ, Ning XH, Li XR, Zhou JF, Jagielska A, Xu BQ, Boons GJ. Chem.-Eur. J. 2012;18:4568–4574. doi: 10.1002/chem.201102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Poloukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. J. Am. Chem. Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Chem. Comm. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]; (c) Gordon CG, Mackey JL, Jewett JC, Sletten EM, Houk KN, Bertozzi CR. J. Am. Chem. Soc. 2012;134:9199–9208. doi: 10.1021/ja3000936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Friscourt F, Ledin PA, Mbua NE, Flanagan-Steet HR, Wolfert MA, Steet R, Boons GJ. J. Am. Chem. Soc. 2012;134:5381–5389. doi: 10.1021/ja3002666. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Friscourt F, Fahrni CJ, Boons GJ. J. Am. Chem. Soc. 2012;134:18809–18815. doi: 10.1021/ja309000s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders BC, Friscourt F, Ledin PA, Mbua NE, Arumugam S, Guo J, Boltje TJ, Popik VV, Boons GJ. J. Am. Chem. Soc. 2011;133:949–957. doi: 10.1021/ja1081519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning X, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL. Angew. Chem. Int. Ed. 2010;49:3065–3068. doi: 10.1002/anie.201000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Jawalekar AM, Reubsaet E, Rutjes FPJT, van Delft FL. Chem. Comm. 2011;47:3198–3200. doi: 10.1039/c0cc04646a. [DOI] [PubMed] [Google Scholar]; (b) Singh I, Heaney F. Chem. Comm. 2011;47:2706–2708. doi: 10.1039/c0cc03985c. [DOI] [PubMed] [Google Scholar]; (c) Heaney F. Eur. J. Org. Chem. 2012:3043–3058. [Google Scholar]
- 25.(a) Mendelsohn BA, Lee S, Kim S, Teyssier F, Aulakh VS, Ciufolini MA. Org. Lett. 2009;11:1539–1542. doi: 10.1021/ol900194v. [DOI] [PubMed] [Google Scholar]; (b) Das B, Holla H, Mahender G, Banerjee J, Reddy MR. Tetrahedron Lett. 2004;45:7347–7350. [Google Scholar]
- 26.Ladmiral V, Legge TM, Zhao YL, Perrier S. Macromolecules. 2008;41:6728–6732. [Google Scholar]
- 27.(a) Sun GR, Cheng C, Wooley KL. Macromolecules. 2007;40:793–795. doi: 10.1021/ma062592x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun G, Fang HF, Cheng C, Lu P, Zhang K, Walker AV, Taylor JSA, Wooley KL. ACS Nano. 2009;3:673–681. doi: 10.1021/nn8007977. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Murray BS, Fulton DA. Macromolecules. 2011;44:7242–7252. [Google Scholar]
- 28.Kamogawa H, Okabe S, Nanasawa M. B. Chem. Soc. Jpn. 1976;49:1917–1919. [Google Scholar]
- 29.Barner-Kowollik C. Handbook of RAFT polymerization. Weinheim: Wiley-VCH; 2008. p. 543. p xi. [Google Scholar]
- 30.(a) Chen M, Moad G, Rizzardo E. J. Polym. Sci. Pol. Chem. 2009;47:6704–6714. [Google Scholar]; (b) Vo CD, Rosselgong J, Armes SP, Tirelli N. J. Polym. Sci. Pol. Chem. 2010;48:2032–2043. [Google Scholar]
- 31.Hanhela PJ, Paul DB. Aust. J. Chem. 1989;42:1257–1272. [Google Scholar]
- 32.Perrier S, Takolpuckdee P, Mars CA. Macromolecules. 2005;38:2033–2036. [Google Scholar]
- 33.Spruell JM, Levy BA, Sutherland A, Dichtel WR, Cheng JY, Stoddart JF, Nelson A. J. Polym. Sci. Pol. Chem. 2009;47:346–356. [Google Scholar]
- 34.Heinenberg M, Menges B, Mittler S, Ritter H. Macromolecules. 2002;35:3448–3455. [Google Scholar]
- 35.Feuer H, Torssell K. Nitrile oxides, nitrones, and nitronates in organic synthesis : novel strategies in synthesis. 2nd ed. Hoboken, N.J: Wiley-Interscience; 2008. p. 753. p xi. [Google Scholar]
- 36.Krishnamurthy VN, Talawar MB, Vyas SM, Kusurkar RS, Asthana SN. Defence Sci. J. 2006;56:551–557. [Google Scholar]
- 37.Ghosh H, Patel BK. Org. Biomol. Chem. 2010;8:384–390. doi: 10.1039/b917096k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.