Abstract
During early postnatal brain development, experience-driven delivery of AMPA receptors to synapses participates in the initial organization of cortical function. By combining virus-mediated in vivo gene delivery with in vitro whole cell recordings, we identified a subunit-specific developmental program of experience-driven AMPA receptor delivery to synapses in rat barrel cortex. We expressed green fluorescent protein (GFP)-tagged AMPA receptors (GFP-GluR1, or GFP-GluR4) into layer 2/3 pyramidal neurons at two distinct developmental periods, postnatal day (P)8–P10 and P12–P14. Two days after viral infection, acute brain slices were prepared, and synaptic transmission from layer 4 to layer 2/3 was analyzed by whole cell recordings. We found that whisker experience drives GluR4 but not GluR1 into these synapses early in postnatal development (P8–P10). However, at P12–14, GluR1 but not GluR4 is delivered into synapses by whisker experience. This precise developmental plan suggests unique plasticity properties endowed in different AMPA receptor subunits which shape the initial experience-driven organization of cortical function.
Keywords: AMPA receptor, Barrel, Cortex, Development, Synapse, Plasticity
1. Introduction
AMPA-type glutamate receptors are mainly responsible for the fast neurotransmission of glutamatergic synapses (Derkach et al., 2007; Malinow and Malenka, 2002). AMPA receptors in the central nervous system (CNS) form tetramers comprised of four subunits (GluR1,2,3 and 4) (Barry and Ziff, 2002; Hollmann and Heinemann, 1994). These four receptors can be classified into two groups. While GluR1 and 4 possess long cytoplasmic carboxyl terminal (C-tail), GluR2 and GluR3 have short cytoplasmic C-tail (Esaki et al., 2005; Sheng and Lee, 2001). In addition, GluR2 has a splicing variant which contains long C-tail, named GluR2long (Kohler et al., 1994).
Previous studies revealed that synaptic activity in vitro (Barry and Ziff, 2002; Boehm et al., 2006; Bredt and Nicoll, 2003; Hayashi et al., 2000; Kakegawa et al., 2004; Malinow and Malenka, 2002; Scannevin and Huganir, 2000; Shi et al., 2001; Zhu et al., 2000) and experience in vivo (Clem and Barth, 2006; Clem et al., 2010; Jitsuki et al., 2011; Kessels and Malinow, 2009; Mitsushima et al., 2011; Rumpel et al., 2005; Takahashi et al., 2003) drive AMPA receptors with long C-tail into synapses. In in vitro hippocampal slice cultures, spontaneous activity is sufficient to drive GluR4 and GluR2long into synapses (Kolleker et al., 2003; Zhu et al., 2000), while synaptic delivery of GluR1 requires robust synaptic activity such as LTP inducing stimuli. In vivo, GluR1 is driven by whisker experience into synapses from layer 4 to layer 2/3 pyramidal neurons in rat barrel cortex (Takahashi et al., 2003). However, the developmental constraints for activity-driven synaptic delivery of GluR1, or other long-C-tail receptor subunits, have not been established.
Here we characterized the experience dependent delivery of long-tailed AMPA receptors into synapses formed from layer 4 to 2/3 in the developing rat barrel cortex in vivo. We identify two periods characterized by activity-driven synaptic incorporation of different long-tailed AMPA receptor subunits. Between P8 and P10, whisker experience drives GluR4 but not GluR1 into synapses. This corresponds to a time when the initial synaptic connection of these layers in the rat barrel cortex is being established (Raymond et al., 2001; Stern et al., 2001). Later, between P12 and P14, GluR1 but not GluR4 is delivered into synapses by whisker experience at a time when the fine mapping in the barrel field is formed. Thus, our results suggest that the initial connections can be made functional rather permissively (given the low level of activity required for Glur4 synaptic incorporation). However, their fine tuning is conducted with a greater amount of quality control, since GluR1 has higher requirements for synaptic incorporation.
2. Results
2.1. Expression of AMPA receptors in the developing rat barrel cortex
In order to elucidate roles of synaptic delivery of long-tailed AMPA receptors on the establishment of cortical circuit in the developing barrel cortex, we first investigated mRNA expression of GluR1 and GluR4 in the developing rat barrel cortex by RT-PCR. mRNA of both long-tailed AMPA receptors was observed at P9 and P14 (Fig. 1A).
Fig. 1.
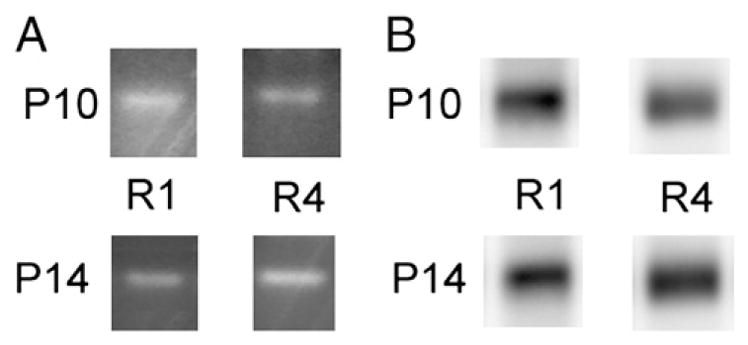
mRNA and protein expression of GluR1 and GluR4 in the barrel cortex during development. (A) RT-PCR was performed with tissue from barrel cortex at P10 and P14. mRNA of both AMPA receptors was observed at both ages. (B) Immunoblot analysis revealed that both GluR1 and GluR4 proteins were expressed in the barrel cortex at P10 and P14.
Next, we examined protein expression of these receptors. Tissues from layers 1 to 3 area of barrel region at either P10 or P14 were obtained, and P2 fraction was prepared for immunoblot analysis. GluR1 and GluR4 proteins were expressed in this region at both P9 and P14 (Fig. 1B).
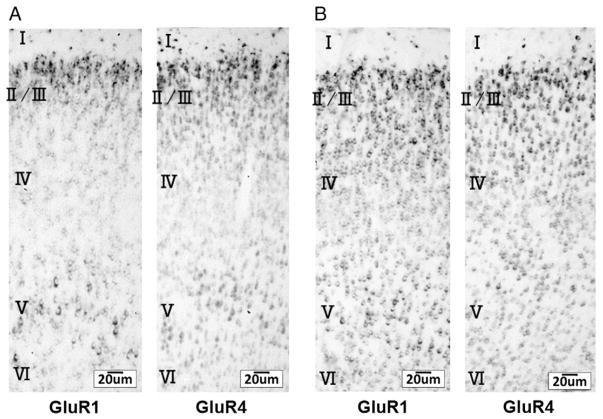
To further examine the developmental profile of GluR1 and 4 expressions, we performed in situ hybridization with GluR1 or GluR4 specific oligonucleotide on sections from rat brains at P10 and P14. We detected mRNA expression of GluR1 and 4 in layer 2/3 of the barrel cortex at P10 and P14 (Figs. 2A, B).
Fig. 2.
mRNA localization of GluR1 and GluR4 in the developing rat barrel cortex. Tissue localization of mRNA of either GluR1 or GluR4 in the developing barrel cortex at P10 (A) and P14 (B) was determined with in situ hybridization. Note that both GluR1 and GluR4 are expressed in layer 2/3 of the barrel cortex at P10 and P14. Scale bars are as indicated.
2.2. Subunit specificity of experience dependent synaptic incorporation of AMPA receptors during development
A previous in vitro study showed that spontaneous activity drives the synaptic incorporation of GluR4, but not GluR1, in hippocampal slices from early postnatal animals (Zhu et al., 2000). This suggests that GluR4 plays a major role of activity-dependent synaptic maturation early in postnatal development. To test whether this is the case with the developing rat barrel cortex in vivo, we examined if experience-driven synaptic incorporation of AMPA receptors follows similar rules using electrophysiological tagging (Hayashi et al., 2000). Expression of recombinant AMPA receptors forms homomeric receptors which, in contrast to endogenous receptors, show little outward current at positive potential and thus synaptic incorporation of recombinant AMPA receptors is detected as an increased rectification (ratio of response at −60 mV to +40 mV) of AMPA receptor mediated synaptic transmission. We microinjected Sindbis virus expressing GFP-tagged GluR4 in layer 2/3 of rat barrel cortex at P8, a time when initial synaptic connection from layers 4 to 2/3 in the barrel cortex is being established (Raymond et al., 2001). After infection, we returned infected rats into their mother’s cage and kept them for 2 days with or without whiskers. Acute coronal cortical brain slices were obtained at P10. Infected neurons in layer 2/3 were identified by GFP fluorescence. Whole cell recordings were obtained from nearby infected and noninfected layer 2/3 pyramidal neurons and transmission was evoked by stimulating layer 4. AMPA receptor mediated responses were isolated pharmacologically. In animals with no whisker trimming, transmission mediated by activation of AMPA receptors on neurons expressing GFP-GluR4 showed increased rectification compared to transmission onto nearby noninfected neurons (153±19%, n=7, p<0.05; Fig. 3A). As rectification is a property of the recombinant receptors, this indicates that GFP-GluR4 receptors were incorporated into synapses. In animals with whiskers trimmed during the 2-day post-infection period, we examined neurons in cortex contralateral to the whisker-trimmed side, which receives little sensory input (Durham and Woolsey, 1984). In this case, GFP-GluR4 infected cells did not show increased rectification (96±9%, n=6, p>0.5; Fig. 3A). This indicates that GFP-GluR4 was not incorporated into synapses in the barrel cortex deprived of whisker input. These results showed that whisker experience drives GFP-GluR4 into layer 4–2/3 synapses between P8 and P10 in the barrel cortex. A previous study showed that GluR1 is incorporated into layer 4–2/3 synapses in the barrel cortex by whisker experience between P12 and P14 (Takahashi et al., 2003). We examined if whisker experience drives GFP-GluR1 into these synapses during P8–P10. GFP-GluR1-infected cells did not show increased rectification even in intact whisker animals, indicating GFP-GluR1 was not incorporated into synapses between P8 and P10 (89±8%, n=8; p>0.2; Fig. 3A).
Fig. 3.
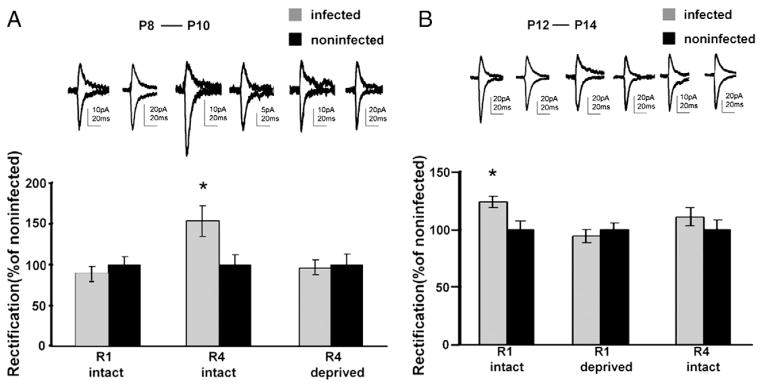
Experience-dependent synaptic delivery of recombinant long-tailed AMPA receptors in the developing rat barrel cortex. Synaptic responses recorded from layer 2/3 neurons (held at −60mV and +40mV and in the presence of APV) of cortical slices obtained from animals with whiskers intact (intact) or trimmed (deprived). Graph of average rectification index (RI, response at −60mV/response at +40mV) of synaptic responses from layer 2/3 pyramidal neurons infected with GFP-AMPA receptors, normalized to RI value of nearby uninfected cells. *indicates p<0.05, Student’s t test. Error bars indicate standard error of the mean (SEM). (A) Whisker experience drives GFP- GluR4 but not GFP-GluR1 between P8 and P10. RI of GFP-GluR4 but not GFP-GluR1 expressing neurons is larger than that of nearby nonexpressing neurons. (B) GFP-GluR1 but not GFP-GluR4 is delivered into synapses during P12 and P14. RI of GFP-GluR1 but not GFP-GluR4 expressing neurons is larger than that of nearby nonexpressing neurons.
We also investigated whether whisker experience drives GFP-GluR4 into synapses at P12–P14, a period when GluR1 is delivered into synapses by whisker experience (intact: 123±5%, n=15, p<0.05; deprived: 94±6%, n=10, p>0.4; Fig. 3B) (Takahashi et al., 2003). GFP-GluR4 infected cells showed no significant increased rectification compared with nearby noninfected neurons in intact whisker animals (111±8%, n=9, p>0.3; Fig. 3B).
To examine whether endogenous AMPA receptors behave in a manner similar to that of recombinant receptors, we expressed a construct encoding GFP-tagged cytoplasmic tail of GluR1 (GFP-GluR1ct) or GluR4 (GFP-GluR4ct). GFP-GluR1ct specifically blocks synaptic potentiation mediated by delivery of endogenous GluR1-containing AMPARs (Shi et al., 2001; Takahashi et al., 2003). When we expressed GFP-GluR1ct in pyramidal neurons of layer 2/3 of the barrel cortex during P8–P10, no synaptic depression was observed at layer 4–2/3 synapses of neurons expressing GFP-GluR1ct in the barrel cortex compared to nearby noninfected neurons, indicating that whisker experience did not induce GluR1-dependent synaptic plasticity at layer 4–2/3 synapses in the barrel cortex at P8–P10 (Fig. 4). On the other hand, GFP-GluR4ct expressing neurons displayed synaptic depression compared to nearby noninfected neurons during P8–P10, consistent with the results from recombinant receptors (Fig. 4). Expression of GFP-GluR4ct during P12–P14 did not depress AMPA-mediated synaptic transmission, indicating no synaptic GluR4 delivery at this age (Fig. 4).
Fig. 4.
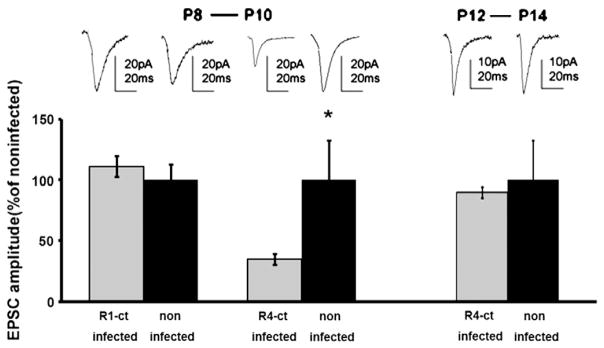
Experience-dependent synaptic delivery of endogenous long-tailed AMPA receptors in the developing rat barrel cortex. (A) (Left) synaptic responses from layer 4 to layer 2/3 pyramidal neurons infected with GFP-GluR4ct expressing virus or noninfected in the barrel cortex of rats at P8–P10 were recorded by simultaneous whole cell recordings. (Right) graph of mean AMPARs-mediated transmission onto infected neurons, normalized to values obtained in noninfected neurons. Note synaptic depression of GFP-GluR4ct expressing neurons compared with nonexpressing neurons. (B) (Left) synaptic responses from layer 4 to layer 2/3 pyramidal neurons infected with GFP-GluR1ct expressing virus or noninfected in the barrel cortex of rats at P8–P10 were recorded by simultaneous whole cell recordings. (Right) graph of mean AMPARs-mediated transmission onto infected neurons, normalized to values obtained in noninfected neurons. Note no synaptic depression of GFP-GluR1ct expressing neurons compared with nonexpressing neurons.
Taken together, whisker experience drives GluR4 but not GluR1 into layer 4–2/3 synapses during P8–P10 in the barrel cortexõ. GluR1 but not GluR4 is delivered into these synapses by experience at P12–P14. This supports the idea that while synaptic delivery of GluR4 is crucial for the initial establishment of synaptic connections early in development, GluR1 incorporation into synapses is important for fine tuning of synaptic connections later in development.
3. Discussion
In this study, we have examined the developmental profile of experience-driven AMPA receptor trafficking at the synapse between cortical layers 4 and 2/3. At a time when synapses are initially being constructed (P8 to P10) (Raymond et al., 2001), GluR4-containing receptors can be driven into synapses by experience; GluR1-containing receptors cannot. A few days later (P12 to P14), when connections at these synapses are being fine tuned, experience can drive GluR1-containing receptors into synapses, but not GluR4. This pattern parallels that seen in organotypic hippocampal slices, where GluR4 is used to deliver receptors to synapses early in postnatal development while GluR1 is used later (Esteban et al., 2003; Zhu et al., 2000). Thus, different AMPA receptor subunits can be used to identify distinct periods in synaptic development.
In hippocampal slice culture, spontaneous activity is enough to deliver GluR4 (Zhu et al., 2000). Although spontaneous neuronal activation is produced in the barrel cortex of animals without stimulation of whiskers (Luo et al., 2003; Stern et al., 2001), synaptic delivery of GluR4 requires whisker experience. This could be due to the nature of spontaneous activity or biochemical signaling in these two brain regions, or differences between in vivo and in vitro conditions.
As previously reported, PKA activation is sufficient to drive GluR4 into CA3–CA1 synapses of organotypic hippocampal slices, while it is necessary but not sufficient for the GluR1 synaptic delivery (Esteban et al., 2003). This suggests that threshold for GluR1 delivery is higher than GluR4. At early postnatal period (P8–P10), whisker-evoked activity in the layer 2/3 of the barrel field is still limited, primarily activating NMDA receptor-mediated transmission (Bureau et al., 2004), but apparently beyond the threshold of synaptic delivery of GluR4. However, this level of activity may not be sufficient to drive GluR1 into synapses. Notably, experience dependent neuronal activity at later stage (P12–P14) is stronger (Stern et al., 2001) and drives GluR1 into synapses but cannot drive GluR4. This could be due to the lack of GluR4-specific molecular machinery required for its synaptic delivery.
In conclusion, our studies have identified a precise use of different AMPA receptor subunits during different phases of synaptic development in rat barrel cortex. GluR4 participates in the initial connections, suggesting that such connections are made functional rather permissively. GluR1, which has a higher threshold for synaptic incorporation, participates during a period of synaptic fine tuning, suggesting that they endow the system with more quality control.
4. Experimental procedures
4.1. RT-PCR
Nine to fourteen-day-old Wistar rats (Charles River) were decapitated under halothane anesthesia and barrel cortex including from layes 1 to 4 were microdissected. Total RNAs were extracted with TriZol (Invitrogen) and reverse transcribed to cDNA with random primers (SuperScript VILO cDNA Syntesis kit, Invitrogen) according to the manufacturer’s instructions. The cDNAs were amplified by PCR (GoTaq Green Master Mix, Promega) with sequence specific primers according to Dijk et al. (Bureau et al., 2004): GluR1, N-5′-CGAGTTCTGCTACAAATCCCG-3′ and C-5′-TGTCCGTATGGCTTCATTGATG-3′ (accession number M38060); GluR2 short, N-5′-TTGAGTTCTGTTACAAGTCAAGGGC-3′ and C-5′-AGGAAGATGGGTTAATATTCTGTGGA-3′ (M38061); GluR2 long, N-5′-GCCTTGGTTTGGCAATGC-3′ and C-5′-GACATCACTCAAGGTCATCTTCATTC-3′ (NM_017261); GluR4, N-5′-CCAGGGCAGAGGCGAAG-3′ and C-5′-CGTTTTCTCCCACACTCCCA-3′ (NM_017263). Primers for the housekeeping gene, cyclophilin were also used: N-5′-ATGGTCAACCCCACCGTGTT-3′ and C-5′-ATGGCGTGTGAAGTCACCAC-3′ (M19533). Amplification condi tions were 95 °C, 30 s; 58 °C, 30 s; 72 °C, 30 s for 33 cycles, followed by a 10-min incubation at 72 °C. The amplified fragments were resolved by electrophoresis on 3% MetaPhor agarose (BioWhittaker Molecular Applications Inc.) and DNA products were visualized with ethidium bromide.
4.2. Biochemical preparations and analysis
Tissue of layers 1–3 region of barrel cortex was obtained from acute brain slices prepared with brain matrix (ASI instruments) at P9 and P14. Synaptoneurosomal fraction was obtained as previously described (Whitlock et al., 2006). After decapitation, tissue samples were homogenized in ice-cold homogenization buffer (10 mM Hepes/1.0 mM EDTA/2.0 mM EGTA/0.5 mM DTT/0.1 mM PMSF/10 mg/liter leupeptin/100 nM microcystin). Tissue was homogenized in a glass/glass tissue homogenizer, and homogenates were passed through two 100-um-pore nylon mesh filters, then through one 5-um-pore filter. Filtered homogenates were centrifuged at 3600 g for 10 min at 4 °C. Resultant pellets were resuspended in 50 ul boiling 1% SDS, boiled for 10 min, and used for western blotting. Same volumes of proteins quantified using BCA protein assay kit(Pierce) were loaded, then proteins were detected with the following antibodies; anti-GluR1/rabbit, 1:500, anti-GluR2-long/rabbit, 1:500 (supplied from Thomas,G), anti-GluR4/rabbit, 1:500 (Millipore). Immunolabeled samples were detected using LAS3000 (Fujifilm). These data were quantified with ImageQuant software.
4.3. Infection of neocortical neurons in vivo
Constructs of AMPA-R subunits tagged with GFP (GluR1-GFP, GluR4-GFP, GluR2long-GFP) and Sindbis viruses were prepared as previously described (Hayashi et al., 2000; Kolleker et al., 2003; Shi et al., 1999, 2001; Takahashi et al., 2003; Zhu et al., 2000). All surgery was performed in accordance with the animal care and use guidelines of CSHL and Yokohama City University. At P8 or P12, rats were anesthetized with a ketamine/xylazine cocktail (ketamine: 0.56 mg/g body weight; xylazine: 0.03 mg/g body weight). The skin overlying the skull was cut and gently pushed to the side. The anterior fontanel was identified and a region 2 mm posterior, 4.5 mm lateral was gently pierced with a dental drill (coordinates were independently ascertained to correspond to barrel cortex by cytochrome oxidase staining). Glass pipettes (tip diameter ~12 μm) were used to inject recombinant Sindbis virus into barrel cortex. After injection, the skin was repositioned and maintained with cyanoacrylate glue. Sensory deprivation was initiated by trimming (to <1 mm) all large whiskers (columns 1–4, α–δ) immediately after injection of the virus and repeated every ~12 h until animals were sacrificed.
4.4. Electrophysiology
2 days after virus injection, rats were anesthetized with isoflurene gas and brains were removed. Brains were quickly transferred into ice-cold dissection buffer (25.0 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 25.0 mM glucose, 110.0 mM choline chloride, 11.6 mM ascorbic acid, 3.1 mM pyruvic acid) gassed with 5%CO2/95%O2. Coronal brain slices were cut (300 μm, Leica vibratome) in dissection buffer and transferred to physiological solution (22–25°, 118 mM NaCl, 2.5 mM KCl, 26.2 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, 1.3 mM MgCl2, 2.5 mM CaCl2, PH7.4, gassed with 5%CO2/95%O2). The recording chamber was perfused with physiological solution containing 0.1 mM picrotoxin, 4 μM 2-chloroadenosine at 22–25°. In order to measure rectification index, we added 0.1 mM D,L-APV to perfusate to block NMDA-Rs. Patch recording pipettes (4–7 MΩ) were filled with intracellular solution (115 mM cesium methanesulfonate, 20 mM CsCl, 10 mM HEPES, 2.5 mM MgCl2, 4 mM Na2ATP, 0.4 mM Na3GTP, 10 mM sodium phosphocreatine, 0.6 mM EGTA at PH7.25). Whole-cell recordings were obtained from infected or uninfected layer 2/3 pyramidal neurons (150–500 μm from pial surface) of rat barrel cortex with Axopatch-1D amplifier (Axon Instruments). There were no significant differences in input or series resistance among groups (infected, non-infected, intact, deprived, isolated, non-isolated). Bipolar tungsten stimulating electrodes were placed in layer 4 ~200–300 μm below recorded cells. Stimulus intensity was increased until a synaptic response of amplitude >~10 pA was recorded. Synaptic AMPA-R mediated responses at −60 mV and +40 mV were averaged over 50–100 trials and their ratio was used as an index of rectification.
4.5. In situ hybridization
The in situ hybridization was performed by digoxigenin-labeled riboprobes. We prepared RNA probes of GluR1 and GluR4 for in situ hybridization. They were the same sequences as the probes used in above-mentioned RT-PCR method according to the previous report (Dijk et al., 2004). The template for GluR1 was a fragment of 91 base pairs (bp); CGAGTTCTGCTACAAATCCCGTAGCGAGTCGAAGCGGATGAAGGGTTTCTGTTTGATCCCACAGCAATCCATCAATGAAGCCATACGGACA. The template for GluR4 was a fragment of 93 bp; CCAGGGCAGAGGCGAAGA-GAATGAAGCTGACTTTTTCCGAAGCCATAAGAAACAAAGCCAGGTTATCCATCACTGGGAGTGTGGGAGAAAACG. These fragments were subcloned into the plasmid pBluescript II SK. To produce antisense or sense strand probes, we linearized the plasmid with an appropriate restriction enzyme. RNA probes were synthesized by in vitro transcription according to the manufacturer’s protocol using the T7 or T3 polymerase (Stratagene, La Jolla, CA) in the presence of digoxigenin-uridine 59-triphosphate (DIG-UTP, Boehringer Mannheim, Indianapolis, IN). SD rats (Charles River Japan, Tokyo, Japan) were deeply anesthetized either at P10 or P14 with sodium pentobarbital (31.5 mg/kg i.p.) and perfused intracardially with 4% paraformaldehyde in PBS. The brains were removed and fixed with 4% paraformaldehyde overnight. After cryoprotected with 30% sucrose, brains were embedded by Tissue-Tek O.C.T Compound (Sakura Finetechnical, Tokyo, Japan), and coronal cryostat sections (16 μm thick) were collected on Superfrost slides (Matsunami Glass, Osaka, Japan) and stored at −80 °C before use. Brain sections were briefly digested with 1 μg/ml proteinase K (Invitrogen) in DW containing 1% 1 M Tris (pH 7.5) and 0.5 M EDTA and refixed with 4% PFA for 30 min, and sections were then hybridized with antisense or sense probes at 50 °C overnight. The final concentration of probes diluted in the hybridization solution (containing 50% formamide, 5% SSC, pH 4.5, 1% SDS, 50 μg/ml yeast tRNA, and 50 μg/ml heparin) was 4 μg/ml. The slides were washed at 50 °C in 50% formamide, 5× SSC, and 1% SDS and then in 50% formamide and 2× SSC twice at 50 °C. Slides were incubated for 1 h at room temperature with 10% BSA and then overnight at 4 °C with alkaline phosphatase-conjugated anti-digoxigenin antibodies (Roche Molecular Biochemicals, Meylan, France). Bound digoxigenin-labeled probes were detected using 5-bromo-4-chlor-indolyl-phosphate and nitroblue-tetrazolium-chloride (Roche Molecular Biochemicals, Meylan, France). No signal was detected with sense probes.
Acknowledgments
We thank Yoshiko Kanno for excellent technical assistance. This project was supported by Grant-in-Aid for Young Scientists (start-up) (18800037), Grant-in-Aid for Scientific Research (B) (20300131) (T.T.), JST, CREST (T.T.), Special Coordination Funds for Promoting Science and Technology (T.T.), the Sumitomo Foundation (T.T.), KANAE Foundation (T.T.), Takeda Science Foundation (T.T.), NARSAD (T.T.), the grant for 2007 Strategic Research Project (K19025) of Yokohama City University (T.T.), “Development of biomarker candidates for social behavior” carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan (TT), and NIH (R.M.).
References
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Precise development of functional and anatomical columns in the neocortex. Neuron. 2004;42:789–801. doi: 10.1016/j.neuron.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 2010;30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dijk F, Kraal-Muller E, Kamphuis W. Ischemia-induced changes of AMPA-type glutamate receptor subunit expression pattern in the rat retina: a real-time quantitative PCR study. Invest Ophthalmol Vis Sci. 2004;45:330–341. doi: 10.1167/iovs.03-0285. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur J Neurosci. 2004;20:101–110. doi: 10.1111/j.1460-9568.2004.03461.x. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Kornau HC, Seeburg PH. The organization of the gene for the functionally dominant alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit GluR-B. J Biol Chem. 1994;269:17367–17370. [PubMed] [Google Scholar]
- Kolleker A, Zhu JJ, Schupp BJ, Qin Y, Mack V, Borchardt T, Kohr G, Malinow R, Seeburg PH, Osten P. Glutamatergic plasticity by synaptic delivery of GluR-B (long)-containing AMPA receptors. Neuron. 2003;40:1199–1212. doi: 10.1016/s0896-6273(03)00722-0. [DOI] [PubMed] [Google Scholar]
- Luo X, Persico AM, Lauder JM. Serotonergic regulation of somatosensory cortical development: lessons from genetic mouse models. Dev Neurosci. 2003;25:173–183. doi: 10.1159/000072266. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:12503–12508. doi: 10.1073/pnas.1104558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]