Abstract
Costimulation blockade protocols are effective in prolonging allograft survival in animal models and are entering clinical trials, but how environmental perturbants affect graft survival remains largely unstudied. We used a costimulation blockade protocol consisting of a donor-specific transfusion and anti-CD154 mAb to address this question. We observed that lymphocytic choriomeningitis virus infection at the time of donor-specific transfusion and anti-CD154 mAb shortens allograft survival. Lymphocytic choriomeningitis virus 1) activates innate immunity, 2) induces allo-cross-reactive T cells, and 3) generates virus-specific responses, all of which may adversely affect allograft survival. To investigate the role of innate immunity, mice given costimulation blockade and skin allografts were coinjected with TLR2 (Pam3Cys), TLR3 (polyinosinic:polycytidylic acid), TLR4 (LPS), or TLR9 (CpG) agonists. Costimulation blockade prolonged skin allograft survival that was shortened after coinjection by TLR agonists. To investigate underlying mechanisms, we used “synchimeric” mice which circulate trace populations of anti-H2b transgenic alloreactive CD8+ T cells. In synchimeric mice treated with costimulation blockade, coadministration of all four TLR agonists prevented deletion of alloreactive CD8+ T cells and shortened skin allograft survival. These alloreactive CD8+ T cells 1) expressed the proliferation marker Ki-67, 2) up-regulated CD44, and 3) failed to undergo apoptosis. B6.TNFR2−/− and B6.IL-12R−/− mice treated with costimulation blockade plus LPS also exhibited short skin allograft survival whereas similarly treated B6.CD8α−/− and TLR4−/− mice exhibited prolonged allograft survival. We conclude that TLR signaling abrogates the effects of costimulation blockade by preventing alloreactive CD8+ T cell apoptosis through a mechanism not dependent on TNFR2 or IL-12R signaling.
Transplantation of allogeneic pancreatic islets has emerged as a promising and clinically feasible treatment for type 1 diabetes (1–4). Unfortunately, current transplantation protocols require chronic administration of immunosuppressive drugs, which poses known risks of infection, organ toxicity (5), and neoplasia (6). It is the goal of transplant immunologists to develop a clinically applicable protocol that will induce donor-specific tolerance leading to the survival of functioning allografts in the absence of chronic immunosuppression.
Our experimental approach to achieving transplantation tolerance is a two-step costimulation blockade protocol consisting of a donor-specific transfusion (DST)3 combined with four peritransplant injections of anti-CD154 mAb (7). Transplantation using DST and anti-CD154 mAb leads to permanent islet and prolonged skin allograft survival in mice (7, 8) and prolonged allograft survival in nonhuman primates (9–12). Anti-CD154 mAb blocks the interaction between CD154 on T cells and CD40 on APCs (13, 14) and, when combined with DST, leads to the deletion of host alloreactive CD8+ T cells (15). Deletion of host alloreactive CD8+ T cells appears to be a necessary component of the mechanism by which costimulation blockade induces prolonged allograft survival (16–18).
Concerns to the potential use of costimulation blockade in the clinic include the response of the host to infectious agents during costimulation blockade and the susceptibility of this protocol to environmental perturbation. We have reported that mice infected with lymphocytic choriomeningitis virus (LCMV) at the time of DST and anti-CD154 mAb are rendered refractory to the effects of costimulation blockade and rapidly reject their allografts (19). In contrast, LCMV infection of mice with healed-in allografts has little effect on allograft survival (20, 21). One possible mechanism by which LCMV may shorten allograft survival is the generation of a virus-specific effector/memory T cell population that cross-reacts with alloantigens (22–25). Alternatively, viruses are known to activate innate immunity through TLRs (26–28), a class of receptors on the surface of APCs that recognize conserved pathogen-associated molecular patterns that occur in bacteria, fungi, and viruses (29). The TLR family is known to consist of at least 11 members that can activate innate immunity through the TLR-associated adaptor molecule MyD88 (e.g., TLR4) as well as MyD88-independent (e.g., TLR3) pathways (29).
Both human (30–32) and murine (33, 34) viruses interact with TLRs, and it has been shown that the antiviral response to LCMV involves MyD88 (33). The TLR-associated adaptor molecule MyD88 is also necessary for the acute rejection of certain minor alloantigens (35). Furthermore, TLR agonists are capable of maturing APCs independently of CD40-CD154 interactions (36). Based on these observations, we hypothesized that activation of TLRs would abrogate the effects of costimulation blockade by bypassing CD40-CD154 interaction requirements for activation of alloreactive T cells.
We observed that treatment with DST plus anti-CD154 mAb activated alloreactive CD8+ T cells, induced their apoptosis, and led to prolonged allograft survival. Coadministration of agonists to TLR2, TLR3, TLR4, or TLR9 at the time of costimulation blockade prevented alloreactive CD8+ T cell apoptosis and shortened allograft survival. Our studies suggest that the underlying mechanism by which the TLR agonists prevent the induction of prolonged skin allograft survival by costimulation blockade is by protecting alloreactive CD8+ T cells from apoptosis through TNFR2 and IL-12R-independent pathways, subsequently leading to alloreactive CD8+ T cell expansion and rapid rejection of the allograft.
Materials and Methods
Animals
C57BL/6 (H2b,), CBA/J (H2k), and BALB/c (H2d) mice were obtained from the National Cancer Institute (Frederick, MD) or The Jackson Laboratory. C57BL/10ScSnJ (H2b,), C57BL/10ScNJ (H2b, Tlr4lps-del, abbreviated as TLR4−/−), C.C3-Tlr4Lps-d/J (H2d, abbreviated as TLR4−/−), B6.129S1-Il12btm1Jm/J (B6.IL-12R−/−, H2b), and B6.129S2-Cd8atm1Mak/J (B6.CD8α−/−, H2b) mice were obtained from The Jackson Laboratory and bred at the animal facility at the University of Massachusetts Medical School. C57BL/10ScNJ mice carry a null mutation of TLR4 and fail to express either TLR4 RNA or protein (37). C.C3-Tlr4Lps-d/J mice are derived from the original C3H/HeJ TLR4−/− strain (37) in which the TLR4 mutation has been backcrossed onto the BALB/c background at The Jackson Laboratory (〈http://jaxmice.jax.org/info/〉). B6.129S2.Tnfrsf1btm1Mwm (B6.TNFR2−/−) mice were the gift of Dr. F. Chan (University of Massachusetts Medical School, Worcester, MA), who originally obtained them from The Jackson Laboratory. (CBA/J × KB5.CBA)F1 CD8+ T cell TCR-transgenic mice were developed by Dr. A. Mellor (Medical College of Georgia, Augusta, GA) and bred in our animal facility (38). The TCR transgene is expressed in CBA (H2k) mice by CD8+ cells, and the transgenic TCR has specificity for native H2-Kb (38).
All animals were certified to be free of Sendai virus, pneumonia virus of mice, murine hepatitis virus, minute virus of mice, ectromelia, lactate dehydrogenase-elevating virus, mouse poliovirus, Reo-3 virus, mouse adenovirus, LCMV, polyoma, Mycoplasma pulmonis, and Encephalitozoon cuniculi. They were housed in a specific pathogen-free facility in microisolator cages, given autoclaved food and acidified water, and maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School and the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences).
Generation of CD8+ KB5 TCR-transgenic synchimeric CBA/J mice
To study the fate of host alloreactive CD8+ T cells in mice treated with costimulation blockade, we used KB5 TCR-transgenic hemopoietic chimeras generated as described previously (39). Briefly, CBA/J nontransgenic mice were treated with 200 cGy of whole body gamma irradiation from a 137Cs source (Gammacell 40; Atomic Energy of Canada, or Mark I-30 Series 2000 Ci; JL Shepherd & Associates) and given a single i.v. injection of 0.5 × 106 KB5 bone marrow cells. To allow sufficient time for hemopoietic chimerism to develop, the KB5 synchimeras received skin allografts at 12–17 wk of age, 8–12 wk after irradiation and injection of KB5-transgenic bone marrow. We refer to these mice as KB5 synchimeras (39).
Transplantation procedures
Recipient mice of the specified strain were treated with a DST, anti-CD154 mAb, and given skin allografts, as described previously (8, 40). Briefly, the DST, consisting of 107 splenocytes from female BALB/c or C57BL/6 mice, was injected i.v. in a volume of 0.5 ml; DST was given on day −7 relative to the day of skin transplantation. Purified hamster anti-mouse CD154 mAb (clone MR1) was obtained from the National Cell Culture Center (Mnneapolis, MN). The concentration of contaminating endotoxin was determined by a commercial firm (Charles River Endosafe) and was uniformly <10 endotoxin units/mg mAb. Mice were injected i.p. with anti-CD154 mAb (0.5 mg/dose) on days −7, −4, 0, and +4 relative to the day of skin transplantation. Full-thickness skin grafts 1–2 cm in diameter were obtained from the flanks of donor mice and transplanted onto the dorsal flanks of recipients (8). Graft rejection was defined as the first day on which the entire graft was necrotic (8, 40).
Viruses and cell lines
LCMV, strain Armstrong, was prepared in baby hamster kidney cells as previously described (41). Mice were inoculated i.p. with 5 × 104 PFU of LCMV within 4 h after DST treatment. The RMA cell line (H2b), a Rauscher virus-induced T cell lymphoma, and the P815 cell line (H2d), a DBA/2-derived, methylcholanthrene-induced mastocytoma, were maintained in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FBS (Invitrogen Life Technologies), 100 U/ml penicillin G, 100 µg/ml streptomycin sulfate, and 2 mM L-glutamine (Sigma-Aldrich).
Preparation and injection of TLR agonists
Polyinosinic:polycytidylic acid (poly I:C; Sigma-Aldrich) was dissolved in Dulbecco’s PBS (D-PBS) at a concentration of 1 mg/ml. Stock was filtered through 0.45-µm sterile nylon mesh (BD Biosciences) and stored at −20°C until needed. The CpG oligonucleotide (52-CT CCC AGC GTG CGC CAT-32) was generated on a phosphorothioate backbone by Trilink BioTechnologies. Upon receipt, lyophilized CpG was suspended in D-PBS and stored at −20°C until needed. LPS from Escherichia coli 0111:B4 (Sigma-Aldrich) was repurified as previously described (42), except that phenol-PBS phase separation was conducted at 2000×g for 30 min to accommodate larger volumes. Repurified (pLPS) was suspended in D-PBS with an assumed 10% loss during repurification (42). Purified LPS was stored at 4°C until used. Pam3Cys-Ser-(Lys)4 (EMC Microcollections) was dissolved in D-PBS and stored at − 20°C until needed. Mice were injected i.p. with the indicated ligand and dose in a volume of 0.5 ml of D-PBS within 1 h of DST treatment using ligand doses determined to be active in preliminary dose-titration experiments.
Flow microfluorometry and Abs
A mouse hybridoma cell line secreting the KB5-specific clonotypic Desire (DES) mAb (43) was a gift from Dr. J. Iacomini (Massachusetts General Hospital, Boston, MA). FITC-conjugated anti-mouse IgG2a developing reagent for DES (clone R19–15), anti-mouse CD8α-PerCP (clone 53–6.7), anti-mouse CD44-allophycocyanin (clone IM7), anti-human/mouse Ki-67-PE (clone B56), anti-mouse IFN-γ-PE (clone XMG1.2), and purified anti-mouse CD16/32 (clone 2.4G2) mAbs were obtained from BD Pharmingen. Isotype control mAbs and the Annexin VPE Apoptosis Detection kit I, which contains Annexin VPE and 7-AAD, were also obtained from BD Pharmingen.
Single-cell suspensions from spleen or heparinized whole blood were made in RPMI 1640 and washed in D-PBS containing 1% fetal clone serum (HyClone) and 0.1% sodium azide (Sigma-Aldrich). Samples were incubated in anti-CD16/32 for 5 min at 4°C before incubation for 20 min with the clonotypic DES mAb. They were washed and incubated for 20 min with fluorescently labeled Abs to cell surface markers and the secondary development Ab for DES. Samples were processed with FACS lysing solution (BD Biosciences) in accordance with the manufacturer’s protocol. Labeled cells were washed, fixed with 1% paraformaldehyde (Polysciences) in D-PBS, and analyzed with a FACSCalibur, FACScan, or LSRII instrument (BD Biosciences) and FlowJo Software (PC version 5.5; Tree Star). Lymphoid cells were gated according to their light-scattering properties.
Annexin V staining
Apoptosis was quantified using the Annexin VPE Apoptosis Detection kit I (BD Pharmingen) according to the manufacturer’s instructions. Briefly, RBC were lysed with 0.84% NH4Cl solution. The samples were stained with the clonotypic Ab DES in D-PBS supplemented with 1% fetal clone serum and 0.1% sodium azide. The samples were then washed with Annexin V Binding Buffer (BD Pharmingen) and stained with anti-mouse IgG2a–FITC, anti-mouse CD8α-PE-Cy7, 7-AAD, and Annexin VPE diluted in binding buffer. Samples were analyzed within 2 h of staining with LSRII (BD Biosciences).
Ki-67 staining
Intracellular Ki-67 expression was determined in splenocytes directly ex vivo. RBC were removed from samples with 0.84% NH4Cl solution and stained with the clonotypic Ab DES. The samples were washed and stained with anti-mouse IgG2a-FITC, anti-mouse CD8α-PerCP, and anti-mouse CD44-allophycocyanin (BD Pharmingen). The samples were then fixed and permeabilized with Cytofix/Cytoperm solution and stained with either anti-human/mouse Ki-67-PE or a mouse IgG1k–PE isotype control (clone MOPC-21; BD Pharmingen) diluted in Perm/Wash Buffer (BD Pharmingen). The samples were analyzed with a FACSCalibur (BD Biosciences).
Intracellular IFN-γ assay
IFN-γ production was assessed in spleen cells using the BD Cytofix/Cytperm kit plus Golgiplug (BD Pharmingen) as previously described (24, 44). Briefly, single-cell suspensions were prepared from spleens and RBC were lysed using 0.84% NH4Cl. Splenocytes (2 × 106 cells) were incubated for 5 h in GolgiPlug and 10 U/ml rIL-2 (R&D Systems) at 37°C in the presence of a syngeneic (RMA, H2b) or allogeneic (P815, H2d) cell line (0.5 × 106 cells per stimulation). Samples were stained with anti-CD8α-PerCP, followed by fixation with BD Cytofix/Cytoperm and staining with anti-IFN-γ-PE.
Statistics
Statistical analyses were performed with GraphPad Prism software (Graphpad Software). Three or more means were compared by one-way ANOVA and Bonferonni’s multiple comparison test. Allograft survival curves were generated by the Kaplan and Meier method and compared by the log-rank test. Duration of allograft survival is presented as the median. Values of p < 0.05 were considered to indicate statistical significance.
Results
TLR agonists administered at the time of DST and anti-CD154 mAb shorten skin allograft survival
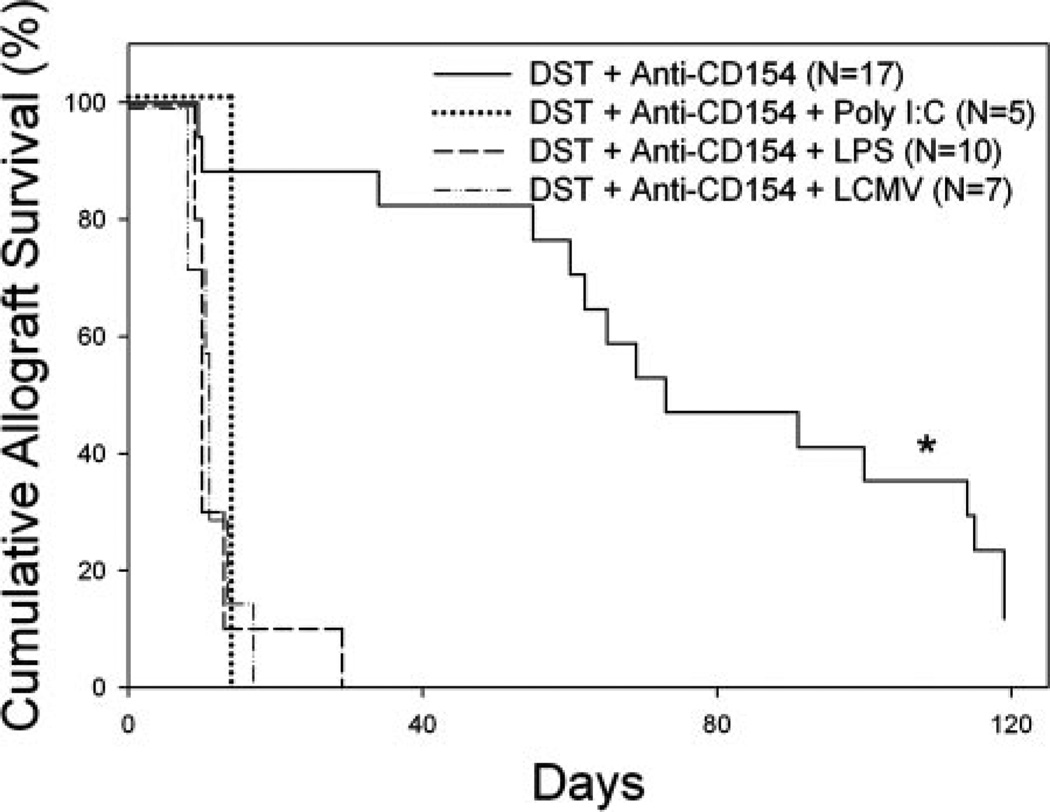
Confirming earlier observations (19–21), we first documented that infection with LCMV at the time of DST in our costimulation blockade protocol abrogates skin allograft survival. C57BL/6 mice were treated with DST, anti-CD154 mAb, and given BALB/c skin allografts, and then randomly divided into four groups. In group 1, no other treatment was given, and these mice exhibited prolonged allograft survival with a median survival time (MST) of 73 days (n = 17, Fig. 1). Group 2 was infected on day −7 with 5 × 104 PFU of LCMV, and skin allograft survival in this group, as expected (19–21), was short (MST = 11 days, n = 7).
FIGURE 1.
LPS and poly I:C abrogate prolonged allograft survival induced by costimulation blockade in C57BL/6 mice. C57BL/6 mice were treated with BALB/c DST and anti-CD154 mAb according to our standard protocol with or without injection of the indicated TLR agonist or infection with LCMV on day −7 relative to transplantation with a BALB/c skin allograft on day 0. *, Statistically significantly different than all others; p < 0.0001.
We next determined whether administration of TLR agonists during initiation of treatment with DST and anti-CD154 mAb would similarly shorten skin allograft survival. To test this, the remaining two groups of C57BL/6 mice were coinjected i.p. with LPS or poly I:C on day −7, the day of DST treatment and the first of four anti-CD154 mAb injections. Administration of either the TLR3 agonist poly I:C (MST = 14 days, n = 5) or the repurified TLR4 agonist LPS (MST = 10 days, n = 10) both led to acute allograft rejection (Fig. 1). Skin allograft survival in mice treated with TLR agonists was similar to that achieved in mice infected with LCMV (p = NS). These data indicate that TLR activation is as effective as LCMV infection in abrogating skin allograft survival induced by costimulation blockade.
TLR agonists impair the deletion of alloreactive CD8+ T cells when administered at the initiation of DST and anti-CD154 mAb treatment
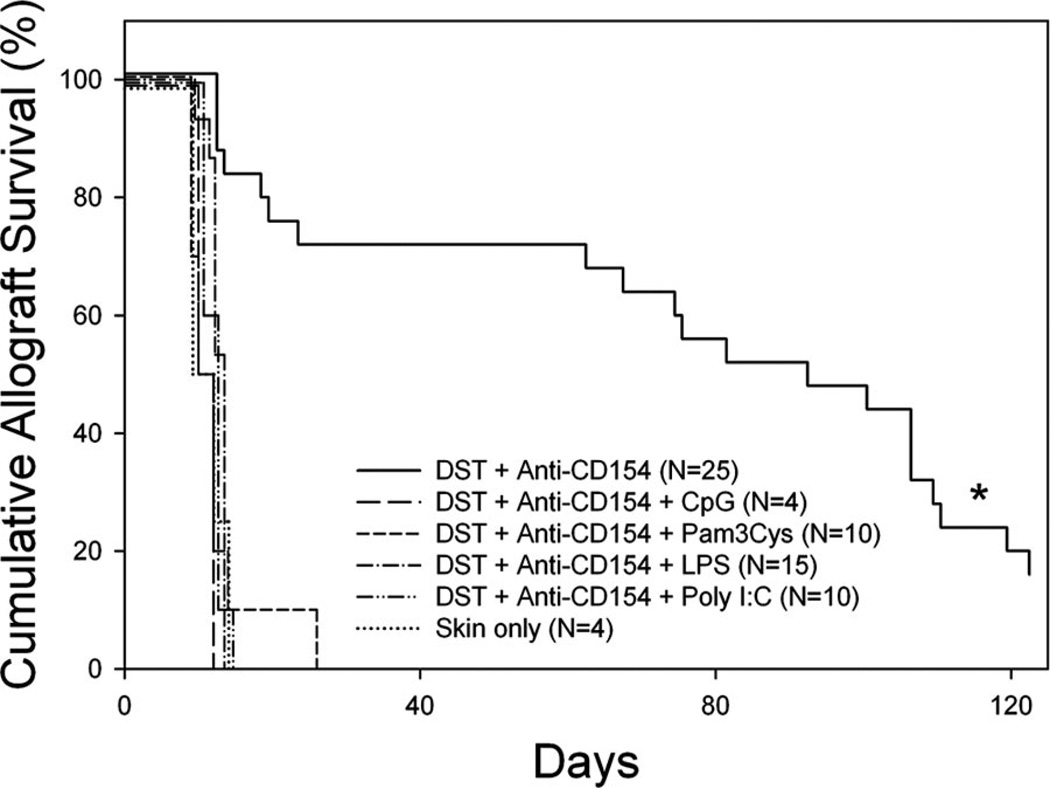
We hypothesized that TLR agonists impair skin allograft survival induced by costimulation blockade by impairing the deletion of alloreactive CD8+ T cells. To determine the fate of alloreactive CD8+ T cells in a normal microenvironment, we used KB5 TCR transgenic hemopoietic “synchimeric” mice (39). The TCR transgene is expressed by CD8+ cells in CBA (H2k) mice and has specificity for H2-Kb. In this system, the mice circulate a self-renewing trace population of anti-H2-Kb alloreactive CD8+ T cells maturing in a normal microenvironment (39). TLR2 (Pam3Cys), TLR3 (poly I:C), TLR4 (LPS), and TLR9 (CpG) agonists were tested for their ability to impair alloreactive CD8+ T cell deletion and to shorten skin allograft survival in synchimeric mice treated with costimulation blockade.
As expected (21, 39, 45, 46), treatment of KB5 synchimeras with DST on day −7 and anti-CD154 mAb on days −7 and −4 led to a marked depletion of alloreactive CD8+ T cells on day −1 relative to skin grafting on day 0 (Table I). The circulating level of CD8+ DES+ T cells fell from an average of 4.69 ± 1.20% of PBLs to 0.55 ± 0.50% (n = 25). Deletion of alloreactive CD8+ T cells was impaired by all TLR agonists tested (p < 0.0001 vs DST and anti-CD154 mAb group, Table I).
Table I.
TLR agonists impair the deletion of alloreactive CD8+ T cells in the blooda
| % Chimerism |
% Chimerism |
||||
|---|---|---|---|---|---|
| Treatment | TLR Agonist (Dose) | n | Pre-DST | Day 6 | % Deletion |
| DST + anti-CD154 | None | 20 | 4.69 ± 1.20 | 0.55 ± 0.50b | 88.3b |
| DST + anti-CD154 | 50 µg of Pam3Cys | 10 | 5.33 ± 0.79 | 4.37 ± 0.78 | 18.0 |
| DST + anti-CD154 | 500 µg of Poly I:C | 10 | 5.38 ± 1.05 | 5.72 ± 1.24 | −6.3 |
| DST + anti-CD154 | 100 µg of LPS | 10 | 4.35 ± 0.93 | 3.14 ± 1.12 | 27.9 |
| DST + anti-CD154 | 50 µg of CpG | 5 | 5.45 ± 0.53 | 3.59 ± 0.54 | 34.1 |
| No treatment | None | 8 | 3.48 ± 1.08 | 4.05 ± 0.67 | − 16.5 |
TLR agonists impair the deletion of alloreactive CD8+ T cells in KB5 synchimeras. KB5 synchimeras were given the treatment indicated on day 0. PBL was collected from mice on day +6 relative to treatment and stained with the clonotypic mAb DES, followed by the DES detection mAb (anti-mouse IgG2a–FITC) and anti-CD8-PerCP mAb as described in Materials and Methods.
P < 0.0001 vs. all other groups.
To confirm that the failure to delete alloreactive CD8+ T cells in KB5 synchimeras treated with DST and anti-CD154 mAb was associated with short skin allograft survival, these mice were then transplanted with C57BL/6 skin allografts and given additional injections of anti-CD154 mAb on days 0 and +4 relative to graft placement on day 0. We observed that, similar to C57BL/6 recipients of BALB/c skin allografts, KB5 CBA synchimeras treated with C57BL/6 DST and anti-CD154 mAb exhibited prolonged C57BL/6 skin allograft survival (Fig. 2, MST = 92 days, n = 25). Corresponding to their failure to delete alloreactive CD8+ T cells, all KB5 synchimeric mice treated with costimulation blockade plus a TLR agonist exhibited short skin allograft survival (Fig. 2, MST = 11–13 days, p < 0.0001 vs DST and anti-CD154 mAb-treated mice).
FIGURE 2.
TLR agonists abrogate prolonged allograft survival induced by costimulation blockade in synchimeric CBA/J mice. KB5 synchimeras were treated with C57BL/6 DST and anti-CD154 mAb according to our standard protocol with or without injection of the indicated TLR agonist on day −7 relative to transplantation with a C57BL/6 skin allograft on day 0. Mice receiving the “‘skin only” treatment received no preconditioning before transplantation. *, Statistically significantly different than all others; p < 0.0001.
CD8+ cells are required for LPS to shorten skin allograft survival induced by costimulation blockade
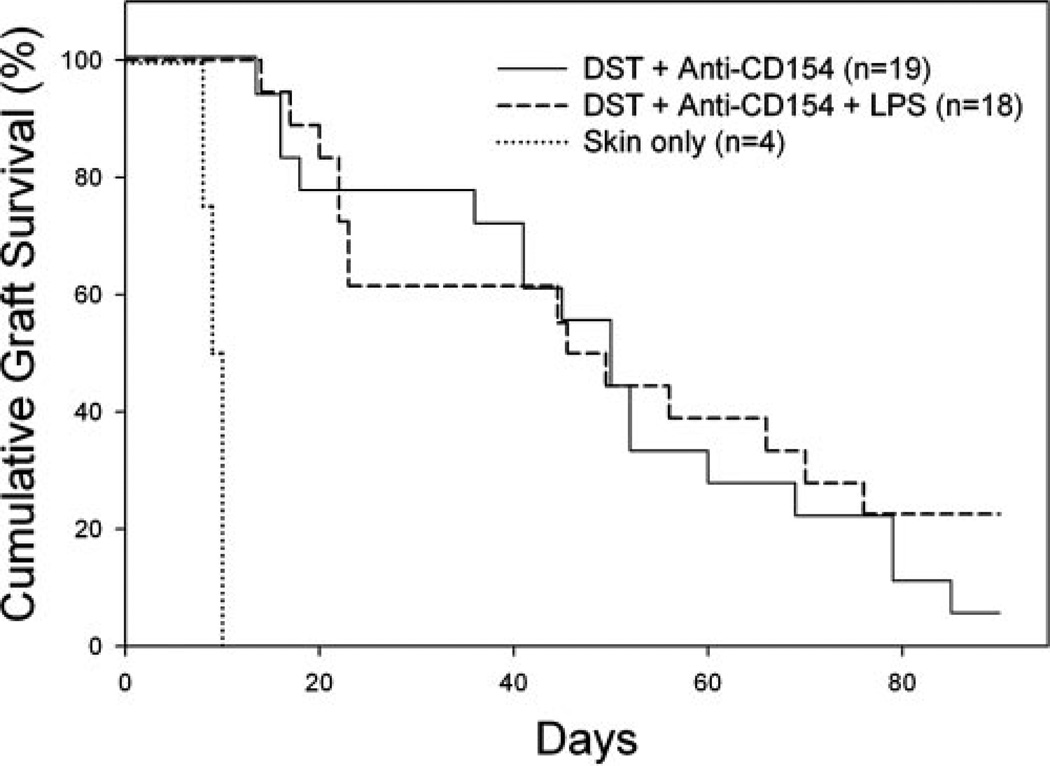
Our finding that TLR agonists impair the deletion of alloreactive CD8+ T cells led us to investigate whether this impaired deletion was directly responsible for the shortened skin allograft survival. To test this, B6.CD8α−/− mice were treated with DST and antiCD154 mAb in the presence of absence of coinjection with LPS. Allograft survival was comparable in tolerized B6.CD8α−/− mice in the absence (MST = 50 days, n = 18) or presence (MST = 48 days, n = 18, p = NS) of LPS (Fig. 3). As expected (39), B6.CD8α−/− mice not treated with costimulation blockade rapidly rejected BALB/c skin allografts (MST = 10 days, n = 4).
FIGURE 3.
CD8+ cells are required for LPS to shorten allograft survival induced by costimulation blockade. B6.CD8α−/− mice were given DST and anti-CD154 mAb according to our standard protocol with or without injection of LPS on day −7 relative to skin grafting on day 0. The difference between groups receiving DST and anti-CD154 mAb or DST, anti-CD154 mAb, and LPS was not significant; p = NS.
To confirm that LPS prevented the deletion of functionally active alloreactive CD8+ T cells, we recovered spleen cells from C57BL/6 mice 22 days after initiation of treatment with costimulation blockade with or without coinjection of LPS, which was 15 days after receiving a BALB/c skin allograft. These cells were tested for their ability to rapidly produce IFN-γ upon in vitro stimulation with alloantigen. Rapid production of IFN-γ is a sensitive marker of functional effector/memory alloreactive CD8+ T cells (24). In C57BL/6 mice treated with BALB/c DST and anti-CD154 mAb, the percentage of CD8+ T cells secreting IFN-γ in response to H2d donor-specific allogeneic stimulation was low (0.03 and 0.02%, n = 2) and similar to that observed after stimulation with syngeneic cells (0.08 and 0.05%, n = 2). In contrast, the percentage of CD8+ T cells secreting IFN-γ in mice treated with DST, anti-CD154 mAb, and LPS was markedly higher after allogeneic stimulation (2.34 and 3.18%, n = 2) than after syngeneic stimulation (0.06 and 0.05%, n = 2). These data indicate that TLR agonists impair the deletion of functional alloreactive effector CD8+ T cells, and that CD8+ T cells are responsible for the shortened skin allograft survival observed.
Costimulation blockade does not prevent the activation and proliferation of alloreactive CD8+ T cells
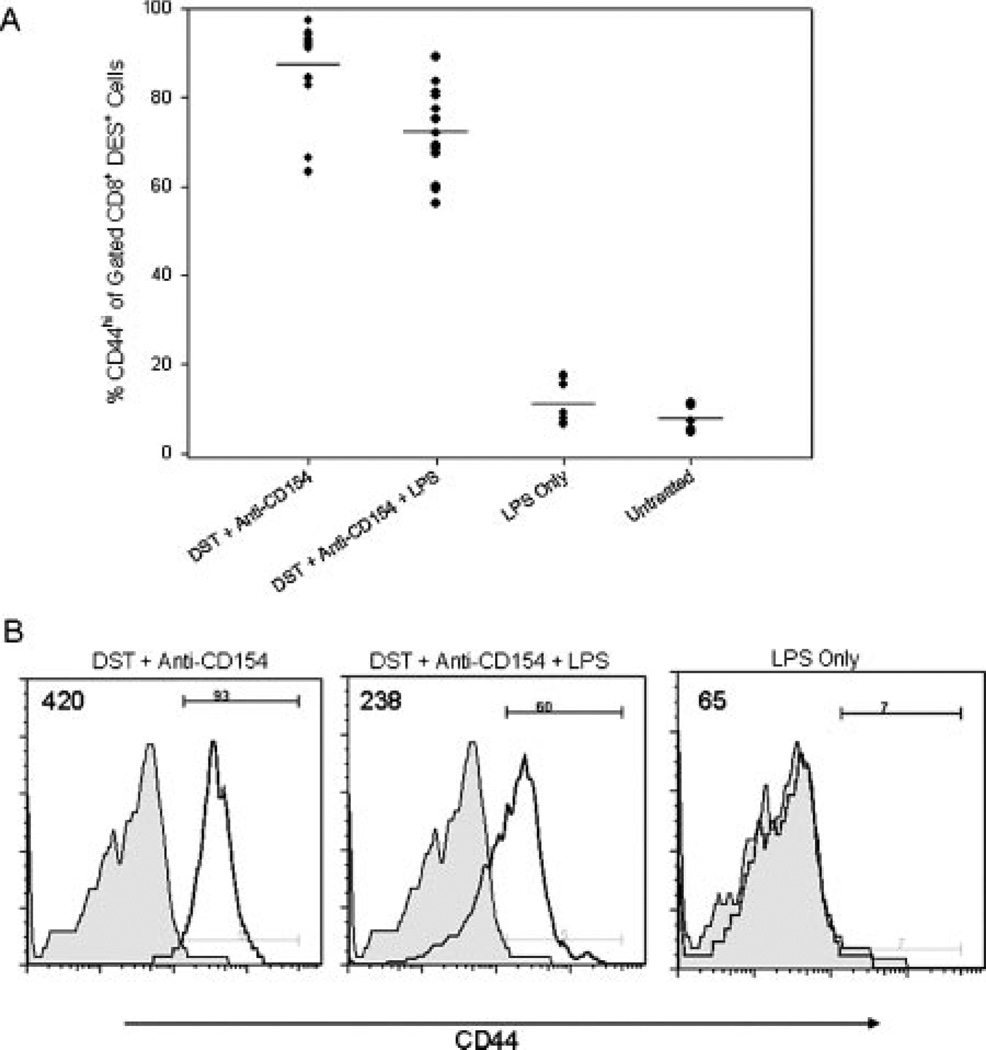
We next investigated the response of alloreactive CD8+ T cells in mice treated with costimulation blockade in the presence or absence of the TLR4 agonist, LPS. We first quantified the expression of the activation marker CD44 on the transgenic KB5 (DES +) CD8+ population in the spleen 72 h after DST plus anti-CD154 mAb treatment. Naive KB5 CD8+ T cells are CD44low (15). We observed that the majority of KB5 CD8+ T cells were CD44high in DST and anti-CD154-treated animals without (87.4 ± 10.8%, n = 13) or with LPS treatment (72.3 ± 10.0%, n = 13, Fig. 4). The median fluorescence intensity (MFI) of CD44 expression in the DST plus anti-CD154 mAb treated group (MFI = 542) was slightly higher than in the group also given LPS (MFI = 337).
FIGURE 4.
Alloreactive CD8+ T cells have an activated phenotype 72 h after treatment with DST and anti-CD154 mAb. KB5 synchimeras were treated with DST and anti-CD154 mAb in the presence or absence of coinjection with LPS and analyzed 72 h later. Splenocytes were stained with purified DES mAb, followed by the DES detection mAb (anti-mIgG2a-FITC), anti-CD8-PerCP mAb, and anti-CD44-allophycocyanin mAb. Samples were fixed and analyzed by flow cytometry. Samples were gated on DES+CD8+ lymphocytes and analyzed for CD44 expression. A, A summary of experimental mice in four independent trials with means indicated by the solid lines (n = 7–13/group). B, Representative flow histograms showing CD44 expression on gated DES+CD8+ lymphocytes. Shaded histograms represent untreated control mice. The MFI of a representative sample from each experimental group is indicated in the upper left corner of each panel. The percentage of CD8+ T cells that was CD44high in the DST and anti-CD154 mAb-treated group was significantly higher than that observed in the DST, anti-CD154 mAb, and LPS-treated group; p < 0.001.
LPS in the absence of DST administration did not lead to a significant increase in the level of CD8+CD44high T cells (11.1 ± 4.77%, MFI = 138, n = 8) above that observed in untreated mice (7.84 ± 2.97%, MFI = 73, n = 7, Fig. 4). These latter results document that LPS in the absence of Ag (DST) does not activate alloreactive CD8+ T cells.
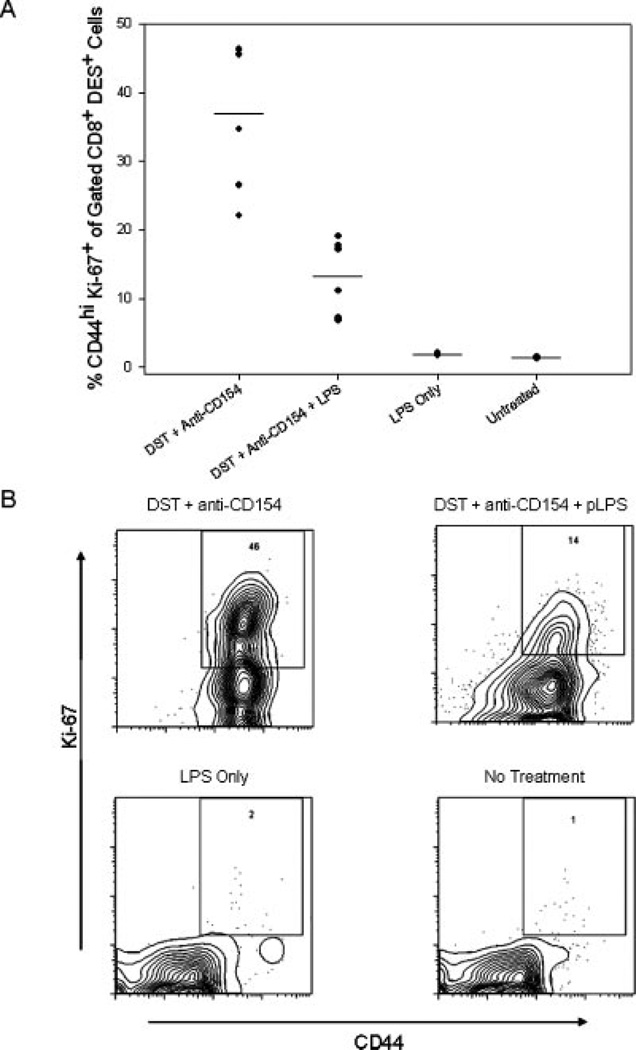
Costimulation blockade did not prevent alloreactive CD8+ T cells from acquiring an activated (CD44high) phenotype, but it was possible that these cells lacked the capacity to proliferate. To address this possibility, the expression of the nuclear proliferation Ag Ki-67 was quantified in the alloreactive CD8+ T cell population in the spleen 72 h after DST and anti-CD154 mAb treatment. At this time point, the absolute number of KB5 alloreactive CD8+ T cells was much reduced in mice treated with costimulation blockade as compared with that observed in mice also given LPS (Table II). We observed a higher proportion of cells expressing the Ki-67 Ag in mice treated with DST and anti-CD154 mAb (36.9 ± 10.8%, n = 6) than in mice treated with DST, anti-CD154 mAb, plus LPS (13.3 ± 5.5%, n = 6, p < 0.01, Fig. 5). In contrast, Ki-67 expression on alloreactive CD8+ T cells in untreated mice (1.4 ± 0.1%, n = 3) and in mice treated with LPS alone (1.9 ± 0.2%, n = 5) was low. These results show that LPS does not increase the activation or proliferative phenotype of alloreactive CD8+ T cells in mice treated with DST and anti-CD154 mAb, and that the few cells still present in mice treated with DST plus anti-CD154 mAb at 72 h were highly activated and proliferating.
Table II.
LPS impairs the deletion of alloreactive CD8+ T cells in the spleena
| 24 h |
48 h |
72 h |
96 h |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | KB5 (×105) | KB5 (%) | n | KB5 (×105) | KB5 (%) | n | KB5 (×105) | KB5 (%) | n | KB5 (×105) | KB5 (%) |
| DST + anti-CD154 | 4 | 6.17 ± 0.89b | 0.99 ± 0.21b | 4 | 7.25 ± 3.92b | 0.97 ± 0.42b | 4 | 18.1 ± 4.90c | 2.56 ± 0.82c | 3 | 7.65 ± 3.41d | 1.09 ± 0.51b |
| DST + anti-CD154 + LPS |
4 | 21.9 ± 2.61 | 3.71 ± 0.47 | 4 | 45.8 ±11.3 | 6.23 ± 1.03 | 4 | 64.0 ± 9.73 | 7.00 ± 0.97 | 3 | 46.2 ± 14.0 | 5.35 ± 1.16 |
| LPS only | 3 | 22.4 ± 1.15 | 3.83 ± 0.10 | 3 | 21.8 ± 4.82 | 2.53 ± 0.25 | 3 | 26.2 ± 9.93 | 2.61 ± 0.50 | ND | ND | |
| No treatment | 3 | 27.9 ±1.85 | 5.27 ± 0.42 | 3 | 34.3 ± 5.33 | 5.72 ± 0.20 | 3 | 36.2 ± 1.67 | 5.48 ± 1.46 | 3 | 33.9 ± 15.4 | 5.79 ± 1.42 |
LPS impairs the deletion and allows the expansion of alloreactive CD8+ T cells. KB5 synchimeras were given the treatment indicated on day −7 and analyzed at the time indicated. Splenocytes were stained with the clonotypic mAb DES, followed by the DES detection mAb (anti-mIgG2a–FITC) and anti-CD8-PerCP mAb
p < 0.01 vs all other groups.
p = N.S. vs LPS only group and p < 0.01 vs all other groups.
p < 0.05 vs untreated group and p < 0.01 vs DST, anti-CD154 mAb, and LPS group.
FIGURE 5.
Alloreactive CD8+ T cells are proliferating in the spleen 72 h after treatment with DST and anti-CD154 mAb. KB5 synchimeras were treated with DST and anti-CD154 mAb in the presence or absence of injection of LPS and analyzed 72 h later. Splenocytes were stained with the clonotypic Ab DES, followed by the DES detection mAb (anti-mIgG2a-FITC), anti-CD8-PerCP mAb and anti-CD44-allophycocyanin mAb. Cells were then fixed, permeabilized, and stained for the nuclear proliferation Ag Ki-67 with an anti-Ki-67-PE mAb. Samples were gated on DES+CD8+ lymphocytes and analyzed for Ki-67 expression. A, A summary of experimental mice from two independent experiments with the mean indicated by the solid lines (n = 3–6 mice/group). B, Representative flow dot plots showing CD44 and Ki-67 expression in gated DES+CD8+ lymphocytes.
LPS protects alloreactive CD8+ T cells from apoptosis in mice treated with DST and anti-CD154 mAb
We next quantified the absolute number of transgenic alloreactive CD8+ T cells in the spleens of KB5 synchimeras at 24, 48, 72, and 96 h after treatment with DST and anti-CD154 mAb in the presence or absence of LPS coadministration. LPS prevented the disappearance of the transgenic population from the spleen as early as 24 h after treatment with DST and anti-CD154 mAb (Table II). In mice treated with DST and anti-CD154 mAb, 6.17 ± 0.89 × 105 splenic KB5 T cells were recovered as compared with 21.9 ± 2.61 × 105 KB5 CD8+ T cells in mice given costimulation blockade plus LPS (Table II, p < 0.01). A small but significant decrease in KB5 cell number was observed in mice treated with DST, antiCD154 mAb, and LPS compared with untreated mice (p < 0.05), but this change was statistically similar to a decrease caused by treatment with LPS alone (p = NS). The transgenic CD8+ T cell population then expanded dramatically in animals treated with DST, anti-CD154 mAb, and LPS at 72 h; this trend was not observed in mice treated with LPS alone (Table II). These data show that LPS 1) prevents the loss of alloreactive CD8+ T cells from the circulation as early as 24 h after costimulation blockade and 2) supports their Ag-specific proliferation and accumulation.
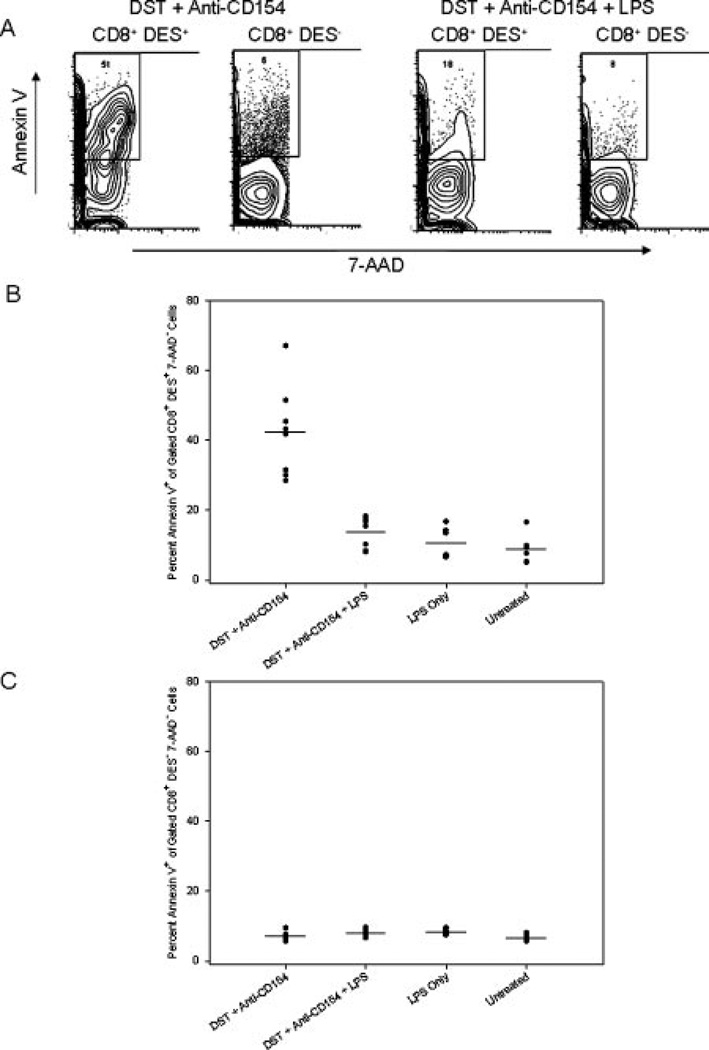
Alloreactive CD8+ T cells were capable of proliferation in the presence of DST and anti-CD154 mAb regardless of LPS treatment (Fig. 5), with no immediate dramatic deletion of alloreactive CD8+ T cells following costimulation blockade in the presence of coadministration of LPS (Table II). These data suggest that LPS prevented the initial deletion of alloreactive CD8+ T cells induced by costimulation blockade and that the accumulation of alloreactive CD8+ T cells at 72 h was more than simply expansion of a small population that survived an initial depletion. This observation led us to hypothesize that LPS might mediate its effect by protecting alloreactive CD8+ T cells from apoptosis. To test this, apoptosis was measured in KB5 transgenic CD8+ T cells 72 h after DST and anti-CD154 mAb treatment using the apoptotic marker annexin V in conjunction with the viability stain 7-AAD.
Although few cells remained at 72 h after DST and anti-CD154 mAb treatment (Table II, Fig. 6A), a high percentage of these remaining cells stained with the apoptosis marker annexin V (42.3 ± 12.9%, n = 8, Fig. 6A). These data suggest that the proliferating, activated alloreactive T cells in mice treated with costimulation blockade were being driven into apoptotic cell death. Strikingly, not only were more transgenic CD8+ cells present in mice treated with DST, anti-CD154 mAb plus LPS at 72 h, but also a significantly lower percentage of these cells were undergoing apoptosis (13.4 ± 4.4%, n = 8, p < 0.0001 vs DST and anti-CD154 mAb, Fig. 6B). This low level of apoptosis was similar to that observed in untreated synchimeric mice (8.8 ± 4.3%, n = 6) and synchimeric mice treated with LPS alone (10.8 ± 4.5%, n = 6). These data suggest that the accumulation of alloreactive T cells at 72 h was likely due to the combined effects of preventing their initial deletion at 24 h and preventing the apoptosis of activated alloreactive T cells at 72 h.
FIGURE 6.
LPS protects alloreactive CD8+ T cells from apoptosis in mice treated with costimulation blockade. KB5 synchimeras were treated with DST and anti-CD154 mAb in the presence or absence of coinjection of LPS and analyzed 72 h later. Splenocytes were stained with the clonotypic Ab DES, followed by the DES detection mAb (anti-mIgG2a–FITC), anti-CD8-PerCP mAb, Annexin VPE, and the viability dye 7-AAD. A, Representative contour plot showing annexin V staining after gating on live (7AAD−) transgenic (CD8+DES+) or nontransgenic (CD8+ DES−) T cells. B, Percent of live (7-AAD−) transgenic CD8+ T cells binding annexin V. C, Percent of live nontransgenic CD8+ T cells binding annexin V. Graphs represent combined data from two independent experiments (n = 6–9 mice/group).
In contrast, the CD8+DES− nontransgenic population in any of the treatment groups showed no increase in the level of apoptosis above that of untreated mice (p = NS, Fig. 6C). This indicates that the apoptosis induced by DST and anti-CD154 mAb is restricted to the Ag-specific alloreactive T cell population and not simply a nonspecific effect of costimulation blockade or LPS treatment on CD8+ T cells.
TLR4 is required on host but not donor origin cells or graft for the detrimental effect of LPS on skin allograft survival
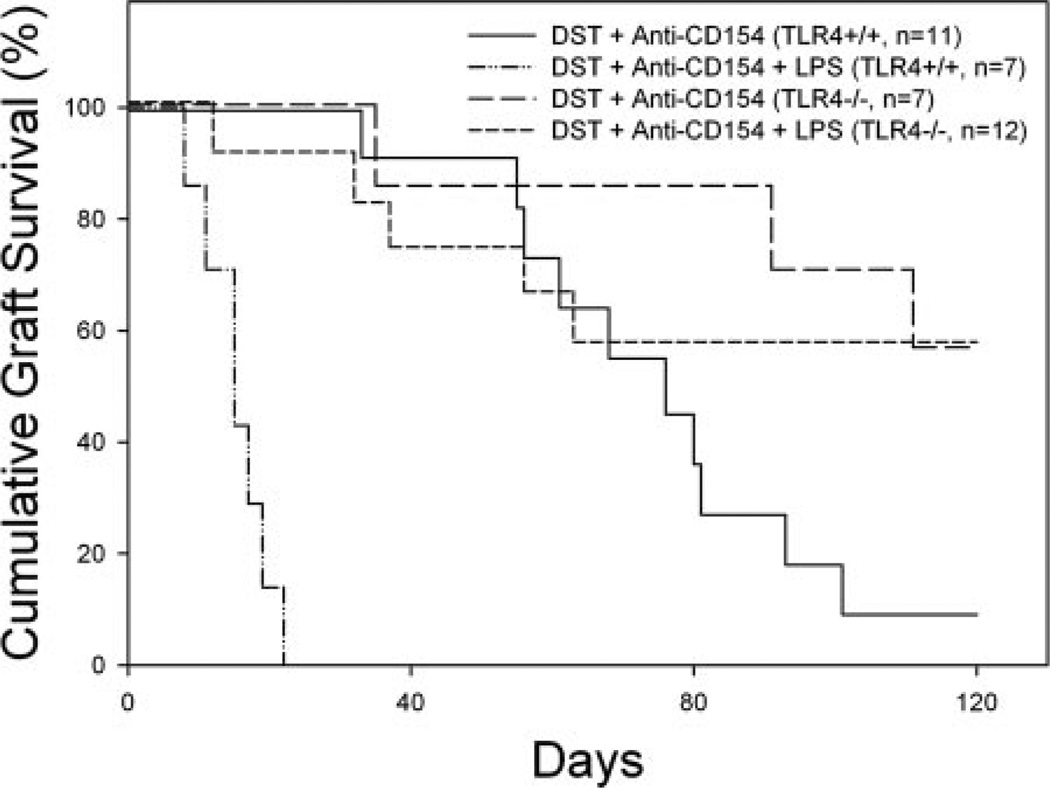
It is possible that LPS abrogates skin allograft survival by engaging TLR4 on cells of host origin, or conversely, by engaging TLR4 directly on cells in the DST or skin allograft. To address the influence of host TLR4 expression on the effects of costimulation blockade, C57BL/10ScSnJ (TLR4+/+) and C57BL/10ScNJ (TLR4−/−) mice were treated with BALB/c DST, anti-CD154 mAb, and given BALB/c skin allografts with or without coinjection of LPS at the time of administration of DST and anti-CD154 mAb.
Treatment of TLR4+/+ C57BL/10ScSnJ mice with DST and anti-CD154 mAb led to prolonged skin allograft survival (MST = 76 days), which was shortened by LPS treatment (MST = 15 days, p < 0.0001, Fig. 7). In contrast, TLR4−/− mice treated with DST and anti-CD154 mAb in the presence (MST >128 days) or absence (MST >128 days) of LPS exhibited comparable skin allograft survival (p = NS, Fig. 7). Interestingly, skin allograft survival was significantly prolonged by costimulation blockade in TLR4−/− mice as compared with that achieved in TLR4+/+ mice (p < 0.05). These data suggest that TLR4 expression is required on host cells for LPS to abrogate prolonged skin allograft survival induced by DST and anti-CD154 mAb.
FIGURE 7.
TLR4 is required on host cells but is not required on donor cells. C57BL/10ScSn (TLR4+/+) and C57BL/10ScN (TLR4−/−) mice were given DST, anti-CD154 mAb, and skin allografts according to our standard protocol with or without coinjection of LPS at the time of co-stimulation blockade. Skin allograft survival in tolerized TLR4+/+ C57BL/ 10ScSn mice was significantly prolonged (MST = 76 days, n = 11) as compared with allograft survival in mice coinjected with LPS (MST = 15 days, n = 7, p < 0.0001). Skin allograft survival in tolerized TLR4−/− C57BL/10ScN mice was similar in the presence (MST >128 days, n = 7) or absence (MST >128 days, n = 12, p = NS) of LPS.
To study the role of TLR4 expression on the DST and graft, the converse experiment was performed. C57BL/6 (TLR4+/+) mice were given C.C3-Tlr4Lps-d/J (TLR4−/−) DST, anti-CD154 mAb, and a C.C3-Tlr4Lps-d/J skin allograft in the presence or absence of LPS administration. Similar to what we observed with TLR4+/+ BALB/c DST and skin graft donors (Fig. 1), allograft survival in the absence of LPS (MST = 48 days, n = 9) was shortened following LPS treatment (MST =11 days, n = 8,p < 0.0001). These data show that the effect of LPS is on host cells, not donor cells or graft.
TNFR2 and IL-12R are not required for LPS to shorten skin allograft survival induced by costimulation blockade
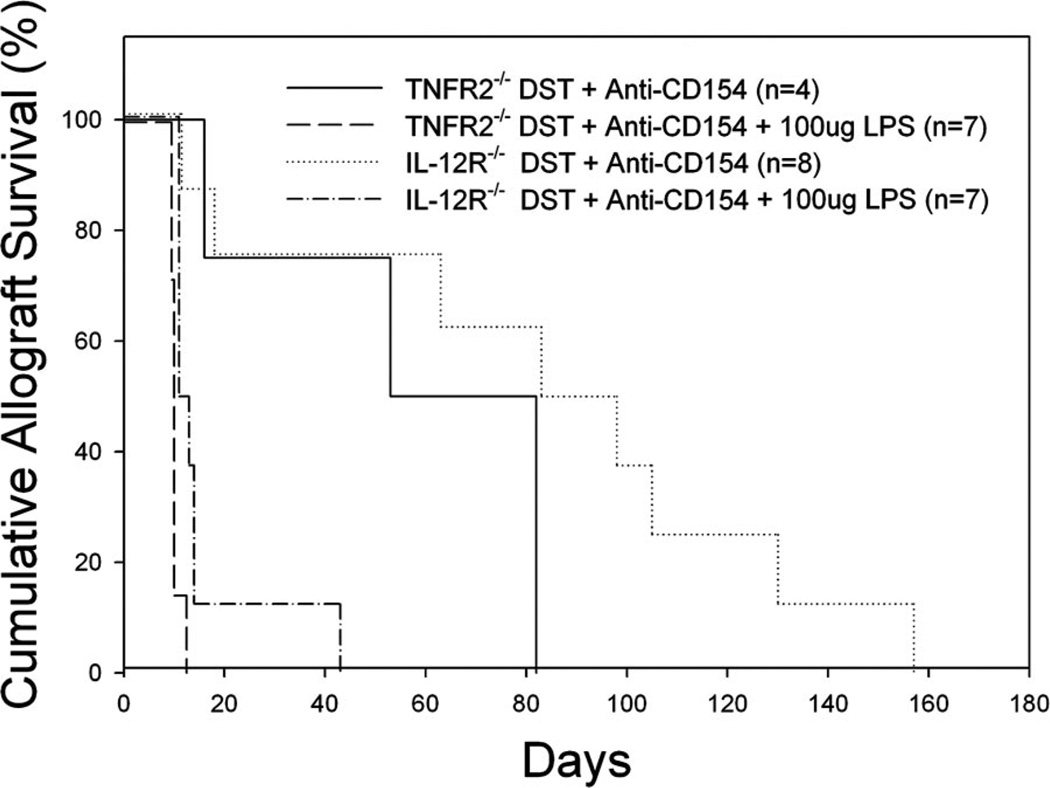
LPS might prevent apoptosis by providing a survival signal to CD8+ T cells by inducing the production of the cytokines IL-12 or TNF-α. CD8+ T cells express IL-12R (47) and TNFR2 (48, 49), and TNF-α and IL-12 are two cytokines that can provide “signal 3” to CD8+ T cells and enhance their activation and the generation of effector function (47–52). To address the possible role of the TNFR2 and IL-12 receptors in mediating the detrimental effects of LPS on graft survival, B6IL-12R−/− and B6.TNFR2−/− mice were treated with DST, anti-CD154 mAb, and given skin allografts in the presence or absence of LPS coadministration.
Treatment of B6.TNFR2−/− mice with DST and anti-CD154 mAb led to prolonged skin allograft survival (MST = 68 days, n = 4), which was shortened by LPS treatment (MST = 11 days, p < 0.005, Fig. 8). Similarly, treatment of B6.IL-12R−/− mice with DST and anti-CD154 mAb led to prolonged skin allograft survival (MST = 91 days, n = 8), which was also shortened by LPS treatment (MST = 12 days, n = 4, p < 0.005, Fig. 8). These data suggest that signaling through neither TNFR2 nor IL-12R is required for the effects of LPS on allograft survival in tolerized mice.
FIGURE 8.
TNFR2 and IL-12R are not required for LPS to shorten allograft survival induced by costimulation blockade. B6.TNFR2−/− and B6.IL-12R−/− mice were given DST and anti-CD154 mAb according to our standard protocol with or without injection of LPS on day −7 relative to skin grafting on day 0. Skin allograft survival in tolerized B6.TNFR2−/− mice was significantly prolonged (MST = 68 days, n = 4) as compared with allograft survival in mice coinjected with LPS (MST = 11 days,p < 0.005). Skin allograft survival in tolerized B6.IL-12R−/− mice significantly prolonged (MST = 91 days, n = 8) as compared with allograft survival in mice coinjected with LPS (MST = 12 days, n = 4, p < 0.005).
Discussion
Costimulation blockade protocols are effective in animal models, but it will be important for their implementation in the clinic to determine how environmental perturbants may affect the prolongation of allograft survival induced by costimulation blockade. We have shown that infection with LCMV at the time of the initial treatment with DST and anti-CD154 mAb abrogates prolonged allograft survival (this paper and Ref. 20); however, the mechanism underlying this observation is not known. We hypothesized that LCMV activates innate immunity through TLRs, an idea supported by the fact that mice deficient in the TLR-associated adaptor molecule MyD88 do not mount a robust anti-LCMV response and fail to clear the virus (33). TLR activation has many of the same effects on APCs as CD40 activation, including up-regulation of the costimulatory molecules CD80 and CD86 (36). Activation of TLRs could mature APCs and provide full activation to alloreactive T cells in the presence of CD40-CD154 blockade. This hypothesis is appealing because it has been demonstrated that treatment of cell lines or allogeneic cells with the TLR3 agonist poly I:C leads to the cross-priming of host APCs (53).
In this study, agonists of TLR2 (Pam3Cys), TLR3 (Poly I:C), TLR4 (LPS), and TLR9 (CpG DNA) were all capable of shortening allograft survival and impairing the deletion of alloreactive CD8+ T cells in mice treated with costimulation blockade. The fact that all TLR agonists had these effects is interesting but not surprising because the TLRs assayed have downstream pathways that are both shared and unique among the receptors tested (26, 54–56). Our data raise the possibility that a shared pathway might be involved. The MyD88-dependent pathway is common to TLR2, TLR4, and TLR9, but TLR3 can signal through a MyD88-independent pathway (29). Interestingly, the MyD88 adaptor molecule has been implicated in the rejection of minor allogeneic mismatches; MyD88 is essential for a female mouse to reject a male skin graft (35). In contrast, MyD88 is not essential for rejection of fully MHC-mismatched allografts, although the nature of the immune response is biased toward a Th2 response (57). We are currently addressing the involvement of MyD88 and other TLR-associated adaptor molecules using genetic knockout mice.
The TLR4 agonist LPS did not increase the capacity of Ag to stimulate alloreactive CD8+ T cells. Alloreactive CD8+ T cells were activated and proliferated in mice treated with DST and antiCD154 mAb in the presence or absence of LPS. It is interesting to note that coadministration of LPS actually decreased the percentage of activated (CD44high) and proliferating (Ki-67+) alloreactive CD8+ T cells at 72 h in mice treated with costimulation blockade. This reduced frequency of activated, proliferating cells may be associated with protection of these cells from apoptosis. It has been reported that activation and proliferation are molecularly linked to apoptosis (58–60), and the increased frequency of proliferating cells in mice treated with costimulation blockade in the absence of LPS at this time point appears to be associated with an increase in apoptosis as determined by annexin V staining. These data mimic our previous findings that alloreactive CD8+ T cells up-regulate CD44 after DST and anti-CD154 mAb treatment in an adoptive transfer model (15). It should also be noted that alloreactive CD8 + T cells from mice treated with LPS alone had a naive CD44lowKi-67− phenotype similar to that observed in untreated mice, confirming that activation and proliferation requires Ag (DST) and is not simply a “nonspecific” response to LPS.
The fact that costimulation blockade does not prevent the initial activation of alloreactive CD8+ T cells suggests that LPS is inducing an essential factor that determines whether the Ag-activated CD8+ T cells will be deleted or survive, and perhaps determine allograft survival and rejection. This is supported by the fact that LPS also prevents the early Ag-dependent loss of alloreactive CD8+ T cells from the circulation at 24 h in mice treated with DST and anti-CD154 mAb. We have previously observed that DST in the absence of anti-CD154 mAb leads to a rapid, but nondeletional, reduction of alloreactive T cells from the circulation (46). This is similar to the redistribution of Ag-activated CD8+ T cells to nonlymphoid tissues that has been described previously (61– 63). In mice treated with DST plus anti-CD154 mAb, we observed a deletional loss of alloreactive T cells, whereas in mice coinjected with LPS, we observed that the Ag-induced redistribution and reduction of activated CD8+ T cells at 24 h is prevented. This observation also excludes the possibility that the accumulation of alloreactive CD8+ cells at 72 h is the result of the proliferation of only a small population of cells that survive deletion.
LPS also decreases the frequency of apoptotic alloreactive CD8+ T cells observed at 72 h after treatment with DST and anti-CD154 mAb. These data suggest that LPS acts to both prevent the early loss of alloreactive T cells in mice treated with costimulation blockade and to protect these cells from apoptosis during Agdriven activation and proliferation. It is important to note that LPS does support the accumulation of these cells later (at 72 h after treatment) and that this likely contributes to allograft rejection. The small decrease in alloreactive CD8+ T cell number observed in LPS-treated mice, regardless of DST and anti-CD154 mAb treatment, is probably due to type IIFN production. It has been established previously that poly I:C induces a type I IFN-dependent attrition of CD8+ T cells (64).
The hypothesis that LPS protects alloreactive CD8+ T cells from death is further buttressed by our findings that LPS prevents apoptosis induced by costimulation blockade. Furthermore, the CD8+ T cells protected from apoptosis are functionally active as evidenced by their ability to rapidly produce IFN-γ upon in vitro allogeneic stimulation. Supporting our observation that LPS prevents apoptosis, we observed that caspase 3 protein expression is much lower in alloreactive KB5 CD8+ T cells in mice treated with DST, anti-CD154 mAb, and LPS as compared with mice that were not injected with LPS (T. B. Thornley, unpublished observations). These data suggest that LPS may alter the balance of proapoptotic and antiapoptotic molecules in Ag-activated T cells, a possibility we are currently investigating. This result also supports earlier evidence that mice defective in apoptotic pathways are resistant to induction of transplantation tolerance (17). More recently, Monk et al. (65) have proposed that the allograft prolonging effects induced by anti-CD154 mAb are mediated by a complement-dependent mechanism. However, the ability of LPS to prevent alloreactive CD8+ T cell deletion argues against a complement-dependent mechanism. Moreover, we have observed that complement-deficient mice treated with DST and anti-CD154 mAb exhibit prolonged allograft survival, further suggesting that complement-mediated cell destruction is not the primary mechanism by which anti-CD154 mAb mediates its effects in this model (T. G. Markees, unpublished observations).
We further established that the impaired deletion of alloreactive CD8+ T cells is a prerequisite for the ability of LPS to abrogate prolonged allograft survival induced by costimulation blockade. Mice that are deficient in CD8+ T cells reject skin allografts with the same kinetics when treated with DST and anti-CD154 mAb with or without coinjection of LPS. These data suggest that TLR agonists act to prevent prolonged allograft survival induced by costimulation blockade by altering the response of alloreactive CD8 but not CD4 T cells, a hypothesis we are currently investigating using an alloreactive CD4+ TCR-transgenic model system. It is unlikely that lack of dendritic cells expressing the CD8αα homodimer is responsible for this observation because it has been shown that dendritic cells isolated from CD8α-deficient mice produce levels of IL-12 that are comparable to those observed in wild-type animals (66). It is also known that CD8+ T cells are not absolutely required for the rejection of skin allografts, because CD8-deficient mice promptly reject them in the absence of preconditioning (67). Therefore, LPS promotes the rejection of allografts through a CD8-dependent mechanism.
Importantly, we document that signaling through TLR4 is the mechanism by which LPS mediates its detrimental effects on allograft survival in mice treated with costimulation blockade. For these effects, TLR4 expression is essential on host cells, but not DST or graft. This is interesting because host transgenic KB5 CD8+ T cells recognize native Ag by direct Ag presentation (38), and the lack of a requirement for TLR4 on DST or the graft suggests that LPS-induced maturation of donor H2b-expressing APCs is not responsible for its effects on graft survival. It is possible, however, that the induction of host cytokines mature donor APCs indirectly, a possibility we are currently investigating. Furthermore, because we used repurified LPS, these data document that LPS is mediating its effect through TLR4, and not through potential lipopeptides that are contaminants of unpurified LPS (42).
Neither IL-12R nor TNFR2 was required for LPS to break the induction of prolonged allograft survival by costimulation blockade. Signal 3 is required for complete T cell activation and effector function; however, it is also known that adjuvant can provide an alternative signal 3 that is not IL-12 (47). We are currently investigating other potential sources of signal 3 induced by TLR agonists and viral infections, including type I IFNs (68).
The ability to identify the specific TLRs involved in LCMV-mediated abrogation of prolonged allograft survival induced by costimulation blockade is complicated by the fact that viruses can interact with multiple TLRs. TLR3, the TLR identified as recognizing dsRNA (69), is a prime candidate for targeting by LCMV. However, despite the fact that MyD88-deficient mice fail to mount an effective anti-LCMV response, TLR3-deficient mice are capable of mounting antiviral responses and clearing LCMV (70). This suggests that LCMV might activate the innate immune system through two or more TLRs. Furthermore, it can be inferred from our previous data that viruses do not always abrogate the effects of costimulation blockade by acting through TLRs. We have previously demonstrated that mice inoculated with LCMV 1 day after transplantation exhibit shortened allograft survival after treatment with DST and anti-CD154 mAb; however, mice injected with poly I:C do not (19). This stands in contrast to our current findings that all TLR agonists assayed shorten skin allograft survival to the same degree as LCMV when administered with the first injection of DST and anti-CD154 mAb (Fig. 1). Therefore, viruses probably shorten allograft survival by different mechanisms when mice are infected at the time of DST vs 1 day after transplantation.
DST and anti-CD154 mAb costimulation blockade induces prolonged allograft survival with a specificity that makes it very appealing; however, the negative impact of viral infections on costimulation blockade has cast some doubt on its efficacy in a nonpathogen-free environment such as that experienced in the “real world” of transplantation in the clinic. Understanding the underlying mechanisms by which viruses and environmental perturbants interfere with costimulation blockade may yield novel strategies that will ultimately overcome this hurdle in making costimulation blockade a clinically viable protocol.
Acknowledgments
We thank Linda Paquin, Jean Leif, Amy Cuthbert, Keith Daniels, and Lisa Giassi for their technical assistance. We also thank Nancy Philips, Evelyn Kurt-Jones, and Sung-Kwon Kim for their helpful insights.
Footnotes
This work was supported in part by National Institutes of Health Grants AI46629, DK53006, CA34461; institutional Diabetes Endocrinology Research Center Grant DK32520; and Grant Nos. 1-2002-396 and 1-2004-548 from the Juvenile Diabetes Foundation, International. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper: DST, donor-specific transfusion; LCMV, lymphocytic choriomeningitis virus; D-PBS, Dulbecco’s PBS; DES, Desirè; MFI, mean fluorescence intensity; MST, median survival time.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. β-cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100–106. doi: 10.2337/diabetes.54.1.100. [DOI] [PubMed] [Google Scholar]
- 2.Street CN, Lakey JRT, Shapiro AMJ, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, Korbutt GS. Islet graft assessment in the Edmonton protocol-implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–3114. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Lakey JRT, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AMJ. Successful islet transplantation-continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 5.Teraoka S, Kawai T, Yamaguchi Y, Fujita S, Tojinbara T, Nakajima T, Hayashi T, Nakagawa Y, Fujikawa H, Honda H, et al. Mechanism of the preventive effect of aminobenzoic acid salt on pancreatic β cell toxicity by cyclosporin. Transplant. Proc. 1990;22:863–866. [PubMed] [Google Scholar]
- 6.Newstead CG. Assessment of risk of cancer after renal transplantation. Lancet. 1998;351:610–611. doi: 10.1016/s0140-6736(98)22009-5. [DOI] [PubMed] [Google Scholar]
- 7.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 9.Elster EA, Xu H, Tadaki DK, Montgomery S, Burkly LC, Berning JD, Baumgartner RE, Cruzata F, Marx R, Harlan DM, Kirk AD. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72:1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han DM, Wagner JL, Ruiz P, et al. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999;48:1473–1481. doi: 10.2337/diabetes.48.7.1473. [DOI] [PubMed] [Google Scholar]
- 11.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc. Natl. Acad. Sci. USA. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston EH, Xu H, Dhanireddy KK, Pearl JP, Leopardi FV, Starost MF, Hale DA, Kirk AD. IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am. J. Transplant. 2005;5:1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsen CP, Pearson TC. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr. Opin. Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 14.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Ann. Rev. Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 15.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Greiner DL, Rossini AA. Treatment of allograft recipients with donor specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J. Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat. Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 17.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nunez G, Tang A, Sayegh M, Hancock WW, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat. Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 18.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1+CD8+ T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turgeon NA, Iwakoshi NN, Meyers WC, Welsh RM, Greiner DL, Mordes JP, Rossini AA. Virus infection abrogates CD8+ T cell deletion induced by donor-specific transfusion and anti-CD154 monoclonal antibody. Curr. Surg. 2000;57:505–506. doi: 10.1016/s0149-7944(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 20.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, Greiner DL, Rossini AA. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J. Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman D, Welsh RM, Markees TG, Woda BA, Mordes JP, Rossini AA, Greiner DL. Viral abrogation of stem cell transplantation tolerance causes graft rejection and host death by different mechanisms. J. Immunol. 2002;168:6047–6056. doi: 10.4049/jimmunol.168.12.6047. [DOI] [PubMed] [Google Scholar]
- 22.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J. Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- 23.Nahill SR, Welsh RM. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J. Exp. Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J. Immunol. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 25.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehme KW, Compton T. Innate sensing of viruses by Toll-like receptors. J. Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devendra D, Eisenbarth GS. Interferon α-a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin. Immunol. 2004;111:225–233. doi: 10.1016/j.clim.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between Toll-like receptor, interferon and viruses. Microbiol. Immunol. 2004;48:147–154. doi: 10.1111/j.1348-0421.2004.tb03500.x. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 30.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 31.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diebold SS, Kaisho T, Hemmi H, Akira S, Sousa CRE. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 33.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 34.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 2001;166:5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 38.Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hämmerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 39.Iwakoshi NN, Markees TG, Turgeon NA, Thornley T, Cuthbert A, Leif JH, Phillips NE, Mordes JP, Greiner DL, Rossini AA. Skin allograft maintenance in a new synchimeric model system of tolerance. J. Immunol. 2001;167:6623–6630. doi: 10.4049/jimmunol.167.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon-γ, and CTLA4. J. Clin. Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 43.Hua C, Boyer C, Buferne M, Schmitt-Verhulst AM. Monoclonal antibodies against an H-2Kb-specific cytotoxic T cell clone detect several clone-specific molecules. J. Immunol. 1986;136:1937–1944. [PubMed] [Google Scholar]
- 44.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 45.Banuelos SJ, Markees TG, Phillips NE, Appel MC, Cuthbert A, Leif J, Mordes JP, Shultz LD, Rossini AA, Greiner DL. Regulation of skin and islet allograft survival in mice treated with costimulation blockade is mediated by different CD4+ cell subsets and different mechanisms. Transplantation. 2004;78:660–667. doi: 10.1097/01.tp.0000130449.05412.96. [DOI] [PubMed] [Google Scholar]
- 46.Markees TG, Pearson T, Cuthbert A, Pearson AL, Shultz LD, Leif J, Phillips NE, Mordes JP, Greiner DL, Rossini AA. Evaluation of donor-specific transfusion sources: unique failure of bone marrow cells to induce prolonged skin allograft survival with anti-CD154 monoclonal antibody. Transplantation. 2004;78:1601–1608. doi: 10.1097/01.tp.0000140847.29917.65. [DOI] [PubMed] [Google Scholar]
- 47.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim EY, Teh HS. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol. 2001;167:6812–6820. doi: 10.4049/jimmunol.167.12.6812. [DOI] [PubMed] [Google Scholar]
- 49.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J. Immunol. 2004;173:4500–4509. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 50.Aspalter RM, Eibl MM, Wolf HM. Regulation of TCR-mediated T cell activation by TNF-RII. J. Leukocyte Biol. 2003;74:572–582. doi: 10.1189/jlb.0303112. [DOI] [PubMed] [Google Scholar]
- 51.Shrikant P, Mescher MF. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J. Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- 52.Kim EY, Teh HS. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol. 2001;167:6812–6820. doi: 10.4049/jimmunol.167.12.6812. [DOI] [PubMed] [Google Scholar]
- 53.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 54.Akira S, Takeda K. Functions of Toll-like receptors: lessons from KO mice. C R. Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 56.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 57.Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a Toll-like receptor signal adaptor protein. Am. J. Transplant. 2004;4:1429–1439. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 58.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 59.Adam-Klages S, Adam D, Janssen O, Kabelitz D. Death receptors and caspases: role in lymphocyte proliferation, cell death, and autoimmunity. Immunol. Res. 2005;33:149–166. doi: 10.1385/IR:33:2:149. [DOI] [PubMed] [Google Scholar]
- 60.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 61.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 62.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 63.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J. Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- 64.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monk NJ, Hargreaves REG, Marsh JE, Farrar CA, Sacks SH, Millrain M, Simpson E, Dyson J, Jurcevic S. Fc-dependent depletion of activated T cells occurs through CD40L–specific antibody rather than costimulation blockade. Nat. Med. 2003;9:1275–1280. doi: 10.1038/nm931. [DOI] [PubMed] [Google Scholar]
- 66.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-α, and IFN-γby mouse dendritic cell subsets. J. Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 67.Desai NM, Bassiri H, Kim J, Koller BH, Smithies O, Barker CF, Naji A, Markmann JF. Islet allograft, islet xenograft, and skin allograft survival in CD8+ T lymphocyte-deficient mice. Transplantation. 1993;55:718–722. doi: 10.1097/00007890-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 69.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 70.Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MBA. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]