Abstract
Fish oil contains the marine ω-3 polyunsaturated fatty acids (ω-3 PUFAs) docosahexaenoic (DHA) and eicosapentaenoic acid (EPA). The consumption of diets rich in these fatty acids is associated with a decreased incidence of prostate cancer. However, there is limited knowledge regarding the non-marine ω-3 PUFA α-linolenic acid (ALA). To study which ω-3 PUFAs are more effective in prostate cancer prevention, and whether the mechanisms of action are conserved between them, we investigated the effect of DHA, EPA and ALA on the human prostate cancer cell lines PC-3 and LNCaP. Different trends of inhibition of PC-3 cell proliferation were observed for the three ω-3 PUFA, with DHA having the most pronounced effects on cell proliferation, while ALA had the minimum effects of the three ω-3 PUFAs. All the ω-3 PUFAs decreased fatty acid synthase (FASN) mRNA. Concerning genes involved in inflammation, cell cycle and apoptosis, DHA regulated the most genes in all categories, followed by EPA and then ALA. In addition, DHA and EPA increased the gene expression of the pro-apoptotic protein activating transcription factor 3 mRNA. Moreover, these two fatty acids significantly induced apoptosis. In conclusion, while some mechanisms of cancer cell inhibition are conserved among ω-3 PUFA, the extent, magnitude, and duration of transcriptional changes vary for each individual fatty acid.
Keywords: prostate cancer, ω-3, docosahexaenoic acid, eicosapentaenoic acid, α-linolenic acid
Introduction
Prostate cancer is one of the most commonly diagnosed types of cancer in males as well as the second leading cause of cancer death in males in the United States. Prostate cancer is a clinically heterogeneous disease that varies in its biological aggressiveness. Metastatic prostate cancer is incurable, and the primary treatment consists of androgen deprivation, which leads to apoptosis of cancer cells and regression of tumors (1,2). However, response to treatment is temporary due to the surviving tumor cells that emerge as androgen-independent. Prostate cancer has long been linked to obesity and nutrition in incidence and mortality (3,4), although the role of dietary fatty acids in the etiology or prevention of this disease has not been fully elucidated yet.
Fatty acids are the primary energy source for prostate cancer cells and androgens upregulate fatty acid synthase (FASN), the enzyme responsible for the de novo synthesis of fatty acids (5). FASN is increased in prostate adenocarcinoma as compared to normal prostatic tissue and is a marker of prostate cancer recurrence, poor prognosis and higher Gleason grade (6). Sterol response element binding protein-1c (SREBP-1c) is a positive regulator of FASN expression through binding elements in the FASN promoter. Diets rich in ω-3 polyunsaturated fatty acids suppress SREBP-1 mRNA and the active nuclear form of the SREBP-1 protein (7–10). Consequent downregulation of FASN has been associated with cell cycle arrest and induction of apoptosis due to nutrient deprivation in several types of cancer, including breast and prostate cancer, which rely on the activity of this enzyme as an energy source (5,11–13).
Androgen ablation and androgen receptor (AR) antagonism therapy in patients with prostate cancer initially induces cell cycle arrest and apoptosis (14,15). However, cancer cells eventually lose dependence on androgens, leading to progression of the androgen-independent tumors (16). Numerous mechanisms have been postulated to account for the conversion from the androgen-dependent to androgen-independent state, including the aberrant activation of androgen receptor by a variety of growth factors. Cytokines and chemokines, produced by activated resident immune cells, are the most important components regulating the tumor growth micro-environment (17–19). Many of these signaling molecules are also able to function in an autocrine manner. Both androgen-dependent and -independent prostate cancer cells produce high levels of the macrophage chemotactic protein-1 (MCP-1) compared to normal prostate epithelial cells (20). MCP-1 acts as an autocrine growth and pro-metastatic factor in prostate cancer (21,22). Notably, pro-inflammatory cytokines such as inter-leukin (IL)-1, IL-6 and tumor necrosis factor (TNF) are able to affect cancer risk (23–26).
Androgen-independent prostate cancer cells do not enter apoptosis upon androgen depletion, however, they maintain the ability to enter the apoptotic pathway (27). An alternative way to modulate apoptosis is by regulating the expression levels of essential apoptotic genes. The anti-apoptotic factor B-cell leukemia/lymphoma 2 (Bcl-2) and the tumor suppressor p53 are two such candidate genes (28). Additionally, activating transcription factor-3 (ATF-3) is a stress-response gene that is involved in numerous cell processes, particularly growth regulation and apoptosis. Wild-type ATF-3 is a transcription factor that regulates the activation of genes involved in cell growth regulation (29). Two isoforms of ATF-3 induce apoptosis through different mechanisms. While the transcription factor ATF-3 derived from splice variant 1 of the gene directly elicits transcriptional changes, variant 4 lacks the leucine zipper region needed to associate with DNA (30). Instead, it induces apoptosis by suppressing the transcription factor nuclear factor-κB (NF-κB), which leads to subsequent inhibition of the downstream anti-apoptotic factors such as Bcl-2 (28,31).
An increase in dietary ω-3 fatty acids has been linked to good prostate health (3,32) and prevention of prostate cancer progression to androgen independence (33). In general, there is good concurrence that fish oil is beneficial in reducing the risk of prostate cancer. However, there are several studies indicating that men with a high dietary intake of ALA have a >3-fold increase in prostate cancer development (34,35). The data on the efficacy of individual fatty acids and their effects on cellular mechanisms are less defined. Therefore, it is important to understand how individual members of the ω-3 fatty acids are able to have discordant effects on cancer risk and prognosis. To better understand the effects of individual fatty acids and to gain insight into potential mechanisms that may benefit prostate cancer patients, the prostate cancer cell lines PC-3 and LNCaP, which represent androgen-independent and -dependent disease, respectively, were used in this study to determine whether PUFAs have a suppressive effect on cell proliferation. We found that, although all the fatty acids decreased the cell viability of androgen-dependent and -independent cell lines, they were demonstrated to have different rates of activity. These findings are significant for dietary recommendations as well as the potential design and use of supplements in the prevention of prostate cancer.
Materials and methods
Cell culture
The prostate cell lines PC-3 and LNCaP were obtained from The University of Texas MD Anderson Cancer Center (Houston, TX, USA). PC-3 cells were grown in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (DMEM/F-12; Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT), 100 units of penicillin and 100 μg/ml of streptomycin (Invitrogen, Carlsbad, CA, USA). LNCaP cells were grown in RPMI medium supplemented with 10% FBS, 100 units of penicillin and 100 μg/ml of streptomycin.
Fatty acid-albumin conjugates
The fatty acids were conjugated to fatty acid-free bovine serum albumin (BSA). This was accomplished following established protocols maintaining a molar ratio of 4:1 (fatty acid:BSA). Fatty acid conjugates were stored under argon at −20°C.
Prostate tissue
Prostatectomy samples were obtained from untreated patients. Samples were flash-frozen and stored at −80°C. Nine sections were obtained, each 10-μm thick, and placed on slides. One section was hematoxylin and eosin (H&E)-stained to confirm pathology (either normal prostate or Gleason grade 4 and 5). The remaining eight sections were examined under light microscopy and compared to H&E staining. Tumor, which was present in >80% of the tissue, was macrodissected away from the remaining fibrous tissue and pooled for RNA isolation. Paraffin-embedded samples from the same prostatectomy samples were sectioned in 5-μm sections placed on charged slides for immunohistochemistry for FASN and SREBP-1.
In vitro proliferation assay
PC-3 (1,000 cells/well) and LNCaP (2,500 cells/well) cells were seeded in white, 96-well culture plates (Greiner Bio One, Frickenhausen, Germany) in the appropriate medium. The following day, the cell media were changed to the appropriate medium supplemented with 0.1% FBS. The following day, the media were removed and fresh media containing 1% FBS and 100 μM-conjugated fatty acid/BSA conjugate was added (n≥4). Cell viability or growth was measured using the CellTiter-Glo® Luminescent Cell Viability assay (Promega, Madison, WI, USA).
RNA analysis
Cells were lysed in Tri-reagent (Sigma, St. Louis, MO, USA) and total RNA was extracted following the manufacturer’s protocol. For normal prostate tissue (NPT) and prostate cancer tissue (PCT), samples were snap-frozen in liquid nitrogen and frozen tissue was stored at −80°C. NPT samples were homogenized in Tri-reagent and total RNA was extracted. Frozen PCT samples were cut into 20-μm sections and mounted on glass slides. A random slide from the dissected tissue was stained with hematoxylin for histological examination. Slides containing ≥80% tumor were analyzed. Tissue from the adjacent 10 slides was scraped into Tri-reagent and total RNA was isolated. One microgram of total RNA from each slide was reverse transcribed using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). cDNA was diluted and real-time PCR was conducted with an ABI 7300 thermal cycler using SYBR-Green PCR Master mix (Applied Biosystems). All the mRNA expression data were corrected using RPL13α expression for normalization prior to graphing and analysis.
The primer sequences used in this study were: ATF3: F, TCACTGTCAGCGACAGACCC and R, CTACCTCGGCTTTTGTGATGG; Bcl-2: F, GCATGCGGCCTCTGTTTGATTTCT and R, AGGCATGTTGACTTCACTTGTGGC; Bcl-6: F, AAGACCGTCCATACCGGTGAGAAA and R, GCAGGTTTCGCATTTGTAGGGCTT; Cox-2: F, AAGTGCGATTGTACCCGGAC and R, CGGTGTTGAGCAGTTTTCTCC; cyclin A2: F, GCATGTCACCG TTCCTCCTT and R, GTGAACGCAGGCTGTTTACTGT; cyclin D1: F, AACCTGAGGAGCCCCAACA and R, GAA GCGTGTGAGGCGGTAGTA; cyclin D2: F, TGTCTCAAAGCTTGCCAGGA and R, CAGGCTATTGAGGAGCACCG; FASN: F, AGGAGCAAGGCGTGACCTT, and R, ACAACGAGCGGATGAGCTG; IL-1β: F, CACGGCCACATTTGGTTCTAA and R, CAGAATGTGGGAGCGAATGAC; IL-6: F, GCCACTCACCTCTTCAGAACG and R, CCGTCGAGGATGTACCGAATT; IL-10: F, GGGAGCCCCTTTGATGATTAA and R, GGGAATCCCTCCGAGACACT; MCP-1: F, ATAGCAGCCACCTTCATTCC and R, TGCACTGAGATCTTCCTATTGG; NF-κB: F, AGGCTATGCAGCTTGCAAAGAG and R, TGTCACCGCGTAGTCGAAAAG; p21: F, GCAGAGGAAGACCATGTGGAC and R, GCGAGGCACAAGGGTACAAG; p57: F, CGGCGATCAAGAAGCTGTC and R, GCTTGGCGAAGAAATCGGA; RPL13α: F, CATCGTGGCTAAACAGGTACTG and R, GCACGACCTTGAGGGCAGCC; SREBP-1c: F, CCATCTGTGAGAAGGCCAGTG and R, GGTGTGGTAGCCAGGCTGTC; TNFα: F, ATCAATCGGCCCGACTATCTC and R, TGGATGTTCGTCCTCCTCACA; TGF-β1: F, CTATTGCTTCAGCTCCACGGA and R, AGGTCCTTGCGGAAGTCAATG.
Immunohistochemistry of prostatectomy samples
Paraffin-embedded tumor tissues were sectioned 5-μm thick and mounted on positively charged gold plus microscope slides. Tissue slides were pre-heated at 60°C for 16 h and dewaxed by immersion in xylene and then in successively diluted solutions of ethanol. Antigen retrieval was achieved by heating the slides at 70°C for 4 h, immersed in Borg Decloaker solution (BioCare Medical, Concord, CA, USA). Endogenous peroxidase activity was blocked by incubating in 3% H2O2 in phosphate-buffered saline (PBS) for 12 min. After rinsing with PBS three times for 3 min each, non-specific tissue binding was blocked for 1 h in protein block solution (Cyto Q Immuno Diluent buffer; Innovex Biosciences, Richmond, CA, USA). The primary antibody was diluted in protein block solution and incubated overnight at 4°C. Dilutions of primary antibodies were as follows: Fatty Acid Synthase antibody (dilution, 1:800; cat. no. NB400-114) (Novus Biologicals, Littleton, CO, USA), and SREBP-1 antibody (dilution, 1:800; cat. no. NB100-2215) (Novus Biologicals). Slides were washed with PBS three times for 3 min each followed by Mach 4™ Universal HRP polymer (BioCare Medical) application for 20 min as a secondary antibody. Staining was visualized by incubation in 3,3′-diaminobenzidine (DAB) and counterstained with Gill’s no. 3 hematoxylin. Internal negative control samples were exposed to protein block solution instead of the primary antibodies and demonstrated no specific signaling. Slides were dried and mounted with Universal Mount solution (Research Genetics, Invitrogen, Carlsbad, CA, USA). Slides were viewed using a Leica DM100 microscope with Leica objectives HCX PL Fluotar 10×/0.30 and HCX PL Fluotar 20×/0.50 with an Applied Scientific Imaging MS-2000 motorized, XY encoded stage. Images were captured with a Qimaging camera and QCapture 2.90.1 imaging software.
Annexin staining
PC3 cells were plated at 300,000 cells/ plate in 60-mm dishes and were serum-starved overnight prior to treatment with fatty acids. Following 24, 48, and 72 h of fatty acid treatment, growth medium was removed from each culture dish and transferred to conical tubes. The cells were removed from plates by scraping, pooled with media from the corresponding plate, and pelleted using centrifugation. The resulting pellet was stained with Annexin CF 488 using the components from a MitoDamage kit obtained from EDM Millipore (Billerica, MA, USA). The manufacturer’s instructions were followed, with the exception of omitting steps for staining with MitoSense Red Dye. 7-AAD DNA intercalating dye was added to identify dead cells, which stained positive for 7-AAD only, and cells in late apoptosis, which stained positive for both 7-AAD and Annexin V. Stained samples were analyzed using the Beckman Coulter Cytomics FC, with only the blue laser turned on. Results are the mean of three independent experiments.
Statistical analysis
Data were presented as the mean of replicates with error bars representing population standard error of the mean (SEM). Statistical significance was determined with all the groups using one-way ANOVA and the paired Student’s t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
ω-3 FAs inhibits androgen-dependent and -independent prostate cancer cell growth
To determine whether all ω-3 fatty acids suppressed proliferation to a similar extent, we investigated the effect of individual fatty acids, (ALA, DHA and EPA) on the growth of prostate cancer cell lines PC-3 and LNCaP. Proliferation was measured using the CellTiter-Glo® Luminescent Cell Viability assay, and results were validated with BrdU staining (data not shown) to ensure that CellTiter-Glo® results were representative of cell proliferation. Androgen-independent PC-3 cells treated with 100 μM ω-3 fatty acids showed a marked decrease in proliferation and survival over a 4-day time course experiment (Fig. 1A). While all the fatty acids caused a decrease in the cell number, the fatty acids worked at different rates, resulting in different overall inhibition patterns. DHA was the most rapid and most effective fatty acid in inhibiting cell viability, followed closely by EPA. ALA was the least active of the ω-3 fatty acids examined at inhibiting cell growth.
Figure 1.
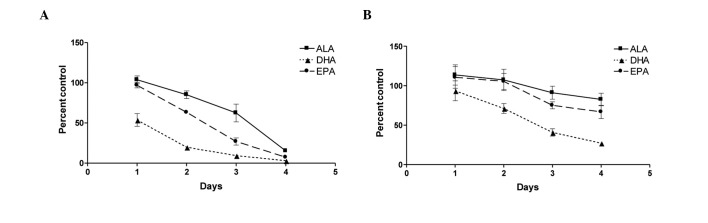
ω-3 fatty acids differentially inhibit androgen-independent and -dependent prostate cancer cell line growth. (A) PC-3 and (B) LNCaP cells were treated with 100 μM α-linolenic (ALA), docosahexaenoic (DHA) or eicosapentaenoic (EPA) acid conjugated to BSA following overnight serum starvation. Cell proliferation was measured using the CellTiter-Glo® Luminescent Cell Viability assay. Three trends of inhibition emerged in PC-3 and LNCaP cells treated with the ω-3 PUFAs. DHA inhibited the two cell lines rapidly and to the greatest extent. EPA also effectively inhibited cancer cell growth, albeit more gradually compared to DHA. PC-3 cells treated with EPA reached approximately the same endpoint compared to DHA-treated cells, ultimately exhibiting a similar magnitude of inhibition. ALA inhibited cells more slowly and to a lesser extent compared to DHA and EPA. While the same inhibitory trends were observed in androgen independent and dependent cell lines, the more protracted response in LNCaP cells may be attributed to their slower rate of cell division. Values are shown as the mean of replicates from four independent experiments with error bars indicating standard error.
The androgen-dependent LNCaP cells exhibited a similar, albeit less pronounced, trend of inhibition of proliferation over the same time period (Fig. 1B). The differences in inhibition are attributed to the slower rate of cell cycle/division of LNCaP cells. Since the inhibitory trends were more pronounced in the more rapidly dividing PC-3 cells, cell cycle arrest, gene analysis and apoptosis experiments were conducted on this cell line.
Transcriptional modulation of cell cycle regulation and apoptotic genes
To elucidate the possible mechanism(s) for the differences between DHA and EPA compared to ALA, the transcript levels of several genes involved in cell cycle regulation and apoptosis were investigated to determine the effects of treatment with ω-3 fatty acids (Table I). Cell cycle gene mRNAs including cyclins A2, D1 and D2, CDK4, p21 (CDKN1A) and p57 (CDKN1C) were examined after a 24-h treatment with ω-3 fatty acids. None of the fatty acids had a significant effect on cyclin D1 or CDK4 transcript levels. A slight, albeit statistically significant increase was identified in cyclin D2 and p57 transcript following treatment with DHA, although not with ALA or EPA. All the fatty acids had an effect on cyclin A2 mRNA; ALA slightly increased cyclin A2 expression while both DHA and EPA decreased its transcript levels. Expression of p21, a cyclin-dependent kinase inhibitor, was significantly increased by treatment with DHA and EPA, but not with ALA.
Table I.
Gene regulation by ω-3 fatty acids.
| Cellular process | Gene | ALA | DHA | EPA |
|---|---|---|---|---|
| Apoptosis | ||||
| ATF-3 | 1.23±0.11 | 11.14±1.95a | 2.82±0.29 | |
| Bcl-2 | 0.73±0.10 | 0.58±0.13 | 0.33±0.06b | |
| Bcl-6 | 0.86±0.09 | 1.82±0.14a | 1.04±0.05 | |
| NF-κB | 0.79±0.10 | 0.70±0.17 | 0.63±0.08 | |
| Cell cycle | ||||
| Cyclin A2 | 1.35±0.04a | 0.20±0.02a | 0.18±0.02a | |
| Cyclin D1 | 0.86±0.08 | 0.87±0.18 | 0.98±0.10 | |
| Cyclin D2 | 1.07±0.36 | 2.55±0.42b | 1.33±0.49 | |
| CDK4 | 1.04±0.06 | 0.72±0.09 | 0.76±0.04 | |
| p21 | 1.26±0.12 | 5.02±0.83a | 2.79±0.35b | |
| p57 | 0.92±0.12 | 1.84±0.27b | 1.02±0.13 | |
| Inflammation | ||||
| Cox-2 | 0.95±0.10 | 5.94±1.54b | 3.02±0.38 | |
| IL-1β | 1.63±0.25 | 2.58±0.87 | 2.91±0.29b | |
| IL-6 | 1.44±0.24 | 13.66±1.33a | 14.16±1.44a | |
| IL-8 | 0.99±0.16 | 1.43±0.08 | 1.47±0.25 | |
| IL-10 | 0.75±0.09 | 5.29±0.98b | 1.94±0.32 | |
| MCP-1 | 0.43±0.04a | 0.04±0.01a | 0.21±0.02a | |
| TGF-β1 | 0.83±0.05 | 0.65±0.06 | 0.93±0.24 | |
| TNF-α | 0.44±0.09 | 0.88±0.28 | 0.60±0.11 | |
| Metabolism | ||||
| FASN | 0.44±0.06a | 0.29±0.017a | 0.49±0.06a | |
| LDLR | 0.67±0.12 | 1.27±0.56 | 0.86±0.18 | |
| SREBP-1 | 0.45±0.09b | 0.46±0.22b | 0.53±0.30b | |
| SREBP-2 | 0.88±0.28 | 1.40±0.46 | 1.30±0.37 |
P<0.001,
P<0.05, compared to the control. Bold values indicate statistical significance which was determined using one-way ANOVA. RNA was isolated from PC-3 cells treated with 100 μM fatty acids for 24 h. Real-time PCR was used to quantify the indicated genes. Levels were normalized to the housekeeping gene RPL13α and fold change is provided. ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Growth inhibition of prostate cancer cell lines by ω-3 fatty acids may also be the result of apoptotic induction. ATF-3, Bcl-2, Bcl-6 and NF-κB mRNA expression levels were examined 24 h after treatment with ω-3 fatty acids (Table I). The greatest change in gene expression was observed in DHA-treated cells, which caused a statistically significant increase in ATF-3 mRNA. EPA had a significant 5-fold induction of ATF-3 expression, which peaked at 12 h (data not shown). DHA-treated cells were also demonstrated to have a modest, albeit significant increase in Bcl-6 gene expression with no significant effect on Bcl-2 gene expression. All the fatty acids decreased Bcl-2 transcript levels, although only EPA treatment resulted in a statistically significant decrease. None of the fatty acids significantly altered NF-κB mRNA expression (Table I). Additionally, NF-κB activity was not altered by treatment with the three fatty acids, which was proven using a NF-κB reporter assay (data not shown).
Fish oil ω-3 fatty acids induce apoptosis
Adherent PC-3 cells were pooled with dead cells from the supernatant in each dish for staining and flow cytometric analysis was performed. Throughout the 3 days, DHA-treated cells exhibited the highest rates of apoptosis, as demonstrated by positive staining with Annexin V. Prostate cancer cells treated with EPA showed intermediate rates of apoptosis throughout the time course, while ALA-treated cells exhibited no apoptosis above baseline levels (Fig. 2). Subsequent comparison of results from flow cytometric and proliferation analyses demonstrated that the rates of apoptosis and death for each fatty acid complemented the live cell counts (Table II). Based on these comparisons, apoptosis was determined to be the primary mechanism of proliferation inhibition in DHA-treated samples. Although ALA did not induce cells to undergo apoptosis, cell death was induced. Data collected on day 3 of ALA treatment showed that, while apoptosis in ALA-treated cells never exceeded baseline rates, the percentage of dead cells was significantly elevated compared to vehicle control-treated samples. Similarly, while apoptosis was observed in EPA-treated cells, the percentages of dead cells exceeded the percentage of apoptotic cells throughout the experiment, indicating that all deaths following EPA treatment cannot be attributed to apoptosis (Table II). Subsequently, we examined additional mechanisms that are potentially be responsible for PC-3 growth inhibition by ω-3 fatty acids.
Figure 2.
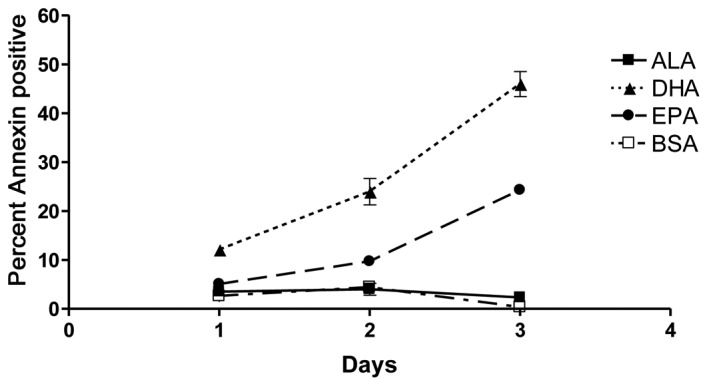
Fish oil ω-3 fatty acids induce cell apoptosis. PC-3 cells were treated with 100 μM α-linolenic (ALA), docosahexaenoic (DHA) or eicosapentaenoic (EPA) acid conjugated to bovine serum albumin (BSA) following overnight serum starvation. Cells and supernatants were collected every 24 h for 3 days and analyzed for apoptosis (annexin staining) and cell death (7-AAD staining) by flow cytometry. DHA significantly induced apoptosis within 24 h, demonstrating the greatest overall induction. EPA also significantly induced apoptosis, but at a later stage compared to DHA and not to the same extent. ALA did not induce apoptosis above BSA control levels. Significance was assessed using one-way analysis of variance (ANOVA). Values are shown as the mean of replicates from three independent experiments with error bars indicating standard error.
Table II.
Cell apoptosis induced by ω-3 fatty acids.
| Day | Treatment | CellTiter-Glo® (% control cells) | Flow cytometry
|
|
|---|---|---|---|---|
| % live cells | % apoptotic cells | |||
| 1 | BSA | 100.00±11.43 | 91.8±1.2 | 2.63±1.01 |
| ALA | 103.75±14.25 | 85.24±1.93 | 3.50±0.82 | |
| DHA | 53.67±24.08a | 74.22±0.67b | 12.10±0.89b | |
| EPA | 97.02±10.32 | 76.12±1.87b | 5.06±1.34 | |
| 2 | BSA | 100.00±24.71 | 87.16±1.95 | 4.42±1.97 |
| ALA | 85.06±14.25 | 85.15±3.50 | 4.01±2.13 | |
| DHA | 19.86±5.40a | 60.34±4.68b | 23.94±4.63b | |
| EPA | 63.17±4.08a | 70.4±1.85b | 9.68±1.60 | |
| 3 | BSA | 100.00±26.77 | 92.44±0.38 | 0.36±0.13 |
| ALA | 62.48±33.17b | 78.62±2.59 | 2.31±0.37 | |
| DHA | 9.35±7.80a | 28.43±3.34b | 45.94±4.46b | |
| EPA | 26.85±13.46a | 35.75±3.39b | 24.29±1.65b | |
P<0.001,
P<0.01, compared to the control. Bold values indicate statistical significance using one-way ANOVA. PC-3 cells were treated with 100 μM fatty acids for 3 days. Cells were cultured in 96-well plates and analyzed by CellTiter-Glo® assay. Values were compared to control (BSA-treated) cells. Cells were grown in 60-mm dishes and analyzed by flow cytometry following annexin staining. Percentage of apoptotic and dead cells represent the percentage of total cells collected. ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Transcriptional modulation of inflammatory genes
Elevated expression levels of pro-inflammatory cytokines are associated with poor prognosis in many tumor types including prostate cancer. Transcript levels of Cox-2, IL-1β, IL-6, IL-8, IL-10, MCP-1, TGF-β1 and TNF-α were examined following a 24-h treatment with 100 μM fatty acids (Table I). None of the fatty acids had a significant effect on IL-8, TGF-β1 or TNF-α. IL-1β transcript levels increased slightly following EPA treatment. All of the fatty acids significantly lowered the expression of MCP-1 in a pattern similar to growth inhibition. In gene expression and proliferation assays, DHA was the most effective, followed by EPA and then ALA. Only DHA significantly increased the mRNA levels of the pro-inflammatory gene Cox-2 and the anti-inflammatory gene IL-10. Notably, DHA and EPA treatment increased IL-6 trascript levels.
ω-3 fatty acids regulate metabolizing genes
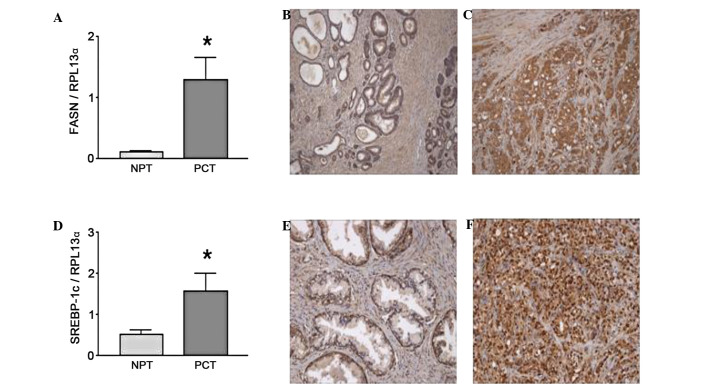
FASN has been suggested to be a prostate cancer oncogene (36). FASN RNA transcript and the protein have been shown to be increased in prostate cancer Gleason grade 4 and 5 tumor samples compared to normal prostate tissue (37), as shown in Fig. 3A by real-time PCR and confirmed by immunohistochemistry (IHC) (Fig. 3B vs. C). FASN mRNA expression was decreased by 50% in PC-3 cells following treatment with ω-3 fatty acids (Table I) with no significant difference between fatty acid treatments. FASN is regulated by the sterol response element binding protein-1c (SREBP-1c), which is also increased in tumor samples (Fig. 3D). In addition, IHC results demonstrated a translocation of SREBP-1 to the nucleus in prostate cancer tumor cells (Fig. 3F) compared to normal prostate tissue (Fig. 3E) where SREBP-1 was predominantly located in the cytoplasm. SREBP-1c mRNA was decreased to a comparable extent by all ω-3 fatty acid treatments (Table I). The expression of caveolin-1 (Cav-1), a gene negatively regulated by SREBP-1c, was examined to validate the downstream effects of SREBP-1c. As expected, the RNA expression pattern of Cav-1 inversely followed the pattern of SREBP-1c levels in both prostate cancer and control cell lines (data not shown). None of the fatty acid treatments had any effect on Low Density Lipoprotein Receptor (LDLR) or SREBP-2.
Figure 3.
Fatty acid synthase (FASN) and its regulator sterol response element binding protein-1c (SREBP-1c) are upregulated in clinical prostate cancer samples. Normal prostate (NPT) and prostate cancer tissues (PCT) isolated from patients at prostatectomy were analyzed and compared by real-time PCR gene expression and immunohistochemistry. RNA was isolated from the clinical samples or PC-3 cells treated with ω-3 PUFAs and gene expression was assessed using quantitative real-time PCR. Clinical samples were demonstrated to have elevated transcript levels of (A) FASN and (D) its regulator SREBP-1c when compared to normal prostate tissue. These trends were immunohistochemically confirmed. Normal prostate tissue was demonstrated to have low levels of (B) FASN and (E) SREBP-1c expression (magnification, ×200), compared to Gleason grade 5 tumor showing (C) FASN in the cytoplasm and (F) nuclear staining of SREBP-1c (magnification, ×400). (A and D) For the clinical samples, statistical significance was assessed using the paired Student’s t-test; *P<0.05.
FASN inhibition suppresses PC-3 proliferation in a similar pattern to ALA
Chemical and siRNA inhibition of FASN leads to slower proliferation and ultimately apoptosis in prostate cancer cells (36). Since all the ω-3 fatty acids inhibited FASN gene transcription to a similar extent, we examined whether this repression resulted in growth inhibition of cells similar to the chemical repression of FASN. Cerulenin, a chemical inhibitor of fatty acid synthase, was used as a positive control. Dose-response data indicated that cerulenin inhibited PC-3 cell growth with an EC50 of 2.5 μM (data not shown). The effect of cerulenin at 2 μM was compared to the effect of the ω-3 fatty acid treatments (100 μM) on PC-3 cell growth (Fig. 4). DHA and EPA inhibited growth more rapidly and to a greater extent compared to either cerulenin or ALA, which showed an identical extent of growth suppression.
Figure 4.
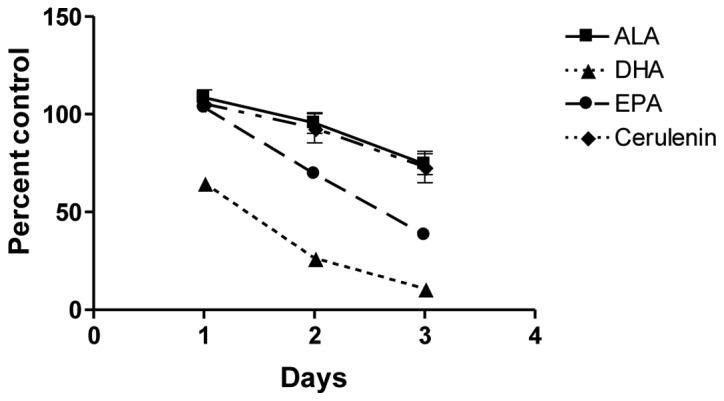
α-linolenic acid (ALA) inhibits PC-3 cell proliferation in an identical pattern as FASN inhibition. PC-3 cells were treated with 100 μM of BSA-conjugated ω-3 fatty acids or 2 μM of the FASN inhibitor cerulenin following overnight serum starvation. Cell proliferation was measured using the CellTiter-Glo® Luminescent Cell Viability assay. In a time course experiment, ALA produced an identical viability curve to cerulenin, while DHA and EPA inhibited PC-3 cell growth more rapidly and effectively, suggesting the activation of additional anti-proliferative pathways by the fish oils. Values are shown as the mean of replicates with error bars representing standard error. ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Discussion
The treatment of androgen-independent (PC-3) and -dependent (LNCaP) prostate cancer cell lines with individual ω-3 fatty acids at 100 μM inhibited cell growth in a time-dependent manner. This concentration was selected since several previous studies have demonstrated that basal level in subjects fed balanced diets yielded plasma concentrations for DHA >80 μM and EPA in the range of 25–30 μM (38–40). Furthermore, supplementation with fish oil increased DHA and EPA to serum concentrations >125 μM (38). For consistency reasons, ALA was examined at the same concentration even though most studies indicate that basal levels are lower compared to those for DHA and EPA with ranges from 7 to 25 μM (39–41) and supplementation levels only reaching 35 μM (40).
All the ω-3 fatty acids effectively inhibited the proliferation of PC-3 and LNCaP prostate cancer cells. The androgen-dependent LNCaP cell line demonstrated a delay in response that may be attributed to the slower cell division observed in this cell line as compared to PC-3 cells. Based on the fact that DHA leads to a more rapid and extensive effect on cell proliferation and gene expression, there appear to be different mechanisms of inhibition by the ω-3 PUFAs. DHA was the most efficient and potent inhibitor of PC-3 proliferation, followed by EPA and then ALA. The observation that ALA was the least toxic of the fatty acids examined indicates that, although the 100 μM concentration has not been reported in vivo, overt toxicity with this molecule in the cell model used in this study is unlikely. To examine whether the differences observed in the viability assay were a result of mechanistic differences, we investigated the pathways involved in fatty acid metabolism, cell cycle regulation, and apoptosis.
All the ω-3 PUFAs examined modulated fatty acid synthesis by decreasing the accumulation of SREBP-1c mRNA. Moreover, additional studies have shown that ω-3 fatty acids inhibit the cleavage of inactive to active SREBP-1c (10). In prostate cancer tissue, the majority of SREBP-1 staining is nuclear compared to cytosolic in the normal prostate tissue indicating the activation of SREBP-1. The inhibition of SREBP-1c, a major regulator of FASN, leads to accumulation of cholesteryl esters within the cell, resulting in cell cycle arrest (42). Since fatty acid metabolism is an important source of energy for prostate cancer cells, the inhibition of SREBP-1c and its target gene FASN is likely to have contributed to the decrease in PC-3 and LNCaP cell viability observed in this study following treatment with the ω-3 fatty acids. The low density lipoprotein receptor pathway is important in providing cells with essential fatty acids, especially those for prostaglandin synthesis. None of the ω-3 fatty acids used in this study altered the transcription of LDLR or SREBP-2, a potent regulator of the LDLR promoter, indicating that this pathway was not involved in the observed inhibition. While all the ω-3 fatty acids examined resulted in equal inhibition of SREBP-1c and FASN (∼50% reduction), they exhibited different efficiencies in the inhibition of proliferation. This suggested that alternative apoptotic or anti-proliferative mechanisms needed to be invoked to cause the more substantial inhibitory trends observed following DHA and EPA treatment of PC-3 and LNCaP cells.
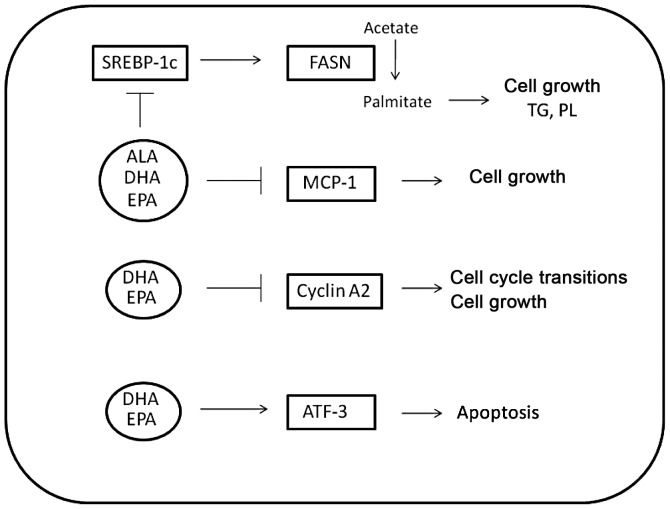
Differences in PC-3 proliferation and cell viability over time may be attributed to the differential regulation by the ω-3 fatty acids of several target genes involved in cell cycle, inflammation and apoptosis (Fig. 5). Of particular importance to the androgen-independent prostate cancer model studied was the inhibition of MCP-1 by all the ω-3 fatty acids used, with DHA and EPA exhibiting inhibition of ∼95 and 80% decrease compared to control, respectively. Specifically, the pattern of MCP-1 mRNA inhibition mimics the pattern of growth inhibition. MCP-1 not only acts as an autocrine growth factor for prostate cancer, but it is also involved in the hypoxic response and angiogenesis of primary tumors (22). In prostate cancer that has metastasized to bone, MCP-1 is involved in the paracrine modulation of osteoblast and osteoclast activity, leading to osteoclastogenesis and alteration of the bone matrix (20). Differential modulation of MCP-1 in prostate cancer cells results in growth inhibition and decrease of metastatic ability as well as success of androgen-independent prostate cancer.
Figure 5.
Cellular pathways involved in fatty acid metabolism, inflammation, cell cycle regulation and apoptosis are differentially modulated by ω-3 fatty acids. The expression of all the genes was quantified in PC-3 cells using quantitative real-time PCR. ALA, DHA and EPA inhibited fatty acid synthase (FASN) and its regulator sterol response element binding protein-1c to 50% of control levels. All three ω-3 PUFAs also inhibited the inflammatory protein and autocrine prostate cancer growth factor MCP-1, although the extent of inhibition varied. DHA caused the most effective inhibition, with MCP-1 levels decreasing to 10% of control levels in DHA-treated PC-3 cells, while EPA inhibited MCP-1 transcript levels by 30%, and ALA caused a decrease to 50% of control. DHA and EPA reduced the transcript levels of cyclin A2 to 20% of control levels to reduce cell proliferation. Only DHA activated ATF-3, leading to the inhibition of anti-apoptotic factors downstream of NF-κB, thereby inducing apoptosis in PC-3 cells.
Considering the known role of ω-3 fatty acids as anti-inflammatory substances, the 13-fold induction of IL-6 in DHA- and EPA-treated samples and the >3-fold induction of COX-2 constituted contradictory findings. Since the chronic expression of IL-6 and Cox-2 has been correlated with the development and progression of prostate cancer and multi-drug resistance (19,24,26), a decreased expression of these genes following fish oil treatment was expected. Although IL-6 is induced at the early 24-h time point, in DHA- and EPA-treated PC-3 cells, its expression rapidly decreased to half the original induction at 48 h (data not shown). Therefore, induction of IL-6 by these ω-3 fatty acids does not appear to cause the chronic inflammatory response that promotes carcinogenesis and growth. Both genes could be regulated by NF-κB. However, there were no alterations in the transcript levels for this transcription factor. In addition, none of the fatty acids increased NF-κB activity as determined by an NF-κB reporter assay. The involvement of NF-κB in sensitizing prostate cells to growth arrest by DHA has been previously reported (43). However, the cell models used in various studies differ and the lack of NF-κB reporter activity in PC-3 cells suggests that NF-κB is not involved in the induction of IL-6 and Cox-2 expression in DHA- and EPA-treated cells.
DHA also increased the expression of genes involved in induction of apoptosis to a greater extent compared to the other fatty acid treatments. The induction of apoptosis is confirmed by the annexin staining of PC-3 cells treated with DHA and EPA. DHA induced annexin staining earlier and to a greater extent compared to EPA, while ALA-treated cells showed no staining above background. In a comparison analysis, cell growth was inhibited by all the fatty acids as measured by CellTiter-Glo® assay and flow cytometry. The differences in the extent of inhibition presented in Table II could be a result of the end point measurement. Flow cytometry measured live or dead cells based on membrane integrity (dye uptake), while the CellTiter-Glo® assay measured the amount of ATP present. The ATP value would decrease prior to loss of membrane integrity. Furthermore, although there appear to be differences in statistical significance at certain time points between the two assays, as shown by ANOVA tests for each assay, most viability values attained are comparable, and may be statistically equivalent (unpaired Student’s t-tests show viability measurements following 72 h of ALA, and EPA treatment according to CellTiter-Glo® and flow cytometry are statistically equivalent). Both fish oils induced apoptosis in PC-3 cells. ATF-3 is the most modulated pro-apoptotic gene identified. It has been reported that the transfection of PC-3 cells with an ATF-3 expression construct resulted in apoptosis (31). Additionally, the activation of KLF-6, which activates ATF-3 in PC-3 and LNCaP cells, resulted in apoptotic death of the cells due to the induced apoptosis by inhibiting NF-κB activity. While our data demonstrate that NF-κB pathways are not involved, these results clearly indicate that DHA activates an ATF-3-dependent apoptotic pathway in androgen-independent prostate cancer cells.
DHA consistently inhibited gene expression of cell cycle promoting genes and affected genes involved in the induction of apoptosis to a greater extent compared to the other fatty acid treatments. DHA was the most effective fatty acid in decreasing prostate cancer cell viability, since it targeted anti-inflammatory/anti-proliferative and pro-apoptotic genes and induced more substantial changes compared to the other ω-3 fatty acids examined in this study. Taken together, these results may indicate that the different efficacies of the fatty acids examined in decreasing cell viability may be due to not only the modification of different pathways within androgen-independent prostate cancer cells, but also to different magnitudes of response. Encouragement of patients to alter their diets in favor of fish products or adding ω-3 fatty acid supplements rich in DHA to their diets should be considered. The ω-3-acid ethyl esters contained in the hypertriglyceridemia medication Lovaza™ could also be considered in prostate cancer treatment or prevention strategies.
Acknowledgments
The authors would like to acknowledge the Microscopy and Cytometry Facilities of the Huck Institutes of the Life Sciences (University Park, PA, USA) for the support in flow cytometry procedures. This study was supported by the National Cancer Institute (grant no. P50 CA140388).
References
- 1.Rambeaud JJ. Intermittent complete androgen blockade in meta-static prostate cancer. Eur Urol. 1999;35(Suppl 1):32–36. [PubMed] [Google Scholar]
- 2.Harris KA, Reese DM. Treatment options in hormone-refractory prostate cancer: current and future approaches. Drugs. 2001;61:2177–2192. doi: 10.2165/00003495-200161150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 4.King IB, Kristal AR, Schaffer S, Thornquist M, Goodman GE. Serum trans-fatty acids are associated with risk of prostate cancer in beta-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2005;14:988–992. doi: 10.1158/1055-9965.EPI-04-0517. [DOI] [PubMed] [Google Scholar]
- 5.Haaland CM, Heaphy CM, Butler KS, Fischer EG, Griffith JK, Bisoffi M. Differential gene expression in tumor adjacent histologically normal prostatic tissue indicates field cancerization. Int J Oncol. 2009;35:537–546. doi: 10.3892/ijo_00000365. [DOI] [PubMed] [Google Scholar]
- 6.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 7.Di Nunzio M, van Deursen D, Verhoeven AJ, Bordoni A. n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells. Br J Nutr. 2010;103:161–167. doi: 10.1017/S000711450999167X. [DOI] [PubMed] [Google Scholar]
- 8.Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Nakamura MT, Cho HP, Clarke SD. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- 10.Nakatani T, Kim HJ, Kaburagi Y, Yasuda K, Ezaki O. A low fish oil inhibits SREBP-1 proteolytic cascade, while a high-fish-oil feeding decreases SREBP-1 mRNA in mice liver: relationship to anti-obesity. J Lipid Res. 2003;44:369–379. doi: 10.1194/jlr.M200289-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 12.Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745–2747. [PubMed] [Google Scholar]
- 13.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 14.Neumann F. Pharmacology and clinical use of antiandrogens: a short review. Ir J Med Sci. 1982;151:61–70. doi: 10.1007/BF02940148. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard-Moguilewsky M. Pharmacology of antiandrogens and value of combining androgen suppression with antiandrogen therapy. Urology. 1991;37:5–12. doi: 10.1016/0090-4295(91)80095-o. [DOI] [PubMed] [Google Scholar]
- 16.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 17.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cansino Alcaide JR, Vera San Martin R, Rodriguez de Bethencourt Codes F, Bouraoui Y, Rodriguez Berriguete G, Oueslati R, Perez-Utrilla M, De la Pena Barthel J, Paniagua Gomez-Alvarez R, Royuela Garcia M. Prostatic specific antigen (PS), pro-inflammatory cytokines, and prostatic pathology (benign prostatic hyperplasia and cancer). Relationship with malignancy. Arch Esp Urol. 2009;62:359–366. doi: 10.4321/s0004-06142009000500005. (In Spanish). [DOI] [PubMed] [Google Scholar]
- 19.Bouraoui Y, Ricote M, Garcia-Tunon I, Rodriguez-Berriguete G, Touffehi M, Rais NB, Fraile B, Paniagua R, Oueslati R, Royuela M. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008;32:23–32. doi: 10.1016/j.cdp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 22.Shi CL, Yu CH, Zhang Y, Zhao D, Chang XH, Wang WH. Monocyte chemoattractant protein-1 modulates invasion and apoptosis of PC-3M prostate cancer cells via regulating expression of VEGF, MMP9 and caspase-3. Asian Pac J Cancer Prev. 2011;12:555–559. [PubMed] [Google Scholar]
- 23.Hsieh TC, Chiao JW. Growth modulation of human prostatic cancer cells by interleukin-1 and interleukin-1 receptor antagonist. Cancer Lett. 1995;95:119–123. doi: 10.1016/0304-3835(95)03876-x. [DOI] [PubMed] [Google Scholar]
- 24.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159:2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Kim JS, Papadopoulos J, Choi HJ, He J, Maya M, Langley RR, Fan D, Fidler IJ, Kim SJ. Consistent interactions between tumor cell IL-6 and macrophage TNF-α enhance the growth of human prostate cancer cells in the bone of nude mouse. Int Immunopharmacol. 2011;11:862–872. doi: 10.1016/j.intimp.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyprianou N. Apoptosis: therapeutic significance in the treatment of androgen-dependent and androgen-independent prostate cancer. World J Urol. 1994;12:299–303. doi: 10.1007/BF00184107. [DOI] [PubMed] [Google Scholar]
- 28.Raffo AJ, Perlman H, Chen MW, Day ML, Streitman JS, Buttyan R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55:4438–4445. [PubMed] [Google Scholar]
- 29.Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med (Berl) 2009;87:1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen BP, Liang G, Whelan J, Hai T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J Biol Chem. 1994;3:15819–15826. [PubMed] [Google Scholar]
- 31.Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283:29795–29801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–543. doi: 10.1093/ajcn/77.3.532. [DOI] [PubMed] [Google Scholar]
- 33.Friedrichs W, Ruparel SB, Marciniak RA, DeGraffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63:771–777. doi: 10.1080/01635581.2011.570892. [DOI] [PubMed] [Google Scholar]
- 34.De Stefani E, Deneo-Pellegrini H, Boffetta P, Ronco A, Mendilaharsu M. Alpha-linolenic acid and risk of prostate cancer: a case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 2000;9:335–338. [PubMed] [Google Scholar]
- 35.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 36.Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 38.Gillies PJ, Bhatia SK, Belcher LA, Hannon DB, Thompson JT, Vanden Heuvel JP. Regulation of inflammatory and lipid metabolism genes by eicosapentaenoic acid-rich oil. J Lipid Res. 2012;53:1679–1689. doi: 10.1194/jlr.M022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawlosky RJ, Hibbeln JR, Salem N., Jr Compartmental analyses of plasma n-3 essential fatty acids among male and female smokers and nonsmokers. J Lipid Res. 2007;48:935–943. doi: 10.1194/jlr.M600310-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Harper CR, Edwards MJ, DeFilippis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136:83–87. doi: 10.1093/jn/136.1.83. [DOI] [PubMed] [Google Scholar]
- 41.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–551. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Schonberg SA, Lundemo AG, Fladvad T, Holmgren K, Bremseth H, Nilsen A, Gederaas O, Tvedt KE, Egeberg KW, Krokan HE. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 2006;273:2749–2765. doi: 10.1111/j.1742-4658.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 43.Cavazos DA, Price RS, Apte SS, deGraffenried LA. Docosahexaenoic acid selectively induces human prostate cancer cell sensitivity to oxidative stress through modulation of NF-κB. Prostate. 2011;71:1420–1428. doi: 10.1002/pros.21359. [DOI] [PubMed] [Google Scholar]