Abstract
Introduction:
Periodontal disease belongs to a group of diseases with more than one cause, it is a disease of a multifactorial etiology. Although bacteria are the main cause of the disease, immunoinflammatory reaction of the host is responsible for the majority of destructive changes in periodontal tissue. The main issue in the evaluation of the success of periodontal therapy is the pluralism of the bacteria and their dynamic changes during the duration, on the one hand, and the possible inaccuracy of classical microbiological analysis in determination of the dominant role of a microorganism, or the success of its reduction or elimination, on the other. Thanks to advances of microbiology and technological development, it is possible to make an assessment of specific microorganisms in a large number of samples of sub-gingival plaque with extreme precision, using checkerboard DNA-DNA hybridization and method of polymerase chain reaction (PCR). The development of laser technology and the discovery of its significant antimicrobial effects have introduced and presented this treatment modality as a possible auxiliary method of periodontitis treatment.
Materials and Methods:
The sample for the study estimating the efficiency of application of diode lasers in the reduction of periodontal pockets consisted of 1164 periodontal pockets in 24 subjects of both sexes. For laser irradiation of periodontal pockets a diode laser was used, a low-power laser (SmilePro 980, Biolitec, Germany), working in a mode precisely tuned for treatment of periodontal pockets. All subjects underwent: general anamnesis, periodontal status, and orthopantogram radiograph analysis. Following a standard periodontal preparation, a sample of subgingival plaque was collected for molecular-biological analysis (real-time PCR method) prior to laser irradiation of periodontal pockets, immediately following the irradiation, and during the control examination 3 months after irradiation.
Results:
The results of the molecular-biological analysis of target periodontal pathogens Actinobacillus (Aggregatibacter) actinomycetemcomitans (AA) and Porphyromonas gingivalis (PG) isolated from periodontal pockets prior to laser irradiation, immediately after laser irradiation, and at the control examination after 3 months were processed statistically (using real-time PCR method). The results showed that there was a statistically significant decrease in CT values for the tested bacteria immediately after treatment and the control examination, compared with the level of CT values for the same bacteria before treatment.
Conclusions:
Based on the obtained results, we concluded that diode laser irradiation reduces the number of active periodontal pathogens. We believe that the use of diode lasers, as a supplementary method in the treatment of periodontal disease, is extremely useful and efficient, and can be recommended as part of standard clinical practice.
Key words: periodontal disease, periopathogen, diode laser, laser therapy.
1. INTRODUCTION
Periodontal disease belongs to a group of diseases with more than one cause, it is a disease of a multifactorial etiology. The disease is caused by the activity of local etiological factors and causes an immune reaction and interaction with systemic and genetic components in the organism. Although bacteria are the main cause of the disease, immunoinflammatory reaction of the host is responsible for the majority of destructive changes in periodontal tissue (1-10).
Resorption of alveolar bone is the most critical moment in the pathogenesis of periodontal disease and indicates the irreversibility of the pathological process. During the development of periodontal disease, complex mechanisms of bone resorption are noted, which are dominant in relation to the formation of new bone. To understand the nature of this complex pathologic process, it is important to emphasize that the destruction of the alveolar bone caused by inflammation is not necrosis of the bone, but the activity of osteoclastic cells that reduce bone mass. This is not a continuous process; instead, it is characterized by alteration of periods of remission and exacerbation (2-20).
In order for the periodontal pathogens to cause the disease, it is important that they are able to colonize the subgingival space and create agents that either directly damage the tissue of the host or lead to self-destruction of tissue. To colonize subgingival space, bacterial species must be able to adhere to a surface, to multiply, to successfully compete with other bacterial species in the environment, and to defend itself against the defense mechanisms of the host (21-23).
Microorganisms in periodontal pockets are in a state of continuous flux. Periodontal destruction can be a result of a combination of bacterial factors that are changing over time. This is in contrast with most other conventional infectious diseases, where the host counters one microorganism, and the diagnosis is determined through the presence of that particular pathogen.
2. OBJECTIVE
The main objective of this study was to precisely evaluate, using methods of polymerase chain reaction (PCR), certain specific microorganisms in a large number of samples of subgingival plaque, and to assess the effect of a combined therapy on active reduction of pathogens in the pockets following the laser therapy (after standard processing methods).
3. MATERIALS AND METHODS
To test the effect of diode laser on the reduction of targeted anaerobic pathogens in periodontal pockets, samples were taken from the pockets of each patient prior to laser treatment, immediately after the treatment, and during the control examination 3 months later; these samples were analyzed using real-time PCR analysis. This study included 24 adult patients of both sexes with chronic periodontitis who had at least one true pockets of 5mm or deeper in at least two quadrants. The study included subjects without systemic diseases who did not take any antibiotics 3 months prior to proposed treatment. All subjects gave their consent to the treatment of periodontitis with complementary diode laser therapy. Each patient underwent the following protocol:
Anamnesis and clinical-radiological evaluation. Following the information about the general health of the patient and habits (smoking) a clinical examination and orthopantogram analysis were performed, followed by the standard initial periodontal therapy.
Microbiological analysis of subgingival plaque. First samples for molecular-biological analysis were taken from the three deepest pockets of each participant during the third visit (after standard methods of preparation), prior to laser irradiation. The same procedure was repeated following the laser therapy and during the control examination after three months. The sampled area was previously isolated and dried using a cotton swab (without the air syringe). Sterile paper points were then introduced to the bottom of the pocket and left in situ for 10 seconds. When removing the points, care was taken to avoid coming into contact with saliva or the epithelium of the oral cavity. The points were then placed in a tightly sealed sterile tube specifically designed for the transport of the sample to a specialized laboratory for molecular-biological analysis (using real-time PCR method). Molecular-biological analysis was done by trained staff at the Institute of Microbiology, Parasitology and Immunology of the University Clinical Center in Sarajevo, following the isolation protocol. DNA was isolated from the obtained samples using QIAamp DNA mini kit. All submitted samples underwent the same protocol, and after the completion of amplification, analysis of the obtained CT values for each bacterium was performed before laser application, immediately after the treatment, and at the control examination after three months.
Laser irradiation of periodontal pockets. Laser irradiation of periodontal pockets was performed after standard periodontal preparation, using a low-power diode laser (SmilePro 980, Biolitec, Germany), twice in a span of five days. Diode laser was set to the “Parodontologye” mode (Puls 25, On time 0.10s, Off time 0.05s, Fiber Range 300-600◄, Set power 2.0W), which is the factory setting for the treatment of periodontal pockets. Laser therapy was performed by introducing the fiber optic working arm of the into the deepest point of the pocket following the longitudinal axis of the tooth, and then using sinusoidal movements to pull it out over a span of 5 seconds (24). The procedure was repeated so that each periodontal pocket received a total of 25s of treatment.
In accordance with the recommended precautions for working with lasers, both the patient and the dentist wore goggles, and the area in which the treatment took place was marked appropriately.
4. RESULTS
The study included 24 patients of both sexes, with an average age of 37.6 years, who had chronic periodontitis; a total of 582 teeth with 1164 periodontal pockets were examined. Molecular-biological analysis using real-time PCR method expressed the obtained numeric values in CT values for each bacterium, according to which 30 CT value was set as the limit at which the bacteria could no longer be detected. Therefore, it can be deduced that lower CT value correlates with the higher positivity of the sample (Table 1).
Table 1.
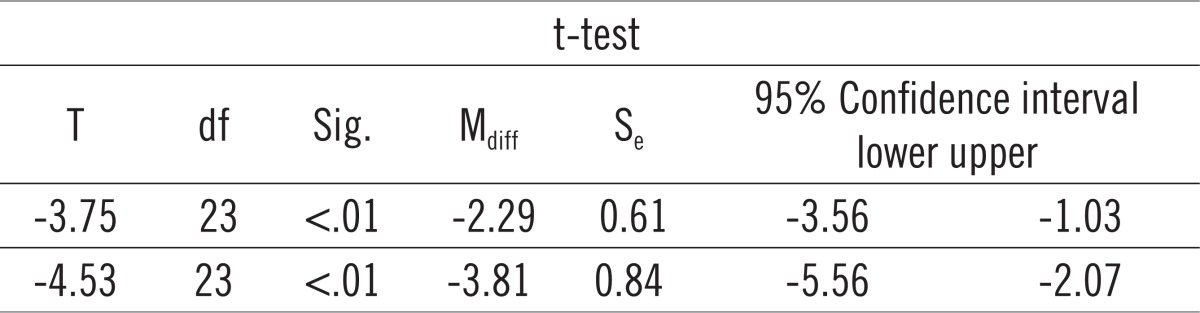
Paierd-samples t-test for Actinobacillus (Aggregatibacter) actinomycetemcomitans (AA).
 |
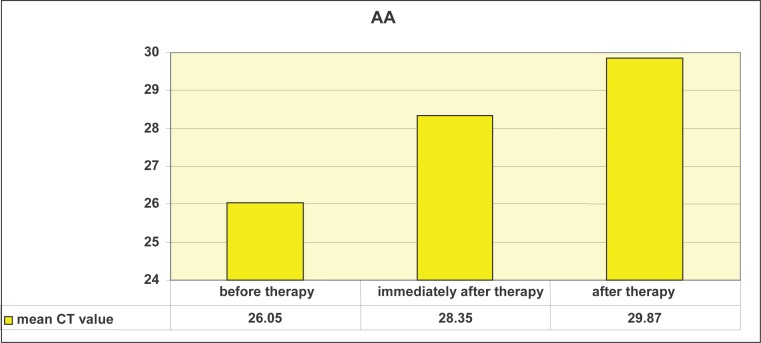
Graph 1 shows that the mean CT values of AA differ before, immediately after, and three months after therapy. We will use paired-samples t-test for to determine whether this is statistically significant.
Graph 1.
Mean CT values for Actinobacillus (Aggregatibacter) actinomycetemcomitans (AA) before laser therapy, immediately after laser therapy, and 3 months later.
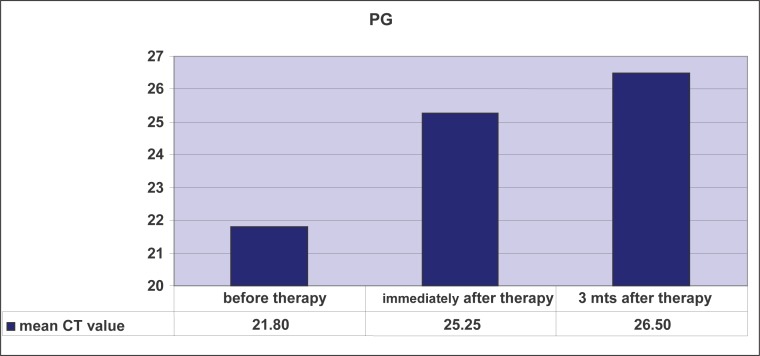
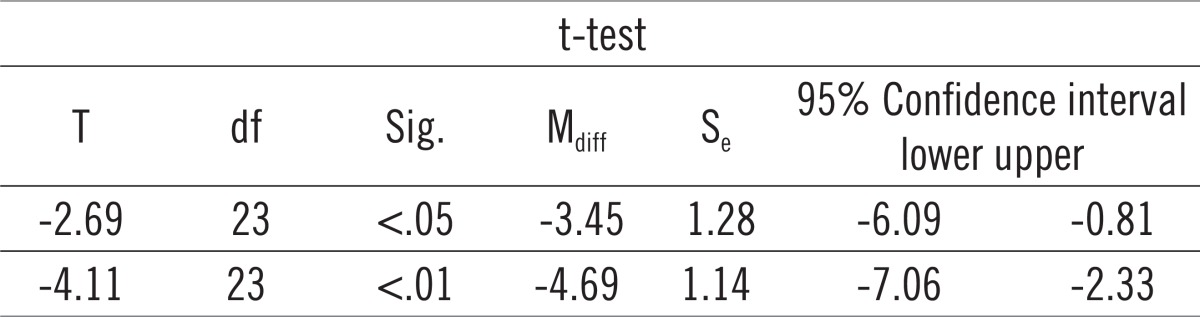
Paired-samples t-test determined that the mean level of CT values for Actinobacillus actinomycetemcomitans (AA) before treatment (M = 26.05) was statistically significantly different from the mean level of CT values of AA immediately after the treatment (M = 28.34): t = -3.75, p <.01, as well as three months after the treatment (M = 29.87): t = -4.53, p <.01, at the 1% risk level. In other words, there was a significant decrease in AA after the treatment compared with the level of AA before treatment. Graph 2 shows that the mean CT values for PG differ before, immediately after, and three months after therapy. We will use paired samples t-test to determine whether this is statistically significant. Paired-samples t-test determined that the level of CT values of PG before treatment (M = 21.80) was statistically significantly different from the mean level of CT values of PG immediately after the treatment (M = 25.25): t = -2.69, p <.05 as well as three months after the treatment (M = 26.49): t = -4.11, p <.01 at the 5% risk level. In other words, there was a significant decrease in PG after the treatment compared with the level of PG prior to therapy (Table 2).
Graph 2.
Mean CT values for Porphyromonas gingivalis (PG) before laser therapy, immediately after laser therapy, and 3 months later.
Table 2.
Paired-samples t-test for Porphyromonas gingivalis (PG).
 |
5. DISCUSSION
It is widely accepted that periodontal pathogens cannot be completely eliminated by conventional scaling and root polishing, and deeper pockets have more incompletely removed subgingival plaque and concrement, especially when using hand-held instruments (25). It is well known that certain periodontal pathogens are able to penetrate the periodontal tissues. For example, A. actinomyc-etemcomitans was found in the connective tissue of active and inactive “loci” of the periodontium, and it is believed that P. gingivalis can penetrate oral epithelial cells (26, 27).
A small number of studies dealt with microbiological analysis of the influence of a low-power laser on periodontal pathogens during nonsurgical periodontal therapy. Explanation for this can be found in the fact that periodontal pathogens are extremely difficult to detect and isolate using the standard laboratory methods. Modern laboratory methods of DNA analysis and real-time PCR analysis which are recommended today are too expensive to be a part of everyday clinical practice. Therefore, studies of this type have a special significance for clinical practice. Findings from several studies have shown a high bactericidal potential of Er:YAG lasers. Aditionally, it is believed that this laser not only eliminates the bacteria, but also deactivates bacterial toxins dispersed in the root cementum without the production of smear layer, which undoubtedly contributes to the improvement of periodontal health (28, 29, 30). These results correspond to the results of our study, although we used a low-power diode laser.
In the first study, Moritz et al. (31) used a diode laser of 2.5 W output power (pulse mode) in treatment of periodontal pockets following an ultrasound. They concluded that radiation causes considerable bacterial elimination from periodontal pockets at a much higher level than the ultrasound, especially of Actinobacillus actinomycetemcomitans. In another study, Moritz et al. (32) used a diode laser as a complementary treatment of periodontitis. Pulse irradiation with 2.5 W (50 Hz, pulse duration 10 msec) was performed three times (after 7 days, after 2 months, and after 4 months) following the application of ultrasound. After 6 months, bacterial reduction was significantly greater in the test group with the improvement of bleeding after probing, compared to the control group in which the ultrasound was followed by rinsing with H2O2. The authors concluded that treatment with diode laser, in combination with ultrasound, improves the process of regeneration of periodontal pockets by eliminating bacteria.
Our results concur with the results of Moritz et al. (31, 32), specifically those related to Actinobacillus actinomycetemcomitans (AA), which is thought to be extremely resistant to many therapeutic procedures, and very difficult to eliminate from medium-deep and very deep periodontal pockets. In our study, we obtained a mean CT value of AA of 26.05 before treatment, 28.35 immediately after treatment, and 29.87 at the control examination after 3 months, which is a statistically significant reduction of the pathogen to the limit of 30 CT values where it is no longer detectable. Our study obtained exceptionally good results, especially in the reduction of Porphyromonas gingivalis ( PG ), which had the highest initial mean CT value (before treatment) of 21.80. Immediately following the completion of therapy, mean CT value was 25.25, and it was 26.50 after 3 months (p < .01 at 5% risk level), which can be explained by a significant prolonged bactericidal effect of complementary laser therapy.
Our results only partially concur with the results obtained by Kamma et al. (33). We believe that the drawback of the study done by Kamma et al. was that the mouth of each subject was divided into four segments in order to evaluate the effect of a variety of therapeutic procedures in the same subject, and that this is the reason why the A. Actinomycetemcomitans was excluded from the study.
Aditionally, Derdilopoulou et al. aimed to evaluate and compare the microbiological effect of four different therapeutic procedures (use of hand-held instruments, Er:YAG-laser, sonic, and ultrasonic scrapers) in patients with chronic periodontitis. Bacterial samples were collected from 72 patients, from the deepest pockets in each quadrant. Using polymerase chain reaction they estimated the amounts of Aggregatibacter (Actinobacillus) actinomycetemcomitans (AA), Porphyromonas gingivalis (PG), Prevotella intermediae (PI), Tannerella forsythensis (TF), and Treponema denticola (TD) at the beginning, as well as 3 and 6 months after treatment. In every patient, individual quadrants were randomly treated with curettes (H-group), Er: YAG laser (L-group), sonic device (S-group), or ultrasonic device (U- group). Three months after the treatment, Pg, Pi, TF, and TD were significantly reduced in all groups. Laser and sonic instruments failed to reduce the AA. Bacterial reductions achieved in this study, were not retained three months after therapy (34). The explanation for these findings could lie in the limited design of the divided mouth study, same as in the study by Kamma et al. (33), where each quadrant was treated with different therapeutic procedure in the same patient. Six months after the active periodontal therapy, the amount of bacteria increased again to a different extent for each treatment group and for each species. The authors believe that it would take an additional clinical trial to determine the microbiological effects of each treatment modality, particularly in relation to modern Er:YAG laser device which was not shown to be superior in this study (34).
Based on the results of Kamma et al. and Derdilopoulou et al. (33, 34), we believe that the efficiency of the applied therapeutic procedures cannot be reliably evaluated in the study of divided mouth. Therefore, we decided to examine the efficacy of a single laser as complementary periodontal therapy.
Our study obtained a statistically significant reduction in the tested periodontal pathogens, detected by real-time PCR methods, and retained them at the control examination after three months. These results speak in favor of the efficiency of complementary periodontal therapy with a diode laser. The obtained results particularly contribute to addressing the concerns regarding the effectiveness of this therapeutic procedure with the complementary use of lasers.
6. CONCLUSION
The results of our study demonstrate the benefits of non-surgical treatment with low-power diode laser in chronic periodontitis, ref lected in a significant reduction of tested periodontal pathogens, not only immediately after the treatment, but also at the control examination three months later.
Based on the above, we may suggest that physicians, after the initial periodontal therapy, should apply complementary laser therapy in the treatment of periodontitis as a safe clinical procedure. The results of such studies encourage us in hoping that the use of complementary lowpower laser in the future will become a part of the standard protocol of non-surgical periodontal therapy.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Metthews DC. Periodontal Medicine : A New Paradigm. J Can Dent Assoc. 2000;66:488–491. [PubMed] [Google Scholar]
- 2.Topic B. Periodontology: biology, immunopahtogenesis, practice. Zagreb: Faculty of Dental Medicine, University of Sarajevo, Medicinska naklada; 2005. [Google Scholar]
- 3.Saiki K, Konishi K, Gomi T, Nishihara T, Yoshikawa M. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol Immunol. 2001;45(6):497–506. doi: 10.1111/j.1348-0421.2001.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 4.Shenker BJ, Hoffmaster RH, Zekavat A, Yamaguchi N, Lally ET, Demuth DR. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G(2) arrest of the cell cycle. J Immunol. 2001 Jul 1;167(1):435–441. doi: 10.4049/jimmunol.167.1.435. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa S, Nakajima T, Ishikura H, Ichinose S, Ishikawa I, Tsuchida N. Novel apoptosis- inducing activity in Bacteroides forsythus: a comparative study with three serotypes of Actinobacillus actinomycetemcomitans. Infect Immun. 2000 Aug;68(8):4611–4615. doi: 10.1128/iai.68.8.4611-4615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato S, Nakashima K, Inoue M, Tomioka J, Nonaka K, Nishihar T, Kowashi Y. Human epithelial cell death caused by Actinobacillus actinomycetemcomitans infection. J Med Microbiol. 2000 Aug;49(8):739–745. doi: 10.1099/0022-1317-49-8-739. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien-Simpson NM, Black CL, Bhogal PS, Cleal SM, Slakeski N, Higgins TJ, Reynolds EC. Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect Immun. 2000 May;68(5):2704–2712. doi: 10.1128/iai.68.5.2704-2712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socransky SS, Haffajee AD. Evidence of bacteial etiology: a historical perspective. In: Socransky SS, Haffajee AD, editors. Microbiology and immunology of periodontal diseases. Periodontol 2000. Vol. 5. 1994. Jun, pp. 7–25. [DOI] [PubMed] [Google Scholar]
- 9.Yasui S, Kojima T, Hata S, Zhang YJ, Umeda M, Ishikawa I. Rapid identification of Porphyromonas gingivalis by bisulfite-modified DNA probe method. J Periodontal Res. 1993;28:98–101. doi: 10.1111/j.1600-0765.1993.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 10.Boutaga K, Savelkoul PHM, Winkel EG, Winkelhoff AJ. Comparison of Subgingival Bacterial Sampling With Oral Lavage for Detection and Quantification of Periodontal Pathogens by Real-Time Polymerase Chain Reaction. J Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 11.Badersten A, Nileus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindhe J, Nyman S. Scaling and granulation tissue removal in periodontal therapy. J Clin Periodontol. 1985;12:374–388. doi: 10.1111/j.1600-051x.1985.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary TJ. The impact of research on scaling and root planning. J Periodontol. 1985;57:69–75. doi: 10.1902/jop.1986.57.2.69. [DOI] [PubMed] [Google Scholar]
- 14.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer K. Long-term evaluation of periodontal therapy: I. Response to four therapeutic modalities. J Periodontol. 1996;67:93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- 15.Badersten A, Nileus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J Clin Periodontol. 1984;11:63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 16.Dickers B, Lamard L, Peremans A, Geerts S, Lamy M, Limme M, Rompen E, de Moor RJ, Mahler P, Rocca JP, Nammour S. Temperature rise during photo-activated disinfection of root canals. Lasers Med Sci. 2009;24(1):81–85. doi: 10.1007/s10103-007-0526-y. [DOI] [PubMed] [Google Scholar]
- 17.Cernavin I, Pugatschew A, Boer N, Tyas MJ. Laser aplications in dentistry: A review of the literature. Australian Dental J. 1994;39:28–32. doi: 10.1111/j.1834-7819.1994.tb05543.x. [DOI] [PubMed] [Google Scholar]
- 18.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg Med. 2006;38(5):468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 19.Jori G. Photodynamic therapy of microbial infections: ‘state of the art and perspectives. J Environ Pathol Toxicol Oncol. 2006;25(1-2):505–519. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.320. [DOI] [PubMed] [Google Scholar]
- 20.Komerik N, MacRobert AJ. Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol. 2006;25(1-2):487–504. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.310. [DOI] [PubMed] [Google Scholar]
- 21.Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: State of the art. J Photochem Photobiol B. 2005;79(2):159–170. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22:302–311. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Moritz A. Oral laser application. Berlin: Quintessenz Verlags GmbH; 2006. [Google Scholar]
- 25.Izumi Y, Hiwatashi-Horinouchi K, Furuichi Y, Sueda T. Influence of different curette insertion depths on the outcome of non-surgical periodontal treatment. J Clin Periodontol. 1999;26:716–722. doi: 10.1034/j.1600-051x.1999.t01-3-261101.x. [DOI] [PubMed] [Google Scholar]
- 26.Saglie FR, Marfany A, Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 27.Sandros J, Papapanou PN, Nannmark U, Dahlén G. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodont Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz F, Sculean A, Georg T, Reich E. Periodontal treatment with an Er: YAG laser compared to scaling and root planing. A controlled clinical study. J Periodontol. 2001;72:361–367. doi: 10.1902/jop.2001.72.3.361. [DOI] [PubMed] [Google Scholar]
- 29.Folwaczny M, Mehl A, Aggstaller H, Hickel R. Antimicrobial effects of 2.94 microm Er:YAG laser radiation on root surfaces: an in vitro study. J Clin Periodontol. 2002 Jan;29(1):73–78. doi: 10.1034/j.1600-051x.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa I, Aoki A, Takasaki AA. Potential applications of Erbium:YAG laser in periodontics. J Periodontal Res. 2004 Aug;39(4):275–285. doi: 10.1111/j.1600-0765.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 31.Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U, Schauer P, Sperr W. Bacterial reduction in periodontal pockets through irradiation with a diode laser: A pilot study. J Clinic Laser Med Surg. 1997;15:33–37. doi: 10.1089/clm.1997.15.33. [DOI] [PubMed] [Google Scholar]
- 32.Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22:302–311. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Kamma JJ, Vasdekis VG, Romanos GE. The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg. 2009 Feb;27(1):2–9. doi: 10.1089/pho.2007.2233. [DOI] [PubMed] [Google Scholar]
- 34.Derdilopoulou FV, Nonhoff J, Neumann K, Kielbassa AM. Microbiological findings after periodontal therapy using curettes, Er:YAG laser, sonic, and ultrasonic scalers. J Clin Periodontol. 2007;34:588–598. doi: 10.1111/j.1600-051X.2007.01093.x. [DOI] [PubMed] [Google Scholar]