Abstract
Applying a next-generation sequencing assay targeting 145 cancer-relevant genes in 40 colorectal cancer and 24 non–small cell lung cancer formalin-fixed paraffin-embedded tissue specimens identified at least one clinically relevant genomic alteration in 59% of the samples and revealed two gene fusions, C2orf44-ALK in a colorectal cancer sample and KIF5B-RET in a lung adenocarcinoma. Further screening of 561 lung adenocarcinomas identified 11 additional tumors with KIF5B-RET gene fusions (2.0%; 95% CI 0.8–3.1%). Cells expressing oncogenic KIF5B-RET are sensitive to multi-kinase inhibitors that inhibit RET.
We analyzed genomic DNA from 40 colorectal cancer (CRC) and 24 non–small cell lung cancer (NSCLC) formalin-fixed paraffin-embedded (FFPE) specimens using an assay that captures and sequences 2,574 coding exons representing 145 cancer-relevant genes (genes that are associated with cancer-related pathways, targeted therapy or prognosis), plus 37 introns from 14 genes that are frequently rearranged in cancer (Supplementary Table 1). We sequenced this 606,676-bp content, selected using solution phase hybridization, to an average coverage of 229 ×, with 84% of exons being sequenced at ≥ 100 × coverage (Supplementary Table 2 and Supplementary Methods). To maximize mutation-detection sensitivity in heterogeneous cancer biopsies, we validated the test to detect base substitutions at a ≥ 10% mutant allele frequency with ≥ 99% sensitivity and to detect indels at a ≥ 20% mutant allele frequency with ≥ 95% sensitivity, with a false discovery rate of < 1% (data not shown).
Among 40 CRCs, we identified 125 alterations in 21 genes. We found at least one alteration in 39 out of 40 tumors (with a range of 1–9 alterations), and 62.5% (25 out of 40) of the tumors harbored at least two classes of DNA alteration (Fig. 1 and Supplementary Table 3). TP53 and APC were altered in 80% (32 out of 40) and 67.5% (27 out of 40) of CRCs, respectively, with both being mutated at higher frequencies than are reported in the Catalogue of Somatic Mutations in Cancer1. Additionally, 11 genes were mutated, amplified or rearranged in multiple CRCs: KRAS (10), BRAF (6), FBXW7 (5), ATM (2), BCL2L1 (2), BRCA2 (2), CDH1 (2), ERBB3 (2), GNAS (2), PIK3CA (2) and SMAD4 (2). ALK, CDK8, LRP1B, MYC, MSH6, RICTOR, SMAD2 and STK11 were each altered in one tumor (Fig. 1a). Notably, 52.5% of CRCs (21 out of 40) harbored at least one alteration that has been linked to a clinical treatment option or is currently being investigated in clinical trials of new targeted therapies. Examples of this include mutations in KRAS and BRAF (cetuximab2,3 or panitumumab resistance3), FBXW7 (anti-tubulin resistance4), BRCA2 (poly-(ADP-ribose) polymerase (PARP) inhibitor trials5), GNAS (MEK or ERK inhibitor trials6) and PIK3CA (phosphoinositide-3-kinase, catalytic, alpha polypeptide (PI3K) and mechanistic target of rapamycin (mTOR) inhibitor trials7) (Supplementary Table 4).
Figure 1.
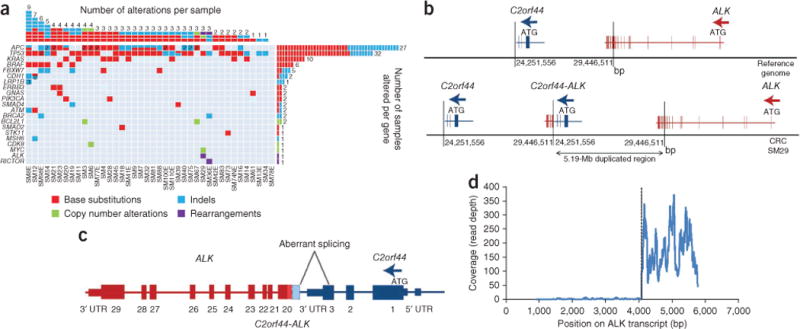
DNA alterations identified in 40 CRC FFPE specimens. (a) The columns in the table denote samples, and the rows denote genes. A number inside the cell indicates the number of alterations of a specific type identified here. (b) A 5,194,955-bp tandem duplication generates an in-frame C2orf44-ALK gene fusion. (c) The RNA sequence of the C2orf44-ALK gene fusion shows aberrant splicing. UTR, untranslated region. (d) RNA sequencing shows an 89.8-fold increase in expression of ALK beginning at exon 20 relative to exons 1–19.
We detected a fusion gene between C2orf44 and ALK in sequencing data from one subject with CRC (Supplementary Fig. 1). This in-frame fusion starts at the canonical exon 20 recombination site that was previously reported for ALK gene fusions8,9. C2orf44, on chromosome 2, contains a coiled-coil domain; the C2orf44-ALK fusion results from a 5,194,955-bp tandem duplication (Fig. 1b,c). Complementary DNA (cDNA) sequencing identified 75 read pairs spanning the fusion junction (data not shown) and an 89.8-fold increase in 3′ ALK expression beginning at exon 20 relative to exons 1–19, suggesting that the C2orf44-ALK fusion transcript results in ALK kinase overexpression (Fig. 1d). Immunohistochemistry (IHC) was negative for ALK staining (data not shown). Clinical detection of ALK rearrangements is currently performed using fluorescence in situ hybridization with ALK break-apart probes9 or by RT-PCR using ALK-rearrangement–specific primers. Given the structure of this gene fusion, probably neither method would have detected it. These findings suggest that a previously unrecognized subset of individuals with CRC may harbor genetic alterations that may respond to treatment with crizotinib or other ALK inhibitors.
Among 24 NSCLCs, we identified 50 alterations in 21 genes, with at least one alteration being present in 83% (20 out of 24) of the tumors (with a range of 1–7 alterations). Twelve genes were altered in multiple tumors: KRAS (10), TP53 (7), STK11 (4), LRP1B (3), JAK2 (3), EGFR (2), BRAF (2), CDKN2A (2), CTNNB1 (2), MDM2 (2), PIK3CA (2) and ATM (2). APC, CCNE1, CDK4, MLH1, MSH6, NF1, RB1, RET, and TSC1 were each altered in one tumor (Fig. 2a and Supplementary Table 3). In 72% (36 out of 50) of the NSCLCs, at least one alteration was associated with a current clinical treatment or targeted therapy trial, including mutations in KRAS (epidermal growth factor receptor (EGFR) kinase inhibitor resistance10 and PI3K and MEK inhibitor trials7), BRAF (v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitor trials, including those with vemurafenib11 and GSK 2118436 (ref. 11)), EGFR (gefitinib or erlotinib sensitivity12), MDM2 (nutlin trials13), CDKN2A, CCNE1 and CDK4 (cyclin-dependent kinase 4 (CDK4) inhibitor trials14,15) and PIK3CA (PI3K and mTOR inhibitor trials7,16) (Supplementary Table 4).
Figure 2.
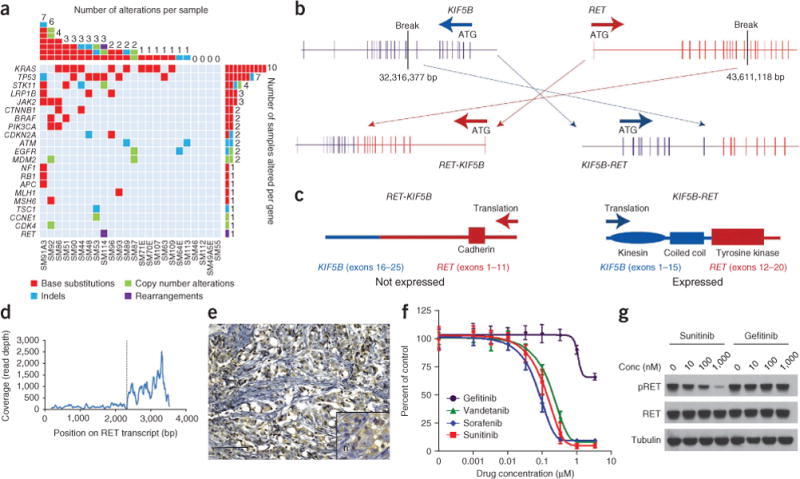
DNA alterations identified in 24 NSCLC FFPE specimens. (a) The columns in the table denote samples, and the rows denote genes. (b) An 11,294,741-bp inversion generates an in-frame KIF5B-RET gene fusion and the reciprocal RET-KIF5B fusion. (c) Protein domain structure of the RET-KIF5B and KIF5B-RET fusions. (d) RNA sequencing shows a 7.3-fold increase in expression of RET beginning at exon 12 relative to exons 1–11. (e) Focal moderate cytoplasmic immunoreactivity for RET protein expression (using avidin-biotin peroxidase). Scale bar, 100 μm; inset, 10 μm. (f) Ba/F3 cells with the KIF5B-REF fusion were treated with different drugs at the indicated concentrations, and viable cells were measured after 72 h of treatment and plotted relative to untreated controls. (g) Cells from e were treated with increasing concentrations of sunitinib or gefitinib for 6 h, and immunoblotting was used to detect the indicated proteins. Conc, concentration; pRET, phosphorylated RET.
In addition to the known NSCLC gene alterations, we made two notable discoveries. The first was a G1849T (V617F) JAK2 mutation present in three subjects at a low allele frequency (4–10%). Although commonly observed in myelodysplastic syndromes, this JAK2 mutation has not been described in solid tumors1. Sequencing additional NSCLCs will be required to characterize the role of JAK2 mutations in NSCLC, and clinical trials will be necessary to assess whether they predict sensitivity to JAK2 inhibitors17.
We also detected an 11,294,741-bp pericentric inversion on chromosome 10 generating a new gene fusion joining exons 1–15 of KIF5B to exons 12–20 of RET (Fig. 2b and Supplementary Fig. 2) in a lung adenocarcinoma from a 44-year-old, male ‘never smoker’ of European ancestry; this fusion is denoted K15;R12, based on the last KIF5B and first RET exons in the fusion. KIF5B exons 1–15 comprise the kinesin motor and coiled-coil domains that mediate homodimerization, whereas exon 15 is a known KIF5B-ALK fusion site in individuals with NSCLC18. RET exons 12–20 encode the tyrosine kinase portion of the PTC-RET fusions observed in ~35% of papillary thyroid carcinomas19 (Fig. 2c). cDNA sequencing identified 490 unique read pairs spanning the fusion junction (data not shown) and detected a 7.3-fold RET expression increase beginning at exon 12 relative to exons 1–11 (Fig. 2d). IHC showed focal moderate cytoplasmic immunoreactivity for RET protein expression (Fig. 2e).
An additional 117 NSCLCs (from 92 individuals of European ancestry, 5 African-American individuals and 20 individuals of unknown ancestry) screened by IHC showed moderate to intense RET staining in 22 samples. RT-PCR and cDNA sequencing of RNA from 15 tumors that had RET expression using IHC identified one additional KIF5B-RET fusion in a male former smoker of European ancestry (Supplementary Table 5).
We evaluated tumors from 121 individuals of European ancestry and 405 Asians, comprising only never or limited former smokers, by RT-PCR for KIF5B-RET fusions, with 1 out of 121 (0.8%) European-ancestry and 9 out of 405 (2%) Asian subjects testing positive for the fusion. None of the fusion-positive tumors contained mutations in EGFR, ERBB2, BRAF or KRAS or rearrangements of EML4-ALK or ROS1, resulting in a total of 10 individuals with RET rearrangements out of 159 (6.3%) subjects without previously known driver mutations (Supplementary Table 5). The lack of known oncogenic mutations in individuals positive for the KIF5B-RET fusion raises the possibility that the RET fusion is a driving oncogenic event. Of note, all additional 12 KIF5B-RET fusions (from the 11 individuals described above) were identified in lung adenocarcinomas, which comprised 561 of the 643 cases screened, in addition to the original 24 individuals, for an overall occurrence rate of 2.0% (95% CI 0.8–3.1%).
The 13 total RET fusions (from 12 individuals) comprise four unique transcripts: eight from K15;R12 (variant 1), three from K16;R12 (variant 2), one from K22;R12 (variant 3) and one from K15;R11 (variant 4) (the subject with variant 4 also had the K15;R12 variant) (Supplementary Figs. 2 and 3). The KIF5B portions of the variants differ, but all retain the coiled-coil domain necessary for homodimerization. No identified RET fusion was amplified.
Thyroid cancers and cell lines harboring PTC-RET translocations are sensitive to sorafenib, which inhibits RET20, suggesting the KIF5B-RET gene fusion in NSCLC may be druggable. KIF5B-RET expression in Ba/F3 cells led to oncogenic transformation, as determined by interleukin-3 (IL-3)-independent growth. These cells were sensitive to sunitinib, sorafenib and vandetanib, which are all multi-targeted kinase inhibitors that inhibit RET but not gefitinib, which is an EGFR kinase inhibitor (Fig. 2f). Sunitinib, but not gefitinib, inhibited RET phosphorylation in Ba/F3 cells with the KIF5B-RET fusion protein (Fig. 2g). These findings suggest that RET kinase inhibitors should be tested in prospective clinical trials for therapeutic benefit in individuals with NSCLC that carry KIF5B-RET rearrangements.
Overall in this study, 52.5% of subjects with CRC and 71% of subjects with NSCLC had genomic alterations that were directly linked to a clinical therapeutic option, including two new gene fusions. Identifying even a small subpopulation of affected individuals with gene fusions who may be potentially responsive to targeted therapy, as exemplified by the discovery of C2orf44-ALK and KIF5B-RET, may have major therapeutic relevance, as highlighted by the recent US Food and Drug Administration’s approval of crizotinib for the treatment of NSCLC that harbors rearrangements in ALK9. These findings in aggregate show the potentially large clinical impact of a single multiplex test that requires minimal DNA from FFPE tumor biopsies.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Supplementary Material
Acknowledgments
We would like to thank M. Hawryluk for his help in preparing this manuscript. This work is supported by the Dana Farber/Harvard Cancer Center Lung Cancer SPORE P50 CA090578 (P.A.J.), the Cammarata Family Foundation Research Fund (M.C. and P.A.J.), the Nirenberg Fellowship at the Dana-Farber Cancer Institute (M.C. and P.A.J.) and the Japan Society for the Promotion of Science (JSPS), grant references 23659674, 21390394 and 21591820 (H.S.).
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
D.L. and R.Y. designed experiments and algorithms, and performed analyses. J.W. and G.M.F. performed data analyses. A.P. and G.O. performed RNA preparations and prepared the cDNA libraries and sequencing. M.J. designed the experiments and performed analyses. J.A.C. and K.C.M. performed laboratory and project management. S.R.D. and T.B. performed tissue pathology preparations and extractions. J.S.R. provided tissue specimens and the pathology review. S.B., K.W.B., A.D., L.G., F.J., E.W. and Z.Z. performed DNA library preparation and sequencing. P.J.S., M.T.C. and P.A.J. designed experiments, analyzed data and wrote the manuscript. T.P., H.N., L.-S.G., C.E.S., J.K., H.S., H.R.K. and S.P. provided subject specimens. M.C. performed genotyping of subjects with lung cancer, conducted in vitro studies and analyzed data. D.E. performed in vitro studies.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
Published online at http://www.nature.com/naturemedicine/.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Forbes SA, et al. Nucleic Acids Res. 2011;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lièvre A, et al. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 3.Di Nicolantonio F, et al. J Clin Oncol. 2008:26, 5705–5712. [Google Scholar]
- 4.Wertz IE, et al. Nature. 2011:471, 110–114. [Google Scholar]
- 5.Turner N, Tutt A, Ashworth A. Curr Opin Pharmacol. 2005;5:388–393. doi: 10.1016/j.coph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Wilson CH, McIntyre RE, Arends MJ, Adams DJ. Oncogene. 2010;29:4567–4575. doi: 10.1038/onc.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman JA, et al. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soda M, et al. Nature. 2007:448, 561–566. [Google Scholar]
- 9.Kwak EL, et al. N Engl J Med. 2010:363, 1693–1703. [Google Scholar]
- 10.Pao W, et al. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PB, et al. N Engl J Med. 2011:364, 2507–2516. [Google Scholar]
- 12.Pao W, et al. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassilev LT, et al. Science. 2004:303, 844–848. [Google Scholar]
- 14.Finn RS, et al. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toogood PL, et al. J Med Chem. 2005:48, 2388–2406. [Google Scholar]
- 16.Di Nicolantonio F, et al. J Clin Invest. 2010:120, 2858–2866. [Google Scholar]
- 17.Wang Y, et al. Blood. 2009:114, 5024–5033. [Google Scholar]
- 18.Wong DW, et al. Cancer. 2011:117, 2709–2718. [Google Scholar]
- 19.Fusco A, et al. Nature. 1987:328, 170–172. [Google Scholar]
- 20.Henderson YC, Ahn SH, Kang Y, Clayman GL. Clin Cancer Res. 2008;14:4908–4914. doi: 10.1158/1078-0432.CCR-07-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


