Abstract
Introduction
Total hip and knee replacement (THR and TKR) are high risk settings for venous thromboembolism (VTE).
Objectives
(1) Summarize cost-effectiveness of VTE prophylaxis regimens for THR and TKR a
Data Sources
Medline (from January 1997 to October 2009), EMBASE (January 1997 until June 2009), and the Economic Evaluation Database[12] (1997- October 2009)
Methods
We identified recent cost-effectiveness studies examining five categories of comparisons: (1) anticoagulants (warfarin, low molecular weight heparin - LMWH, or fondaparinux) vs. aspirin; (2) LMWH vs. warfarin; (3) fondaparinux vs. LMWH; (4) comparisons with new oral anticoagulants; and (5) extended (≥3 weeks) vs. short duration prophylaxis (< 3 weeks). We abstracted information on cost and effectiveness for each prophylaxis regimen in order to calculate an incremental cost-effectiveness ratio. Because of variations in effectiveness units reported and horizon length analyzed, we calculate two cost-effectiveness ratios, one for the number of symptomatic, proximal VTE events avoided at 90 days and the other for quality adjusted life-years (QALYs) at the one year mark or beyond.
Results
We identified 33 studies with 67 comparisons. After standardization, comparisons between LMWH and warfarin were inconclusive whereas fondaparinux dominated LMWH in nearly every comparison. The latter results were derived from radiographic VTE rates. Extended duration prophylaxis after THR was generally cost-effective. Small numbers prohibit conclusions about aspirin, new oral anticoagulants, or extended duration prophylaxis after TKR.
Conclusions
Fondaparinux after both THR and TKR and Extended duration LMWH after THR appear to be cost cost-effective prophylaxis regimens. Small numbers for other comparisons and absence of trials reporting symptomatic endpoints prohibit comprehensive conclusions.
Keywords: thrombosis, total joint replacement, antithrombotic therapy, preventive medicine
Introduction
In 2005, there were 580,000 total hip or knee replacements (THR or TKR) performed in the U.S[1] and that number is projected to increase to 4.5 million by 2030[2]. Although THR and TKR are generally safe procedures[3], they have been identified[4] as high-risk events for venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE). For almost 20 years, physicians have been offering pharmacological prophylaxis to patients undergoing THR and TKR. Uncertainty exists, however, about the optimal pharmacological regimen for prophylaxis.
Guidelines[5] published by the American College of Chest Physicians (ACCP) in 2008 support using potent anticoagulant regimens with agents such as fondaparinux, low molecular weight heparin (LMWH), and warfarin (target INR 2-3) and discourage aspirin therapy alone. Guidelines[6] by the American Academy of Orthopedic Surgeons (AAOS), in contrast, support use of aspirin or a lower potency warfarin regimen (INR < 2) in addition to LMWH and fondaparinux, stating that the latter agents do not offer increased protection against PE but substantially raise the rate of bleeding complications. New oral anticoagulants such as rivaroxaban, a factor Xa inhibitor, and dabigatran, a direct thrombin inhibitor, are expected to gain FDA approval within the next several months and it is anticipated that they too will be supported by the above professional societies.
Several studies have attempted to address these risk-benefit and cost issues using decision analysis methodology regarding specific strategies implementing VTE prophylaxis. Some studies[7-9] substantiate the cost-effectiveness of newer regimens more potent in terms of preventing VTE while others[10] do not. Individual study results vary depending on the setting, economic perspective (e.g., groups for which cost and effects will be aggregated – patients, payers, or others), horizon (time course over which cost and effectiveness information was assessed), and effectiveness outcome analyzed (e.g., VTE events averted, life-years gained, quality adjusted life years (QALYs) gained). Measuring effectiveness in QALYs, particular over a horizon of one year or greater, permits comparison of cost-effectiveness of interventions across diseases but some authors may choose not to measure QALYs because their focus resides in the economics related to the period immediately following surgery. To more meaningfully compare VTE prophylaxis regimens, we systematically reviewed recently published studies that evaluated the cost-effectiveness of the different pharmacologic options in patients undergoing THR and TKR. We abstracted information about cost and effects both for a short and long horizon. In each case, we calculated the incremental cost-effectiveness ratios using our abstractions and then converted our estimates based in many currencies into 2009 US dollars (USD).
Methods
Study Selection
Using published recommendations[11] for identification of cost-effectiveness studies, we searched Medline (from January 1997 to October 2009), EMBASE (January 1997 until June 2009), and the Economic Evaluation Database[12] (1997- October 2009). (Appendix 1) We also searched the bibliographies of included studies.
We included studies that evaluated the cost-effectiveness of pharmacological agents in patients undergoing THR or TKR. Specifically, we focused our search on recent (1997- October 2009) studies published in English that contained complete documentation of methods (as compared with abstracts or brief reports), had discrete information available for TKR or THR (i.e., not combined with other orthopedic surgeries), and contained enough information to calculate an incremental cost-effectiveness ratio for at least one of five important comparisons. The five comparisons were: (1) anticoagulants (fondaparinux, LMWH, warfarin) vs. aspirin; (2) LMWH vs. warfarin; (3) fondaparinux vs. LMWH; (4) comparisons with new oral anticoagulants and (5) extended duration prophylaxis (3 weeks or more with any agent) vs. short duration prophylaxis (< 3 weeks with any agent). We did not analyze information about regimens not routinely recommended as sole therapy by the ACCP or AAOS. These include unfractionated heparin, parenteral thrombin inhibitors, or non-pharmacological means such as intermittent pneumatic compression or graduated stockings. Two authors (A.K. and N.R.) evaluated each study for inclusion. Disagreements were resolved by discussion.
Study Abstraction and Quality Assessment
We derived an abstraction instrument based on the recommendations of the Panel on Cost-Effectiveness[13-15]. Two abstractors (N.R. and W.C.) assisted the primary author (A.K.) in recording, in duplicate, the description of the study setting, cohort age, economic perspective, and presence of pharmaceutical industry sponsorship.
To summarize the cost-effectiveness information of our 5 main comparisons, we abstracted data on the incremental cost and effectiveness for both a short and longer horizon when available. The horizon represents the period of time over which costs and effectiveness are aggregated. For certain diseases such as the common cold, a short-horizon analysis may suffice. In other cases, long-term consequences must be accounted for, even for short-term interventions.[13-15] For the short horizon, we abstracted data on the projected costs incurred and VTE events avoided for the period closest to 90 days from surgery. For the purpose of calculating effectiveness, we abstracted data on the combined incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE) that would be detected in routine clinical practice. If a study did not report such an outcome, we also accepted the incidence of radiographically detected events and noted the distinction. If effectiveness was defined only by the life-years or QALYs, we recorded that information.
For the long horizon, we accepted any information that projected the cost and effectiveness for one year or more. We abstracted effectiveness information preferentially for the outcome of QALYs or unadjusted life-years.
For each study with missing information about drug regimen, dosage, duration of therapy, horizon of analysis, major bleeding rate, DVT, PE, and death rate, we contacted corresponding authors first by email and then by letter. If the authors did not respond, we recorded the information as not specified.
We adjusted all cost information to 2009 USD by inflating or deflating to the year 2005 according to readily available consumer price indices for each country [16, 17], converted to USD via World Health Organization purchasing power parity indices [18], and then inflated to USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov [19]. This approach followed the example of Bachmann et al.[20]
Study Quality
To assess study quality, we created an instrument adapted from “Drummond’s List”[21] and one other instrument from Brauer et al.[22] These included items about the use of cost data from a randomized controlled trial or other primary source, use of efficacy data from pooled results of a systematic review, identification of credible sources for all input parameters, appropriate calculation of an incremental cost-effectiveness ratio (ICER), and use of comprehensive one-way sensitivity analyses. The ICER is an expression of how much additionally it costs (in dollars) to achieve an additional unit of benefit (e.g. one more QALY). Policy makers are interested in the ICER value because it facilitates determination about whether newer, more effective interventions represent good value compared to existing, less expensive programs.[23] Interpreting the results of cost-effectiveness analysis can be problematic, making it difficult to decide whether to adopt a diagnostic test or treatment. The threshold for adoption in the United States is thought to be somewhere between $20,000 /QALY gained and $100,000 /QALY gained, with a threshold of $50,000 /QALY gained frequently proposed.[24] In one-way sensitivity analysis, the decision analyst examines what change will occur in the ICER if the value of an input parameter varies across a range of plausible values.
We also recorded quality items specific to VTE including assessment of joint function following hemarthrosis, propagation of asymptomatic DVT to symptomatic PE, incidence of post-thrombotic syndrome, costs of major and minor bleeding, and future costs related to VTE including blood monitoring and physician visits. Studies ignoring downstream bleeding consequences could make newer, more potent regimens appear more cost-effective whereas studies ignoring downstream costs of treating VTE will bias our interpretation in the other direction. We did not specifically document if individual studies included death costs related to VTE or bleeding. On the whole, death events were rare and the associated costs would be largely paid by the family of the patient and not the institution or health system which was the economic perspective chosen by all but three of the studies analyzed.
We did not pool the results of individual studies given the various modeling assumptions adopted by each author. Instead, we qualitatively compared studies to determine trends in the cost-effectiveness of certain regimens in comparison to others.
Results
We identified 370 titles and abstracts meeting our search criteria. Of the 370, 56 were relevant and were entered for full text review. Of these, 33 studies met all inclusion criteria.[8-10, 25-46] [7, 10, 31, 47-53] (Figure 1)
Fig. 1.
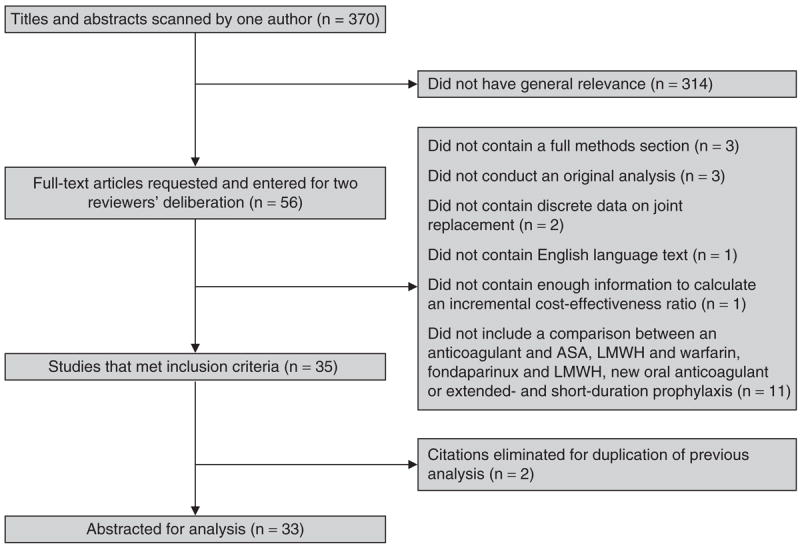
Flow diagram of article selection. ASA = acetylsalicylic acid (aspirin); LMWH = low-molecular-weight heparin.
Most studies were set in the United States (14 of 33)[28-30, 32-38, 41, 42, 44, 46] or Europe (14 of 33).[7-9, 26, 27, 31, 39, 45, 47-50, 52, 53]. Twenty studies[7, 8, 25, 28-38, 41, 42, 44-47] adopted an institutional perspective; only three[10, 49, 50] adopted a societal perspective. Ten studies[7, 9, 25, 29, 32, 37, 47, 48, 51, 53] reported pharmaceutical company sponsorship.
There was substantial variation in the quality of reporting. Only six of the 30 studies reported performing a systematic review and meta-analysis of efficacy data.[10, 27, 29, 36, 37, 51] In addition, only 12 studies documented comprehensive use of one-way sensitivity analysis.[7, 10, 26, 27, 29, 33-36, 45, 48, 49, 53]] Only three of 30 studies[10, 29, 50] measured effectiveness in quality-adjusted life-years to at least the one year horizon. (Table 1 and Appendix Table 2).
Table 1.
Descriptive Characteristics of Studies Included in Systematic Review of VTE Pharmacologic Prophylaxis after Total Hip and Knee Replacement
| Characteristic | Number of Studies (%)* |
|---|---|
| Setting | |
| USA | 14 (42) |
| Canada | 4 (12) |
| Europe | 14 (42) |
| South Africa | 1 (3) |
| Economic Perspective | |
| Institutional | 20 (61) |
| National health system | 10 (30) |
| Societal | 3 (9) |
| Sponsorship† | |
| Pharmaceutical sponsor | 10 (30) |
| Pharmaceutical grant | 9 (27) |
| Pharmaceutical consultants | 2 (6) |
| Government agency | 3 (9) |
| None reported | 9 (27) |
| Comparison Type | |
| Anticoagulant vs. aspirin | 2 (6) |
| LMWH‡ vs. warfarin | 15 (45) |
| Fondaparinux vs. LMWH | 10 (30) |
| Comparisons with new oral anticoagulants | 2 (6) |
| Extended vs. short duration prophylaxis | 9 (27) |
| Quality Inventory¶ | |
| Costs measured through primary source? | 16 (48) |
| Effectiveness calculated using pooled results of systematic review? | 6 (18) |
| Data sources comprehensively documented and credible? | 29 (88) |
| Costs and effects discounted (for studies with horizon 1 year or more)? | 6 (18) |
| Incremental cost-effectiveness ratio (ICER) calculated correctly? | 30 (91) |
| One-way sensitivity analysis used comprehensively? | 13 (39) |
| Other Distinguishing Features | |
| Effectiveness measured in QALYs at a horizon of at least one year? | 7 (21) |
| Asymptomatic VTE adequately addressed? | 18 (54) |
| Post thrombotic syndrome adequately addressed? | 10 (30) |
| Major Bleeding included in cost calculation? | 28 (85) |
Abbreviations: LMWH = low molecular weight heparin, VTE = venous thromboembolism, QALY = quality adjusted life-year,
Out of 33 studies.
If both pharmaceutical and government sponsorship, pharmaceutical sponsorship was recorded
Low molecular weight heparin
Derived from quality scales published separately by Drummond20 and Brauer21.
Comparison of Anticoagulants to Aspirin
We included two studies[25, 26] with three comparisons of an anticoagulant to aspirin. (Table 2) In all three comparisons, results were available for THR exclusively. Sarasin et al.[26] found that the ICER was $1700 /VTE avoided for four weeks of warfarin compared to aspirin and $1300 /VTE avoided for four weeks of LMWH compared to aspirin. There was no apparent pharmaceutical company sponsorship for that study. The final comparison, sponsored by Sanofi-Aventis, the manufacturer of enoxaparin, was set in South Africa, reported an ICER of $7200 /VTE avoided for 10 days of enoxaparin compared with 10 days of aspirin.
Table 2.
Summary of Cost-effectiveness Studies Comparing Anticoagulants with Aspirin
| Study ID | Comparator #1 | Aspirin regimen | Horizon | Major Bleeding rate (%) Anticoagulant / ASA | DVT rate (%) Anticoagulant rate / ASA | PE rate (%) Anticoagulant / ASA | Death Rate (%) Anticoagulant / ASA | Cost-Effectiveness Result Reported by Study Authors, converted to 2009 USD* |
|---|---|---|---|---|---|---|---|---|
| THR Results from Short Horizon Analysis | ||||||||
| Sarasin 2002 | Enoxaparin 40 mg qd × 10 days | 300 mg qd × 10 days | 90 days | 0.49 / 0.48 | 5.59 / 8.79 ‡ | 1.12 / 1.76 | ns /ns | LMWH ICER = $1300 / VTE avoided |
| Abdool-Carrim 1997 | Enoxaparin 40 mg or Dalteparin 4,000-5,000 IUs qd × 28 days post-discharge | 160 mg qd × 28 days post-discharge | ns | 2.00 / 0.70 | 21.0 / 35.0 ‡ | ns / ns | 1.10 / 1.50 | LMWH ICER = $7200 / VTE avoided |
| Sarasin 2002 | Warfarin dose not specified × 28 days post-discharge | 160 mg qd × 28 days post-discharge | 90 days | 0.59 / 0.48 | 6.52 / 8.79 ‡ | 1.30 / 1.76 | ns /ns | Warfarin ICER = $1700 / VTE avoided |
Abbreviations: LMWH = low molecular weight heparin, ns = not specified, VTE = venous thromboembolism, QALY = quality adjusted life-year
Cost-effectiveness result is the incremental cost-effectiveness ratio (ICER). To arrive at ICER values, incremental costs reported in foreign currencies were inflated or deflated according to readily available consumer price indices, converted to USD via 2005 World Health Organization purchasing price parity indices, and then inflated to 2009 USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov.
DVT rate not specified (i.e. clinical vs. radiographic and proximal vs distal)
Comparison of LMWH to Warfarin
We included 15 studies with comparisons of LMWH and warfarin.[10, 26-38, 51] (Table 3) Twelve of 14 compared these agents in patients receiving THR. Of those documenting a short horizon cost-effectiveness result, 3 studies [26, 35, 51] found that the ICER for LMWH was ≤$2,000 / VTE avoided compared with warfarin. In two other studies[29, 30], LMWH cost an additional $2100 /VTE avoided. In a sixth study,[33] LMWH cost $5,200 /VTE avoided. In the next study[10], LMWH cost $109,000 /VTE avoided. This study by Skedgel et al. examined four additional weeks (in addition to the hospital period) of LMWH compared with four additional weeks of warfarin. It found that the cost, in Canada, would be almost ten-fold higher for LMWH given the significant proportion of patients (39% at baseline) that would require daily nursing supervision of LMWH injection in their homes compared with the same proportion that would require weekly home phlebotomy for monitoring INR while using warfarin. In the remaining four studies of short horizon[27, 31, 32, 37], warfarin dominated LMWH.
Table 3.
Summary of Cost-effectiveness Studies Comparing LMWH to Warfarin
| study ID | Horizon | LMWH regimen | Warfarin regimen | Major Bleeding Rate (%) LMWH / Warfarin | DVT rate (%) LMWH / Warfarin | PE rate (%) LMWH / Warfarin | Death Rate (%) LMWH / Warfarin | Cost-Effectiveness Result in 2009 USD* |
|---|---|---|---|---|---|---|---|---|
| THR Results from Short Horizon Analysis | ||||||||
| Sarasin 2002 | 90 days | Enoxaparin 40 mg + tinzaparin 4,000 - 5,000 IUs qd × 28 days post-discharge | INR 2-3 × 28 days post-discharge | 0.49 / 0.59 | 5.59 / 6.52¥ | 1.12 / 1.30 | ns /ns | LMWH ICER = $312 /VTE avoided |
| Hull 1997 | ns | Tinzaparin 5250 IUs qd × 9 days | INR 2-3 × 9 days | 2.80 / 1.50 | 20.8 / 23.2§ | ns / ns | ns / ns | LMWH ICER $970 /VTE avoided |
| Dranitsaris 2009 | 10 days | Dalteparin 5,000 IUs × 10 days | Warfarin INR 2-3 × 10 days | 6.6 / 4.5 | 4.4 / 6.7 | 0 / 0 | 0 / 0 | LMWH ICER $1300 /VTE |
| Botteman 2002 | ns | 30 mg bid × 7 days | 5 mg × 7 days | ns / ns | 13.6 / 21.3¥ | ns / ns | 0.70 / 1.10 | LMWH ICER = $2100 /VTE avoided |
| Caprini 2002 | 7 days | 30 mg bid × 7 days | INR 2-3 × 10 days | 1.20 / 0.50 | 2.00 / 4.00 | 0.50 / 0.90 | ns / ns | LMWH ICER = $2100 /VTE |
| Friedman 2000 | 21days | 40 mg qd × 21 days | 5 mg × 21 days | 0 / 1.00 | 8.00 / 10.0 | 0 / 0.99 | ns/ ns | LMWH ICER= $5200 /VTE avoided |
| Skedgel 2007 | 90 days | 40 mg qd × 28 days post-dicharge | 5 mg × 28 days post-discharge | 0.11 / 0.54 | 1.10 / 1.57 | 0.24/ 0.34 | 0.03/ 0.05 | LMWH ICER ? = $109,000 /VTE avoided |
| Dahl 2003 | 35 days | Dalteparin 5,000 IUs × 7-15 days | 5 mg × 7-15 days | ns / ns | 8.50 /8.30 | 2.30 / 0.90 | ns / ns | Warfarin dominates |
| Anderson 1998 | 90 days | 30 mg bid × 7-14 days | 5 mg; duration not specified | 2.04/ 0.98 | 2.40 / 2.40 | 1.10 / 1.10 | 0.10/ 0.09 | Warfarin dominates |
| Francis 1999 | 7 days | Dose and duration not specified | ns | 1.70 / 1.00 | 8.30 / 5.30 | 0.50 / 0 | ns / ns | Warfarin dominates |
| Wade 2000 | 30 days | 40 mg × 30 days | 5 mg × 30 days | 0 / 0 | 6.20 / 2.90 | 0 / 0 | ns /ns | Warfarin dominates |
| THR Results from Long Horizon Analysis | ||||||||
| Botteman 2002 | lifetime | 30 mg bid × 7 days | 5 mg × 7 days | ns / ns | ns / ns | ns / ns | ns / ns | LMWH dominates |
| Wade 1997 | 1 year | 30 mg bid × 7 days | 5 mg × 7 days | 2.80 / 1.30 | 4.75 / 3.45¥ | 1.95 / 0.75 | ns / ns | Warfarin dominates |
| TKR Results from Short Horizon Analysis | ||||||||
| Nerurkar 2002 | hospital period | 5-6 days dose not specified | 5-6 days dose not specified | 2.00 /3.00 | 5.85 / 3.55§ | ns / ns | 1.22/ 1.92 | LMWH dominates |
| Hull 1997 | ns | Tinzaparin 5250 IUs × 9 days | INR 2-3 × 9 days | 2.80 / 0.90 | 45.0 / 54.9§ | ns / ns | ns / ns | LMWH ICER = $950 /VTE avoided |
| Hawkins 1998 | ns | 30 mg bid × 4 days | 5 mg × 4 days | 2.10 / 1.80 | 20.90 / 35.1§ | ns / ns | 0 / 0 | LMWH ICER = $1100 /VTE avoided |
| Dranitsaris 2009 | 35 days | Dalteparin 5,000 | Warfarin INR 2-3 | 6.6 / 4.5 | 4.4 / 5.8 | 0 / 0 | 0 / 0 | LMWH ICER $1400 /VTE avoided |
| Anderson 1998 | 90 days | 30 mg bid × 7-14 days | 5 mg; duration not specified | 2.04/ 0.98 | 1.92 / 1.92 | 0.87 / 0.87 | 0.23 / 0.22 | Warfarin dominates |
| THR Combined with TKR Result | ||||||||
| Bell 2001 | 6 month | Ardeparin (dose and duration not specified) | ns | ns / ns | ns / ns | ns / ns | ns / ns | LMWH dominates |
Abbreviations: LMWH = low molecular weight heparin, ns = not specified, VTE = venous thromboembolism, QALY = quality adjusted life-year
Cost-effectiveness result is the incremental cost-effectiveness ratio (ICER). To arrive at ICER values, incremental costs reported in foreign currencies were inflated or deflated according to readily available consumer price indices, converted to USD via 2005 World Health Organization purchasing price parity indices, and then inflated to 2009 USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov.
ICER compares extended LMWH v extended duration warfarin
Radiographic VTE rate
DVT rate not specidied
In two studies of long horizon, results conflicted with one study[29] finding that LMWH dominated warfarin while the other [38] found the opposite.
In comparisons that analyzed cost-effectiveness in the setting of TKR (or TKR cases combined with THR cases), LMWH dominated or cost less than $2,000 / VTE avoided in four studies.[28, 34-36] In the final study[27], warfarin dominated LMWH.
Eight of 15 studies comparing LMWH to warfarin reported some pharmaceutical company sponsorship, grant support, or involvement of pharmaceutical company consultants. In each case, the pharmaceutical company was the manufacturer of LMWH, either Sanofi-Aventis, Pfizer, or a company which merged with these two. All but two[32, 37] of these eight found favorable cost effectiveness ratios for LMWH. The two studies by government agencies indicated that LMWH was either poor value for its cost or was dominated by warfarin.
Comparisons of Fondaparinux to LMWH
We included 10 studies with comparisons of fondaparinux to LMWH.[8, 9, 39-46] (Table 4) Nine of 10 analyzed prophylaxis for THR. Six studies [8, 9, 39, 40, 42, 46] analyzed cost-effectiveness over a short horizon. In all 6, fondaparinux dominated or cost less than $1300 /VTE avoided. In four studies with a long horizon, fondaparinux dominated LMWH. In a fifth, LMWH cost $40 /VTE avoided.
Table 4.
Summary of Cost-effectiveness Studies Comparing Fondaparinux to LWMH
| Study ID | Horizon | Fondaparinux regimen | LMWH regimen | Major Bleeding (%) rate Fondaparinux/LMWH | DVT rate (%) Fondaparinux/LMWH | PE rate (%) Fondaparinux/LMWH | Death Rate (%) Fondaparinux/LMWH | Cost-Effectiveness Result Reported by Study Authors, converted to 2009 USD * |
|---|---|---|---|---|---|---|---|---|
| THR Results from Short Horizon Analysis | ||||||||
| Dranitsaris 2004 | 90 days | Dose not specified; 7 days | Dose not specified; 7 days | difference = 0% | Fondaparinux 0.69% more averted | difference =0.41 | ns / ns | Fondaparinux dominates |
| Bjorvatn 2005 | 90 days | 2.5 mg qd × 7 days | 40 mg qd × 7 days | ns / ns | 1.84 / 2.71 | 0.58 / 1.09 | 0.14 / 0.22 | Fondaparinux dominates |
| Spruill 2004 (1) | 10 days | 3 mg; duration not specified | 30 mg bid; duration not specified | 4.5 / 3.5 | 0.90 / 2.90 | ns /ns | 0 / 0 | Fondaparinux dominates |
| Sullivan 2004 | 90 days | 2.5 mg bid × 7 days | 30 mg bid × 7days | ns / ns | Fondaparinux 1.16 % more VTEs averted | ns / ns | ns / ns | Fondaparinux dominates |
| Wade 2003 ‡ | 11 | 2.5 mg qd × 5-9 days | 30 mg bid × 5-9 days | 2.88 / 2.71 | 2.02 / 3.01 | 0.58 / 1.09 | 0.10 / 0.18 | Fondaparinux dominates |
| Annemans 2004 | 90 days | Dose not specified; 7 days | Dose not specified; 7 days | 2.87 / 2.70 | 1.85 / 2.73 | 0.58 / 1.09 | 0.10 / 0.18 | Fondaparinux ICER = $1300 /VTE avoided |
| THR Results from Long Horizon Analysis | ||||||||
| Annemans 2004 | 5 years | Dose not specified; 7 days | Dose not specified; 7 days | 2.88 / 2.71 | 2.02 / 3.01 | 0.58 / 1.09 | 0.10 / 0.18 | Fondaparinux dominates |
| Gordois 2003 | 5 years | Dose not specified; 7 days | 40 mg qd × 7 days | 2.80 / 2.60 | Fondaparinux 1.50 % more total VTEs averted | 0.58 / 1.09 | difference = 0.8 | Fondaparinux dominates |
| Sullivan 2004 | 5 years | 2.5 mg bid × 7 days | 30 mg bid × 7days | ns / ns | Fondaparinux 1.16 % more VTEs averted | ns / ns | ns / ns | Fondaparinux dominates |
| Szucs 2005 | 5 years | Dose not specified; 7 days | Dose not specified; 7 days | 2.85 / 2.69 | 1.96 / 2.88 | 0.59 / 1.09 | 0.11 / 0.18 | Fondaparinux dominates |
| Lundkvist 2003 | 5 years | Dose not specified; 7 days | 40 mg qd × 7 days | ns / ns | 1.84 / 2.71 | 0.58 / 1.09 | 0.11 / 0.19 | Fondaparinux ICER = $40 /VTE avoided |
| TKR Results from Short Horizon Analysis | ||||||||
| Bjorvatn 2005 | 90 days | 2.5 mg qd × 7 days | 40 mg qd × 7 days | ns / ns | 1.49 / 2.73 | 0.66 / 1.19 | 0.18 / 0.35 | Fondaparinux dominates |
| Dranitsaris 2004 | 90 days | Dose not specified; 7 days | Dose not specified; 7 days | difference =0% | Fondaparinux aparinux 1.27% more averted | difference = 0.54 | ns / ns | Fondaparinux dominates |
| Spruill 2004 (2) | ns | 2.5 mg qd × 4-5 days | 30 mg bid × 4-5 days | 2.1 / 0.20 | 2.40 / 5.40 | 0.20 / 0.80 | 0/0 | Fondaparinux dominates |
| Sullivan 2004 | 90 | 2.5 mg qd × 7 days | 30 mg bid × 7 days | ns / ns | Fondaparinux 1.78% more VTEs averted | ns / ns | ns / ns | Fondaparinux dominates |
| Annemans 2004 | 90 days | Dose not specified; 7 days | Dose not specified; 7 days | 2.87 / 2.71 | 1.50 / 2.75 | 0.66/ 1.19 | 0.12 / 0.19 | Fondaparinux ICER = $660 /VTE avoided |
| TKR Results from Long Horizon Analysis | ||||||||
| Annemans 2004 | 5 years | Dose not specified; 7 days | Dose not specified; 7 days | 2.87 / 2.71 | 1.68 / 3.11 | 0.66/ 1.19 | 0.12 / 0.19 | Fondaparinux dominates |
| Gordois 2003 | 5 years | 2.5 mg qd × 7 days | 40 mg qd × 7 days | 2.8 / 2.6 | Fondaparinux 1.95% more total VTEs averted | 0.66/ 1.19 | difference = 0.7 | Fondaparinux dominates |
| Lundkvist 2003 | 5 years | 2.5 mg qd × 7 days | 40 mg qd × 7 days | ns / ns | 1.49 / 2.73 | 0.66 / 1.19 | 0.12 / 0.20 | Fondaparinux dominates |
| Sullivan 2004 | 5 years | 2.5 mg qd × 7 days | 30 mg bid × 7 days | ns / ns | Fondaparinux 1.78% more VTEs averted | ns / ns | ns / ns | Fondaparinux dominates |
| Szucs 2005 | 5 years | Dose not specified; 7 days | Dose not specified × 8 days | 2.85 / 2.69 | 1.60 / 2.92 | 0.65 / 1.20 | 0.11 / 0.19 | Fondaparinux dominates |
Abbreviations: LMWH = low molecular weight heparin, ns = not specified, VTE = venous thromboembolism, QALY = quality adjusted life-year
Cost-effectiveness result is the incremental cost-effectiveness ratio (ICER). To arrive at ICER values, incremental costs reported in foreign currencies were inflated or deflated according to readily available consumer price indices, converted to USD via 2005 World Health Organization purchasing price parity indices, and then inflated to 2009 USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov.
Of the 8 studies reporting cost-effectiveness results for TKR[8, 9, 39, 40, 43-46], all but one found that fondaparinux dominated LMWH over the short and long horizon. In this study [43], fondaparinux, cost an additional 660 / VTE avoided.
Among the 10 studies comparing fondaparinux to LMWH, a pharmaceutical company sponsored one and supported five more through grants. In each case the sponsor or grantor was Sanofi-Aventis, the manufacturer of enoxaparin (the inferior comparator). Each result demonstrated good value with dominance by the use of fondaparinux.
Comparisons with New Oral Anticoagulants
Only two studies to date have made comparisons with new oral anticoagulants. In the only one which made this comparison in patients undergoing THR, Wolowacz et al.[53] found that dabigatran dominated LMWH over a 60 year horizon (equivalent to a lifetime analysis given the elderly age of the average patient undergoing THR).
In the setting of TKR, McCullagh et al.[52], found that in the short horizon of 180 days, rivarobaxan dominated both LMWH and dabigatran; dabigatran cost only an additional $750 /VTE avoided compared with LMWH. In the long horizon, Wolowacz et al. found that dabigatran dominated LMWH.
The study by Wolowacz et al. was sponsored by Boehringer Ingelheim, the manufacturer of dabigatran, whereas McCullagh reported no sponsorship or support.
Comparisons of Extended Duration to Short Duration Prophylaxis
We found nine studies[7, 10, 31, 47-52] with comparisons of extended duration vs. short duration prophylaxis in patients undergoing THR. (Table 5) Among short horizon results, three studies [31, 47, 52] with 5 comparisons, found that extended duration therapy after THR either dominated short duration prophylaxis or the ICER was less than $120 /VTE avoided. In Skedgel et al.[10], extended duration warfarin prophylaxis cost an additional $3,200 / VTE avoided but extended duration LMWH cost an additional $27,400 /VTE avoided. In five other studies, the ICER for extended duration therapy was between $7800 and 13,200 /VTE avoided. In McCullagh et al., dabigatran administered for 35 days cost $730,000 /VTE avoided compared with short duration LMWH; the high ICER results mainly from the many fold increased bleeding rates found with dabigatran compared with LMWH (2.0% vs. 0.08%).
Table 5.
Summary of Cost-effectiveness Studies with Comparisons Including New Oral Anticoagulants
| Study ID | Exact Horizon | Comparator 1 | Comparator 2 | Major Bleeding rate (%) Extended / Short | DVT rate Major Bleeding rate (%) Extended / Short | PE rate Major Bleeding rate (%) Extended / Short | Death Rate Major Bleeding rate (%) Extended / Short | Cost-Effectiveness Result in 2009 USD* |
|---|---|---|---|---|---|---|---|---|
| THR Result from Long Horizon Analysis | ||||||||
| Wolowacz 2009 | 60 years | Dabigatran 220 mcg × 28-35 days | Enoxaparin 40 mg × 28-35 days | 2.0 / 1.6 | 4.6 / 4.8 | 0.9 / 0.9 | 0.4 / 0.4 | Dabigatran dominated LMWH |
| TKR Results from Short Horizon Analysis | ||||||||
| McCullagh 2009 | 180 days | Rivarobaxan 10 mg × 14 days | Enoxaparin 40 mg × 10 days | 0.57 / 0.08 | 0.87 / 1.8 | 0 / 0.10 | 0 / 0 | Rivaroxaban dominated LMWH |
| McCullagh 2009 | 180 days | Rivarobaxan 10 mg × 14 days | Dabigatran 220 mcg × 10days | 0.57 / 1.5 | 0.87 / 2.7 | 0 / 0 | 0 / 0 | Rivaroxaban dominated dabigatran |
| McCullagh 2009 | 180 days | Dabigatran 220 mcg × 10 days | Enoxaparin 40 mg × 10 days | 1.5 / 1.3 | 2.7 / 1.8 | 0 / 0.10 | 0 / 0 | Dabigatran ICER $750 / VTE avoided |
| TKR Result from Long Horizon Analysis | ||||||||
| Wolowacz 2009 | 60 years | Dabigatran 220 mcg × 6-10 days | Enoxaparin 40 mg × 6-10 days | 1.5 / 1.3 | 12.1 / 12.4 | 2.1 / 2.2 | 1.7 / 1.7 | Dabigatran dominated LMWH |
Abbreviations: LMWH = low molecular weight heparin, ns = not specified, VTE = venous thromboembolism, QALY = quality adjusted life-year
Cost-effectiveness result is the incremental cost-effectiveness ratio (ICER). To arrive at ICER values, incremental costs reported in foreign currencies were inflated or deflated according to readily available consumer price indices, converted to USD via 2005 World Health Organization purchasing price parity indices, and then inflated to 2009 USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov.
Among two THR studies with long horizon results available, Bischof [7] found that extended duration fondaparinux dominated short duration fondaparinux. Haentjens et al. [50] found that extended duration enoxaparin cost an additional $9,300 /QALY gained compared with short duration enoxaparin.
For TKR, we found only two studies. At a 35 day horizon, Dranitsaris et al.[51] found that the extended duration dalteparin cost an additional $14,600 /VTE compared with short duration warfarin and $60,000 /VTE compared with short duration dalteparin. At a one year horizon, Haentjens et al.[50] found that extended duration enoxaparin cost an additional $73,000 /QALY gained compared with short duration enoxaparin.
Six of the 10 studies comparing extended duration to short duration therapy included pharmaceutical company sponsorship or grant support. Their was no clear trend among the results with respect to the presence of sponsorship although two of the three studies sponsored exclusively by a government agency found that extended duration therapy with LMWH or dabigatran delivered improved effectiveness at a relatively high cost (between $27,400 and $730,000 /VTE avoided). As mentioned above, the third study by Haentjens et al. found that extended duration LMWH was clearly cost-effective after THR but much less good value after TKR.
Discussion
Although multiple VTE prophylaxis regimens are supported by the American College of Chest Physicians (ACCP) and the American Academy of Orthopedic Surgeons (AAOS), our systematic review suggests that not all of them may be cost-effective relative to other regimens. There was no consensus about the cost-effectiveness of LMWH compared with warfarin. By contrast, fondaparinux dominated LMWH in nearly every comparison we found. Extended duration prophylaxis with LMWH after THR appeared to be cost-effective with multiple studies indicating extended duration prophylaxis dominates short duration LMWH or cost no more than an additional $10,000 /VTE avoided. Small numbers, predominance of studies analyzing only a short horizon, lack of established cost-effectiveness thresholds for VTE based effectiveness units, and reliance by study authors on venographic endpoints prohibit robust conclusions about the comparisons analyzed.
Comparisons of our work with previous reviews of the economic literature are limited by differences in type of surgery included and publication dates of the included articles. Sullivan et al.[54] summarized the prophylaxis literature between 1984 and 2000 and found that most studies presented consistent findings including that LMWH is cost-effective compared with warfarin. Our results do not support this conclusion. Sullivan et al. based their conclusions on many studies that we excluded because they were published prior to 1997 or which included outcomes from patients undergoing hip fracture surgery. We believe temporal trends[55, 56] in the care of total hip and knee replacement necessitated excluding earlier studies. We also felt that hip fracture surgery identified a distinctive patient population with respect to cost, risk, and benefit issues.[4] Similar to our findings, Sullivan et al. also found that extended duration LMWH was generally cost-effective compared with short duration therapy.
Ivanovic et al.[57] summarized the literature about fondaparinux. These authors concluded that fondaparinux was more cost-effective than LMWH (enoxaparin) 40 mg daily initiated preoperatively but less cost-effective than LMWH 30 mg twice daily initiated postoperatively. Our review did not specifically compare the cost-effectiveness of regimens with LMWH initiated at different times but we found that fondaparinux dominated LMWH in all but one when considering the longer horizon. LMWH dosages in the included studies were evenly distributed between 40 mg daily and 30 mg twice daily. Ivanovic et al. also report not being able to calculate ICERs for two studies whereas we were able to calculate them based on data presented in tables included by the study authors.
Wolowacz et al.[58] also published a review discussing the evolution of model building over a twenty year time span (1987-2006). In terms of quality, the findings of that review were generally consistent with the abstractions we performed, particularly with respect to the paucity of studies measuring QALYs over a sufficiently long period. Unlike their review, we abstracted cost and effect information and independently calculated incremental cost effectiveness ratios for each comparison discussed. We converted costs to 2009 USD and measured effects in common units (total VTE events avoided for short horizon studies and QALYs for long horizon studies). This facilitated comparisons between the multiple regimens supported by major professional societies.
The most salient finding of our review is that fondaparinux dominates LMWH. These results should, however, be interpreted cautiously. There have been only four randomized controlled trials comparing fondaparinux with enoxaparin[59-62] and only one[59] involved patients with TKR surgery. A summary estimate of risk calculated by Turpie et al[63] suggested that fondaparinux offers a 55% reduction in the odds of venographic VTE but no difference in the incidence of symptomatic VTE at postoperative day 11 when screening venography was performed. The studies of cost-effectiveness evaluating fondaparinux generally extrapolated these short horizon venographic rates to estimate the number of symptomatic VTE events. Recent evidence[64] suggests that the ratio of asymptomatic venographic DVT rate to symptomatic DVT rate is between 3 and 7 for THR and between 15 and 24 for TKR. These ratios, however, came from trials using enoxaparin only. Although they do not address this point specifically for fondaparinux, the 2008 ACCP guidelines[4] state that initial efficacy studies using venographic endpoints should be followed with trials that use symptomatic (and objectively confirmed) VTE as endpoints.
There is less conclusive evidence about the duration of prophylaxis although extended prophylaxis with LMWH appears cost-effective compared with short duration therapy in the case of THR surgery. Authors of cost-effectiveness studies included in this review generally summarized efficacy of extended duration prophylaxis with LMWH using one or more of the seven randomized controlled trials[65-71] which reported on the efficacy of extended duration prophylaxis. At least two of these trials[65, 66] did not require venography at the time of discharge from the hospital, permitting assessment of symptomatic VTE rates from four to seven weeks after operation. We cannot draw firm conclusions on the question of extended duration versus short duration of therapy with other agents which have not been studied extensively. Our review also suggests that there is insufficient cost-effectiveness evidence to support extended prophylaxis for TKR. The most recent update of the ACCP guidelines “recommends” extended prophylaxis for THR and “suggests” extended prophylaxis for TKR.
Limitations to our work include differences in economic perspective and setting. As our results overwhelmingly suggest that fondaparinux dominates LMWH, we believe our conclusions are sound for this comparison keeping in mind the absence of trial data measuring symptomatic endpoints. The economic perspective did not appear to explain the variations in results found but we did not have sufficient numbers of studies within each major comparison to make firm statements about the influence of individual differences in analytic methods. Although we converted from foreign currencies to USD using purchasing power parity, cost structures between countries may not be comparable as highlighted by Drumond and Tang[72].
We also acknowledge the potential bias exerted by pharmaceutical company sponsorship of multiple studies. This bias could have played a role in the comparisons between LMWH and warfarin and extended duration with short duration therapy. They do not appear to have played a role in the comparisons including fondaparinux. Multiple studies sponsored by the manufacturer of LMWH found fondaparinux to be dominant to LMWH. In general, however, we did not have sufficient numbers within each comparison type to determine if variation in study results was related to pharmaceutical company sponsorship
Another major limitation is that there is no established threshold for declaring a prophylaxis regimen cost-effective when disease based units are used to express effectiveness. The QALY permits comparing the value of interventions across diseases given that the utilities which are used to calculate them are standardized to estimates between 0 and 1 where 1 represents perfect health and 0 represents death.
Another limitation includes absence of cost-effectiveness analyses about certain comparisons such as fondaparinux versus warfarin, fondaparinux versus aspirin, and low intensity warfarin (INR < 2) vs. any of the other regimens. We also acknowledge the possibility of English language and publication bias as with any systematic review.
The demand for cost-effectiveness research is growing at a fervent pace. In early 2009, the U.S. government dedicated $1.1 billion to comparative effectiveness research including cost-effectiveness research.[73] The U.S. Centers for Disease Control adopted the results of cost-effectiveness research when it prepared guidelines[74] about screening for HIV infection. Similarly, the United States Preventive Services Task Forces incorporated model results when it updated its most recent colorectal cancer screening recommendations[75]. As the demand for cost-effectiveness work grows, the need to be able to summarize and standardize the information will grow as well. Our work was a comprehensive, systematic review of the cost-effectiveness literature regarding VTE prophylaxis for patients undergoing total joint replacement. In addition, we improved upon previous reviews by standardizing cost-effectiveness information to a common currency and effectiveness unit.
In summary, we found that fondaparinux dominated LMWH in virtually all studies we analyzed but firm conclusions cannot be made until trial data are available which measure symptomatic VTE rates. Extended duration LMWH prophylaxis also appears cost-effective compared with short duration prophylaxis in the case of THR. There is limited evidence to determine the cost-effectiveness of other regimens including extended duration fondaparinux, extended duration LMWH after TKR, prophylaxis with new oral anticoagulants, low-intensity warfarin therapy, or aspirin. These knowledge gaps represent important areas for future research.
Supplementary Material
Table 6.
Summary of Cost-effectiveness Studies Comparing Extended Duration Therapy with Short Duration Therapy
| study ID | Exact Horizon | Comparator 1 | Comparator 2 | Major Bleeding rate (%) Extended / Short | DVT rate Major Bleeding rate (%) Extended / Short | PE rate Major Bleeding rate (%) Extended / Short | Death Rate Major Bleeding rate (%) Extended / Short | Cost-Effectiveness Result in 2009 USD* |
|---|---|---|---|---|---|---|---|---|
| THR Results from Short Horizon Analysis | ||||||||
| Bergqvist 1999 | 19-23 days post discharge | Enoxaparin 40 mg × 30 days | Enoxaparin | 2.04 / 4.17 | 1.53 / 5.34 | 0 / 1.53 | 0 / 0 | Extended duration LMWH dominated |
| Dahl 2003 | 35 days | Dalteparin 5,000 IU qd × 28-35 days | Dalteparin 5,000 Ius × 7-15 days | ns / ns | 5.50 / 8.5 | 0.5 / 2.3 | ns / ns | Extended duration LMWH dominated |
| McCullagh 2009 | 180 days | Rivarobaxan 10 mg × 35 days | Dabigatran 220 mcg × 14 days | 0.08 / 2.0 | 0.29 / 0.93 | 0.40 / 0.50 | 0 / 0 | Extended duration rivaroxaban dominated |
| McCullagh 2009 | 180 days | Rivarobaxan 10 mg × 35 days | Enoxaparin 40 mg × 14 days | 0.08 / 0.08 | 0.29 / 2.2 | 0.12 / 0.50 | 0 / 0 | Extended duration rivaroxaban dominated |
| Dahl 2003 | 35 days | Dalteparin 5,000 IU qd × 28-35 days | Warfarin | ns / ns | 5.5/ 8.3 | 0.5 / 0.9 | ns / ns | Extended duration LMWH ICER = $120 /VTE avoided |
| Skedgel 2007 | 90 days | Warfarin 5 mg qd × 28 days post discharge | Regimen for hospital period not specified | 0.54 / 0.11 | 1.57 / 3.28 | 0.29 / 0.61 | 0.05 / 0.10 | Extended duration warfarin ICER = $3,200 /VTE avoided |
| Davies 2000 | 90 days | Enoxaparin 40 mg qd × hospitalization period + 21 days | Enoxaparin 40 mg qd for hospitalization period | ns / ns | 1.8 / 7.4 | ns / ns | 0.1 / 0.7 | Extended duration LMWH ICER = $7800 /VTE avoided |
| Dranitsaris 2009 | 35 days | Dalteparin 5,000 IUs × 35 days | Warfarin INR 2-3 × 10 days | 6.6 / 4.5 | 3.72 / 6.7 | 0 / 0 | 0 / 0 | Extended duration LMWH ICER $8,000 /VTE avoided |
| Detournay 1998 | 30-35 days | Enoxaparin 40 mg qd × 30-35 days | Enoxaparin 40 mg qd × 7-14 days | ns / ns | Extend-ed 16.0-21.1% more events avoided | ns / ns | Extended 0.60-0.78% more events avoided | Extended duration LMWH ICER= $10,000 / VTE avoided |
| Dranitsaris 2009 | 35 days | Dalteparin 5,000 IUs × 35 days | Dalteparin 5,000 IUs × 10 days | 6.6 / 6.7 | 3.72 / 5.3 | 0 / 0 | 0 / 0 | Extended LMWH ICER $13,200 / VTE avoided |
| Bischopf 2006 | 30 days | Fondaparinux × 28 days; dose not specified | Fondaparinux × 7 days; dose not specified | ns / ns | Fond 1.6% more events avoided | Fond 0.5% more events avoided | 0 / 0.1 | Extended duration fondaparinux ICER = $13,300 / Life-year gained |
| Skedgel 2007 | 90 days | LMWH 40 mg qd × 28 days post discharge | Regimen for hospital period not specified | 0.11 / 0.11 | 1.10 / 3.28 | 0.20 / 0.61 | 0.03 / 0.10 | Extended duration LMWH ICER = $27,400 / VTE avoided |
| McCullagh 2009 | 180 days | Dabigatran 220 mcg × 35 days | Enoxaparin 40 mg × 14 days | 2 / 0.08 | 0.93 / 2.2 | 0.40 / 0.50 | 0 / 0 | Extended duration dabigatran ICER = $730,00 / VTE avoided |
| THR Results from Long Horizon Analysis | ||||||||
| Bischof 2006 | 5 years | Fondaparinux × 28 days; dose not specified | Fondaparinux × 7 days; dose not specified | ns / ns | ns / ns | ns / ns | ns / ns | Extended duration fondaparinux dominates |
| Haentjens 2004 | 1 year | Enoxaparin × 42 days; dose not specified | Enoxaparin × 12 days; dose not specified | 1.7 / 1.7 | 5.12 / 8.95 | ns / ns | ns / ns | Extended duration LMWH ICER = $9,300 / QALY gained |
| TKR Results from Short Horizon Analysis | ||||||||
| Dranitsaris 2009 | 35 days | Dalteparin 5,000 IUs × 35 days | Warfarin INR 2-3 × 10 days | 6.7 / 4.8 | 4.0 / 5.8 | 0 / 0 | 0 / 0 | Extended LMWH ICER $14,600 / VTE |
| Dranitsaris 2009 | 35 days | Dalteparin 5,000 IUs × 35 days | Dalteparin 5,000 IUs × 10 days | 6.7 / 6.9 | 4.0 / 4.4 | 0 / 0 | 0 / 0 | Extended duration LMWH ICER $60,600 / VTE avoided |
| TKR Result from Long Horizon Analysis | ||||||||
| Haentjens 2004 | 1 year | Enoxaparin 40 mg qd × 42 days | Enoxaparin × 12 days; dose not specified | 0.5 / 0.5 | 6.81 / 7.70 | ns / ns | ns / ns | Extended duration LMWH ICER = $73,300 / QALY gained |
Abbreviations: LMWH = low molecular weight heparin, ns = not specified, VTE = venous thromboembolism, QALY = quality adjusted
Cost-effectiveness result is the incremental cost-effectiveness ratio (ICER). To arrive at ICER values, incremental costs reported in foreign currencies were inflated or deflated according to readily available consumer price indices, converted to USD via 2005 World Health Organization purchasing price parity indices, and then inflated to 2009 USD using the Bureau of Labor Statistics consumer price calculator available at www.bls.gov.
Acknowledgments
This work was performed at Boston Medical Center affiliated with Boston University School of Medicine
Abbreviation List
- AAOS
American Academy of Orthopedic Surgeons
- ACCP
American College of Chest Physicians
- DVT
Deep Venous Thrombosis
- ICER
Incremental cost-effectiveness ratio
- INR
International normalized ratio
- LMWH
Low molecular weight heparin
- PE
Pulmonary embolism
- PTS
Post-thrombotic syndrome
- QALY
Quality-adjusted life-year
- THR
Total hip replacement
- TKR
Total knee replacement
- USD
United States Dollar
- VTE
Venous thromboembolism
Footnotes
Conflicts of Interest: Dr. Kapoor has no conflicts of interest to report
Dr. Chuang has no conflicts of interest to report
Dr. Radhakrishnan has no conflicts of interest to report
Dr. Smith has no conflicts of interest to report
Dr. Berlowitz has no conflicts of interest to report
Dr. Segal has no conflicts of interest to report
Dr. Katz has no conflicts of interest to report
Dr. Losina has no conflicts of interest to report
Contributor Information
Alok Kapoor, Email: alok.kapoor@bmc.org.
Warren Chuang, Email: warren.chuang@bmc.org.
Nila Radhakrishnan, Email: nila.radhakrishnan@bmc.org.
Kenneth J. Smith, Email: smithkj2@upmc.edu.
Dan Berlowitz, Email: dberlow@bu.edu.
Jodi B Segal, Email: jsegal@jhmi.edu.
Jeffrey N. Katz, Email: jnkatz@partners.org.
Elena Losina, Email: elosina@partners.org.
References
- 1.Kapoor A, Chuang Warren, Radhakrishnan Nila, Silliman Rebecca, Smith Kenneth J, Berlowitz Daniel, et al. Predicting Total Injury and Venous Thromboembolism in Older Adults with Comorbidities Following Total Hip and Knee Replacement (awaiting manuscript review) Boston, MA: 2009. [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007 Apr 1;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. Journal of Bone & Joint Surgery - American Volume. 2007 Mar;89(3):526–33. doi: 10.2106/JBJS.F.00952. [DOI] [PubMed] [Google Scholar]
- 4.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Eighth ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2008;133(3 Suppl):381S–453. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 5.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl) doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty. [July 10, 2007];2007 doi: 10.5435/00124635-200903000-00007. Available from: http://www.aaos.org/Research/guidelines/PE_guideline.pdf. [DOI] [PubMed]
- 7.Bischof M, Leuppi JD, Sendi P. Cost-effectiveness of extended venous thromboembolism prophylaxis with fondaparinux in hip surgery patients. Expert Review of Pharmacoeconomics and Outcomes Research. 2006;6(2):171–80. doi: 10.1586/14737167.6.2.171. [DOI] [PubMed] [Google Scholar]
- 8.Szucs TD, Kaiser WE, Mahler F, Gutzwiller F. Thromboembolic prophylaxis with fondaparinux in major orthopaedic surgery: Outcomes and costs. HeartDrug. 2005;5(3):121–30. [Google Scholar]
- 9.Bjorvatn A, Kristiansen F. Fondaparinux sodium compared with enoxaparin sodium: a cost-effectiveness analysis. American Journal of Cardiovascular Drugs. 2005;5(2):121–30. doi: 10.2165/00129784-200505020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Skedgel C, Goeree R, Pleasance S, Thompson K, O’Brien B, Anderson D. The cost-effectiveness of extended-duration antithrombotic prophylaxis after total hip arthroplasty. Journal of Bone & Joint Surgery American Volume. 2007;89(4):819–28. doi: 10.2106/JBJS.F.00092. [DOI] [PubMed] [Google Scholar]
- 11.Alton V, Eckerlund I, Norlund A. Health economic evaluations: how to find them. Int J Technol Assess Health Care. 2006;22(4):512–7. doi: 10.1017/S0266462306051452. [DOI] [PubMed] [Google Scholar]
- 12.Centre for Reviews and Dissemination. Economic Evaluation Database. [2008 November 17];2008 Available from: http://www.crd.york.ac.uk/crdweb/
- 13.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996 Oct 9;276(14):1172–7. [PubMed] [Google Scholar]
- 14.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996 Oct 23-30;276(16):1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996 Oct 16;276(15):1253–8. [PubMed] [Google Scholar]
- 16.Bank of Canada. Consumer Price Index 1995 to present. [2008 July 12];2008 Available from: http://www.bankofcanada.ca/en/cpi.html.
- 17.European Commission. Harmonised Indices of Consumer Prices. [2009 November 22];2009 Available from: http://epp.eurostat.ec.europa.eu/portal/page/portal/hicp/data/main_tables.
- 18.World Health Organization. Purchasing Power Parity 2005. [2009 November 22];2009 Available from: http://www.who.int/choice/costs/ppp/en/index.html.
- 19.Bureau of Labor Statistics. Consumer Price Index. [2008 June 13];2008 Available from: http://www.bls.gov/cpi/
- 20.Bachmann M. Cost effectiveness of community-based therapeutic care for children with severe acute malnutrition in Zambia: decision tree model. Cost Effectiveness and Resource Allocation. 2009;7(1):2. doi: 10.1186/1478-7547-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond MF, O’Brien B, Stoddart G, Torrance G. Methods for Economic Evaluation of health Care Programmes. Oxford: Oxford University Press; 1997. [Google Scholar]
- 22.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. Journal of Bone & Joint Surgery - American Volume. 2005 Jun;87(6):1253–9. doi: 10.2106/JBJS.D.02152. [DOI] [PubMed] [Google Scholar]
- 23.Hunink M, Glasziou P, Siegel J. Decision Making in Health and Medicine. First. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 24.Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, et al. Bias in published cost effectiveness studies: systematic review. Bmj. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdool-Carrim T, Adler H, Becker P, Carides M, Ginsberg J, Golele R, et al. The cost and benefit of prophylaxis against deep vein thrombosis in elective hip replacement. DVT/PE Prophylaxis Consensus Forum. South African Medical Journal Suid Afrikaanse Tydskrif Vir Geneeskunde. 1997;87(5):594–600. [PubMed] [Google Scholar]
- 26.Sarasin FP, Bounameaux H. Out of hospital antithrombotic prophylaxis after total hip replacement: low-molecular-weight heparin, warfarin, aspirin or nothing? A cost-effectiveness analysis. Thrombosis & Haemostasis. 2002;87(4):586–92. [PubMed] [Google Scholar]
- 27.Anderson DR, O’Brien B, Nagpal S. Economic evaluation comparing low molecular weight heparin with other modalities for the prevention of deep vein thrombosis and pulmonary embolism following total hip or knee arthroplasty. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1998. [Google Scholar]
- 28.Bell GK, Goldhaber SZ. Cost implications of low molecular weight heparins as prophylaxis following total hip and knee replacement. Vascular Medicine. 2001;6(1):23–9. [PubMed] [Google Scholar]
- 29.Botteman MF, Caprini J, Stephens JM, Nadipelli V, Bell CF, Pashos CL, et al. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clinical Therapeutics. 2002;24(11):1960–86. doi: 10.1016/s0149-2918(02)80091-1. [DOI] [PubMed] [Google Scholar]
- 30.Caprini JA, Arcelus JI, Kudrna JC, Sehgal LR, Oyslender M, Maksimovic D, et al. Cost-effectiveness of venous thromboembolism prophylaxis after total hip replacement. Phlebology. 2002;17(3-4):126–33. [Google Scholar]
- 31.Dahl OE, Pleil AM. Investment in prolonged thromboprophylaxis with dalteparin improves clinical outcomes after hip replacement. Journal of Thrombosis & Haemostasis. 2003;1(5):896–906. doi: 10.1046/j.1538-7836.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 32.Francis CW, Pleil AM, Reinhart SP, Cohen B. A pharmacoeconomic evaluation of low-molecular-weight heparin in patients after total hip-replacement surgery. P and T. 1999;24(3):136–45. [Google Scholar]
- 33.Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clinical Orthopaedics & Related Research. 2000;370:171–82. doi: 10.1097/00003086-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins DW, Langley PC, Krueger KP. A pharmacoeconomic assessment of enoxaparin and warfarin as prophylaxis for deep vein thrombosis in patients undergoing knee replacement surgery. Clinical Therapeutics. 1998;20(1):182–95. doi: 10.1016/s0149-2918(98)80045-3. [DOI] [PubMed] [Google Scholar]
- 35.Hull RD, Raskob GE, Pineo GF, Feldstein W, Rosenbloom D, Gafni A, et al. Subcutaneous low-molecular-weight heparin vs warfarin for prophylaxis of deep vein thrombosis after hip or knee implantation. An economic perspective. Archives of Internal Medicine. 1997;157(3):298–303. [PubMed] [Google Scholar]
- 36.Nerurkar J, Wade WE, Martin BC. Cost/death averted with venous thromboembolism prophylaxis in patients undergoing total knee replacement or knee arthroplasty. Pharmacotherapy. 2002;22(8):990–1000. doi: 10.1592/phco.22.12.990.33609. [DOI] [PubMed] [Google Scholar]
- 37.Wade WE, Hawkins DW. Cost effectiveness of outpatient anticoagulant prophylaxis after total hip arthroplasty. Orthopedics. 2000;23(4):335–8. doi: 10.3928/0147-7447-20000401-15. [DOI] [PubMed] [Google Scholar]
- 38.Wade WE, Spruill WJ. Cost analysis of the American College of Chest Physicians guidelines for deep vein thrombosis prophylaxis in patients undergoing orthopedic arthroplastic surgery. Pharmacotherapy. 1997;17(6):1286–91. [PubMed] [Google Scholar]
- 39.Gordois A, Posnett J, Borris L, Bossuyt P, Jonsson B, Levy E, et al. The cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against thromboembolism following major orthopedic surgery. Journal of Thrombosis & Haemostasis. 2003;1(10):2167–74. doi: 10.1046/j.1538-7836.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Dranitsaris G, Kahn SR, Stumpo C, Paton TW, Martineau J, Smith R, et al. Pharmacoeconomic analysis of fondaparinux versus enoxaparin for the prevention of thromboembolic events in orthopedic surgery patients. American Journal of Cardiovascular Drugs. 2004;4(5):325–33. doi: 10.2165/00129784-200404050-00005. [DOI] [PubMed] [Google Scholar]
- 41.Wade WE, Spruill WJ, Leslie RB. Cost analysis: fondaparinux versus preoperative and postoperative enoxaparin as venous thromboembolic event prophylaxis in elective hip arthroplasty. American Journal of Orthopedics. 2003;32(4):201–5. [PubMed] [Google Scholar]
- 42.Spruill WJ, Wade WE, Leslie RB. Cost analysis of fondaparinux versus enoxaparin as venous thromboembolism prophylaxis in elective hip replacement surgery. Blood Coagulation & Fibrinolysis. 2004;15(7):539–43. doi: 10.1097/00001721-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Annemans L, Minjoulat-Rey MC, De Knock M, Vranckx K, Czarka M, Gabriel S, et al. Cost consequence analysis of fondaparinux versus enoxaparin in the prevention of venous thromboembolism after major orthopaedic surgery in Belgium. Acta Clinica Belgica. 2004;59(6):346–57. doi: 10.1179/acb.2004.050. [DOI] [PubMed] [Google Scholar]
- 44.Spruill WJ, Wade WE, Leslie RB. A cost analysis of fondaparinux versus enoxaparin in total knee arthroplasty. American Journal of Therapeutics. 2004;11(1):3–8. doi: 10.1097/00045391-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Lundkvist J, Bergqvist D, Jonsson B. Cost-effectiveness of fondaparinux vs. enoxaparin as venous thromboembolism prophylaxis in Sweden. European Journal of Health Economics. 2003;4(4):254–62. doi: 10.1007/s10198-003-0175-4. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SD, Davidson BL, Kahn SR, Muntz JE, Oster G, Raskob G. A cost-effectiveness analysis of fondaparinux sodium compared with enoxaparin sodium as prophylaxis against venous thromboembolism: use in patients undergoing major orthopaedic surgery. Pharmacoeconomics. 2004;22(9):605–20. doi: 10.2165/00019053-200422090-00005. [DOI] [PubMed] [Google Scholar]
- 47.Bergqvist D. Cost-Effectiveness of Prolonged Administration of a Low Molecular Weight Heparin for the Prevention of Deep Venous Thrombosis Following Total Hip Replacement. Value in Health. 1999;2(4):288–94. doi: 10.1046/j.1524-4733.1999.24003.x. [DOI] [PubMed] [Google Scholar]
- 48.Davies LM, Richardson GA, Cohen TA. Economic evaluation of enoxaparin as postdischarge prophylaxis for deep vein thrombosis (DVT) in elective hip surgery. Value in Health. 2000;3(6):397–406. doi: 10.1046/j.1524-4733.2000.36005.x. [DOI] [PubMed] [Google Scholar]
- 49.Detournay B, Planes A, Vochelle N, Fagnani F. Cost effectiveness of a low-molecular-weight heparin in prolonged prophylaxis against deep vein thrombosis after total hip replacement. Pharmacoeconomics. 1998;13(1 Pt 1):81–9. doi: 10.2165/00019053-199813010-00008. [DOI] [PubMed] [Google Scholar]
- 50.Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. A cost-utility analysis. Archives of Orthopaedic & Trauma Surgery. 2004;124(8):507–17. doi: 10.1007/s00402-004-0720-3. [DOI] [PubMed] [Google Scholar]
- 51.Dranitsaris G, Stumpo C, Smith R, Bartle W. Extended Dalteparin Prophylaxis for Venous Thromboembolic Events: Cost-Utility Analysis in Patients Undergoing Major Orthopedic Surgery. American Journal of Cardiovascular Drugs. 2009;9(14):45–8. doi: 10.2165/00129784-200909010-00005. [DOI] [PubMed] [Google Scholar]
- 52.McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics. 2009;27(10):829–46. doi: 10.2165/11313800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Wolowacz SE, Roskell NS, Maciver F, Beard SM, Robinson PA, Plumb JM, et al. Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clinical Therapeutics. 2009;31(1):194–212. doi: 10.1016/j.clinthera.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan SD, Kahn SR, Davidson BL, Borris L, Bossuyt P, Raskob G. Measuring the outcomes and pharmacoeconomic consequences of venous thromboembolism prophylaxis in major orthopaedic surgery. Pharmacoeconomics. 2003;21(7):477–96. doi: 10.2165/00019053-200321070-00003. [DOI] [PubMed] [Google Scholar]
- 55.Anderson FA, Jr, Audet AM. Physician practices in the prevention of deep vein thrombosis: the MassPRO DVT Study. Orthopedics. 1996;19:9–11. [PubMed] [Google Scholar]
- 56.Anderson FA, Jr, Hirsh J, White K, Fitzgerald RH, Jr Hip and Knee Registry I. Temporal trends in prevention of venous thromboembolism following primary total hip or knee arthroplasty 1996-2001: findings from the Hip and Knee Registry. Chest. 2003;124(6 Suppl) doi: 10.1378/chest.124.6_suppl.349s. [DOI] [PubMed] [Google Scholar]
- 57.Ivanovic N, Beinema M, Brouwers JR, Naunton M, Postma MJ. Thromboprophylaxis in total hip-replacement surgery in Europe: acenocoumarol, fondaparinux, dabigatran and rivaroxban. Expert Review of Pharmacoeconomics & Outcomes Research. 2007;7(1):49–58. doi: 10.1586/14737167.7.1.49. [DOI] [PubMed] [Google Scholar]
- 58.Wolowacz SE, Hess N, Brennan VK, Monz BU, Plumb JM. Cost-effectiveness of venous thromboembolism prophylaxis in total hip and knee replacement surgery: the evolving application of health economic modelling over 20 years. Current Medical Research and Opinion. 2008;24(10):2993–3006. doi: 10.1185/03007990802443255. [DOI] [PubMed] [Google Scholar]
- 59.Bauer KA, Eriksson BI, Lassen MR, Turpie AGG the Steering Committee of the Pentasaccharide in Major Knee Surgery S. Fondaparinux Compared with Enoxaparin for the Prevention of Venous Thromboembolism after Elective Major Knee Surgery. New England Journal of Medicine. 2001 Nov 1;345(18):1305–10. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson BI, Bauer KA, Lassen MR, Turpie AGG the Steering Committee of the Pentasaccharide in Hip-Fracture Surgery S. Fondaparinux Compared with Enoxaparin for the Prevention of Venous Thromboembolism after Hip-Fracture Surgery. New England Journal of Medicine. 2001 Nov 1;345(18):1298–304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 61.Lassen MR, Bauer KA, Eriksson BI, Turpie AG, Steering C, Lassen MR, et al. European Pentasaccharide Elective Surgery Study. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002 May 18;359(9319):1715–20. doi: 10.1016/S0140-6736(02)08652-X. [DOI] [PubMed] [Google Scholar]
- 62.Turpie AG, Bauer KA, Eriksson BI, Lassen MR, Committee PSS, Turpie AGG, et al. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002 May 18;359(9319):1721–6. doi: 10.1016/S0140-6736(02)08648-8. [DOI] [PubMed] [Google Scholar]
- 63.Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Archives of Internal Medicine. 2002;162(16):1833–40. doi: 10.1001/archinte.162.16.1833. [DOI] [PubMed] [Google Scholar]
- 64.Quinlan DJ, Eikelboom JW, Dahl OE, Eriksson BI, Sidhu PS, Hirsh J. Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. Journal of Thrombosis and Haemostasis. 2007;5(7):1438–43. doi: 10.1111/j.1538-7836.2007.02571.x. [DOI] [PubMed] [Google Scholar]
- 65.Bergqvist D, Benoni G, Bjorgell O, Fredin H, Hedlundh U, Nicolas S, et al. Low-Molecular-Weight Heparin (Enoxaparin) as Prophylaxis against Venous Thromboembolism after Total Hip Replacement. New England Journal of Medicine. 1996 Sep 5;335(10):696–700. doi: 10.1056/NEJM199609053351002. [DOI] [PubMed] [Google Scholar]
- 66.Comp PC, Spiro TE, Friedman RJ, Whitsett TL, Johnson GJ, Gardiner GA, et al. Prolonged Enoxaparin Therapy to Prevent Venous Thromboembolism After Primary Hip or Knee Replacement. J Bone Joint Surg Am. 2001 Mar 1;83(3):336–45. doi: 10.2106/00004623-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Dahl OE, Andreassen G, Aspelin T, Muller C, Mathiesen P, Nyhus S, et al. Prolonged thromboprophylaxis following hip replacement surgery--results of a double-blind, prospective, randomised, placebo-controlled study with dalteparin (Fragmin) Thrombosis & Haemostasis. 1997 Jan;77(1):26–31. [PubMed] [Google Scholar]
- 68.Heit JA, Elliott CG, Trowbridge AA, Morrey BF, Gent M, Hirsh J, et al. Ardeparin Sodium for Extended Out-of-Hospital Prophylaxis against Venous Thromboembolism after Total Hip or Knee Replacement: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Intern Med. 2000 Jun 6;132(11):853–61. doi: 10.7326/0003-4819-132-11-200006060-00002. [DOI] [PubMed] [Google Scholar]
- 69.Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, et al. Low-Molecular-Weight Heparin Prophylaxis Using Dalteparin Extended Out-of-Hospital vs In-Hospital Warfarin/Out-of-Hospital Placebo in Hip Arthroplasty Patients: A Double-blind, Randomized Comparison. Arch Intern Med. 2000 Jul 24;160(14):2208–15. doi: 10.1001/archinte.160.14.2208. [DOI] [PubMed] [Google Scholar]
- 70.Planes A, Vochelle N, Darmon JY, Fagola M, Bellaud M, Huet Y. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet. 1996 Jul 27;348(9022):224–8. doi: 10.1016/s0140-6736(96)01453-5. [DOI] [PubMed] [Google Scholar]
- 71.Prandoni P, Bruchi O, Sabbion P, Tanduo C, Scudeller A, Sardella C, et al. Prolonged Thromboprophylaxis With Oral Anticoagulants After Total Hip Arthroplasty: A Prospective Controlled Randomized Study. Arch Intern Med. 2002 Sep 23;162(17):1966–71. doi: 10.1001/archinte.162.17.1966. [DOI] [PubMed] [Google Scholar]
- 72.Drummond M, M A, editors. Economic evaluation in health care: merging theory with practice. New York: Oxford University Press; 2001. [Google Scholar]
- 73.Reinhardt U. Cost-Effectiveness Analysis and U.S. Health Care. New York Times. 2009 Mar 13; [Google Scholar]
- 74.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55(RR-14):1–17. [PubMed] [Google Scholar]
- 75.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2008 Nov 4;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


