Abstract
Purpose
Diet is a potentially modifiable risk factor for endometriosis. It has been hypothesized that vitamins C, E, and the B vitamins may influence factors involved in the pathogenesis of endometriosis, such as oxidative stress and steroid hormone metabolism. In this large, prospective cohort study, we examined the relation between intake of vitamins C, E, the B vitamins, and the use of multivitamin supplements and diagnosis of endometriosis.
Methods
Data were collected from women in the Nurses’ Health Study II between 1991 and 2005. Diet was assessed via food frequency questionnaire. Incidence rate ratios (RR) and 95% confidence intervals (CI) were estimated using time-varying Cox proportional hazards models.
Results
A total of 1383 incident cases of laparoscopically-confirmed endometriosis were observed among 70,617 women during 735,286 person years of follow-up. Intakes of thiamine (B1) (RR = 0.84, CI = 0.72–0.99; P-value, test for linear trend [P] = 0.04), folate (B9) (RR = 0.79, CI = 0.66–0.93; P = 0.003), vitamin C (RR = 0.81, CI = 0.68–0.95; P = 0.02), and vitamin E (RR = 0.70, CI = 0.59–0.83; P<0.0001) solely from food sources were inversely related to endometriosis diagnosis. However, intakes of these nutrients from supplements alone were unrelated to endometriosis.
Conclusion
Thiamine, folate, vitamin C, and vitamin E from food sources are inversely related to endometriosis risk. Our results suggest that the protective mechanism may not be related to the nutrients themselves but rather other components of foods rich in these micronutrients or factors correlated with diets high in these vitamin-rich foods.
Keywords: B vitamins, Diet, Endometriosis, Multivitamins, Prospective cohort, Vitamin C, Vitamin E
INTRODUCTION
Endometriosis is the third leading cause of gynecologic hospitalization in the United States (1). It is characterized by the deposition and growth of endometrial tissue outside the uterus and can lead to debilitating pain and infertility, both of which contribute to the high health care costs and substantial physical and psychological burden among those who suffer from the disease (2). Endometriosis has a complex and multifaceted etiology that is poorly understood, but likely involves hormonal, anatomic, genetic, immune, and inflammatory factors. Because retrograde menstruation occurs in <90% of women (3), factors that influence the volume of retrograde menstruation or affect a woman’s susceptibility to implantation are of etiologic interest (4).
Diet is a potentially modifiable risk factor for endometriosis. Few studies have explored the influence of nutrition on endometriosis risk, even though numerous books and websites suggest dietary changes for the prevention and control of endometriosis. Studying the intake of specific nutrients versus foods provides a way to assess etiologic hypotheses (5) regarding the impact of diet on elements of endometriosis pathogenesis, including oxidative stress, estrogen levels, and prostaglandin metabolism. For example, the antioxidant vitamins C and E may be involved in clearing free radicals and reactive oxygen species, which have been implicated in the growth and adhesion of endometrial cells in the peritoneal cavity in women with endometriosis (6). In addition, the B vitamins, particularly pyridoxine (B6), both enhance the metabolism of estrogen into an inactive form (7) and support the conversion of linoleic acid to gamma linolenic acid, an essential component of the production of series 1 anti-inflammatory prostaglandins (8). The action of these prostaglandins may inhibit the growth of endometrial tissue.
The literature on diet and endometriosis is sparse, and few studies have assessed the impact of individual nutrients on this condition. Smaller clinic-based cross-sectional studies have reported positive associations between self-reported vitamin C and E intake and endometriosis (9, 10), but another small clinical study looking at serum vitamin E levels did not find any association in multivariable analysis (6). A population-based case-control study found no association between any of the B vitamins, or vitamins C and E and odds of endometriosis diagnosis (11).
In this prospective study, we investigated the relation between intake of vitamins B, C, and E and the use of multivitamin supplements on the incidence of laparoscopically-confirmed endometriosis in the Nurses’ Health Study II cohort.
MATERIALS AND METHODS
Study population and data collection
The study population consists of participants in the Nurses’ Health Study II (NHSII) prospective cohort. In 1989, the baseline questionnaire was completed by 116,430 registered nurses ages 25–42 residing in 14 states. More than 90% of the cohort has consistently completed biennial questionnaires regarding incidence of disease outcomes and biologic, environmental, dietary, and lifestyle risk factors. Data for these analyses were collected from this cohort starting in 1991, when dietary data was first collected, and concluding in 2007.
Case ascertainment
On follow-up questionnaires beginning in 1993, participants were asked whether they had “physician-diagnosed endometriosis.” Those reporting the diagnosis were asked to provide further information regarding the time of diagnosis and whether they had received laparoscopic confirmation of the diagnosis. In 1994, a validation study of self-reported endometriosis was conducted (12). From the 1766 cases who reported an incident diagnosis on the 1993 questionnaire, 200 women were randomly selected and asked to complete a supplementary questionnaire and grant permission for review of their medical records. Among those who reported laparoscopic confirmation and for whom records were received and reviewed (n = 105), a laparoscopic diagnosis of endometriosis was confirmed in 96%. However, among those women without laparoscopic confirmation (n = 26), evidence of clinical diagnosis was found in only 54% of the records. Severity data (defined by the staging system outlined by the American Society for Reproductive Medicine) suggested that most laparoscopically-confirmed cases (61%) had minimal or mild disease. Endometriosis was the primary indication for hysterectomy in only 6% (n = 9/163) of women with documented hysterectomy indications. For women diagnosed with endometriosis during hysterectomy, it is not possible to discern whether any associated risk factors are related to endometriosis or to the hysterectomy indication. Only women with laparoscopically-confirmed disease were therefore included in analyses of incident diagnosis of endometriosis in order to minimize misclassification and avoid confounding by indication for hysterectomy.
Endometriosis has a complex relation with infertility among those with laparoscopically-confirmed diagnoses. The baseline prevalence of infertility (defined as the inability to become pregnant after attempting to conceive for one year or longer) among those with a laparoscopically-confirmed diagnosis was 20% compared with 4% among those who never received laparoscopic confirmation (12). It is likely the study population over-represents women with otherwise “asymptomatic” endometriosis secondary to the primary causes of infertility and these women would not have received a laparoscopic diagnosis of endometriosis had they not attempted to become pregnant. To examine potential etiologic heterogeneity between endometriosis in the setting of infertility and endometriosis among those who were never infertile, the risk factors for these two “subtypes” of endometriosis – as defined by the indication for diagnosis – were examined separately.
Dietary assessment
Participants completed a previously validated (13) semi-quantitative food frequency questionnaire (FFQ) in 1991, 1995, 1999, and 2003. The questionnaire asked participants to report the frequency of consumption of specific serving sizes of over 130 food items during the past year. In addition, participants were asked to report whether they used multivitamins or other nutrient supplements, and to provide the brand and dose. The nine possible frequency responses listed ranged from never or less than once per month to six or more times per day. Intake of thiamine (B1), niacin (B3), pyroxidine (B6), folate (B9), cobalamine (B12), C, and E was derived by multiplying frequency of consumption of specific food items by the nutrient composition according to the U.S. Department of Agriculture food composition database (14). The nutrient residual method was used to adjust for total energy intake and reduce other extraneous sources of variation (15).
To study differences in latent, short-term, and cumulative diet effects, micronutrient intake was classified according to three different durations of exposure: baseline intake reported in 1991, most recent intake, and cumulative averaged intakes. Cumulative averaged intakes were calculated by assigning the 1991 intake to the 1991–1995 follow-up period, the average of the 1991 and 1995 intake to the 1995–1999 follow-up period, the average of the 1991, 1995 and 1999 intake to the 1999–2003 follow-up period, and the average of the 1991, 1995, 1999, and 2003 intake to the 2003–2007 follow-up period. This approach provides the best approximation of long-term intake. Because risk of endometriosis diagnosis did not differ by the timing of exposure for any of the micronutrients examined (i.e. latent, most recent, and cumulative averaged intakes did not yield different effect estimates), only results for cumulative average intakes (which are least vulnerable to within-person variation [5]) are presented.
Statistical analysis
The initial FFQ was completed by 97,807 women in 1991. Women who had an implausible total energy intake (<800 or >4200 kcal/day) or who left more than 70 food items blank in the 1991 FFQ were excluded. Because endometriosis rarely develops after natural or surgical menopause, all analyses were restricted to premenopausal women with intact uteri. Women who reported a prior diagnosis of endometriosis, history of infertility, diagnosis of cancer other than non-melanoma skin cancer, or hysterectomy before 1991 were also excluded.
Women were followed from the return of the 1991 questionnaire until self-report of laparoscopically-confirmed endometriosis diagnosis or one of the following censoring events: death, cancer diagnosis (other than non-melanoma skin cancer), hysterectomy, the onset of menopause, or end of the study period, whichever occurred first. Women were also censored at the time of self-reported infertility, as infertility is strongly associated with laparoscopic diagnosis of endometriosis. The resulting comparison groups therefore do not contain women diagnosed with endometriosis or past infertility.
Age- and multivariable-adjusted incidence rate ratios (RR) for laparoscopically-confirmed endometriosis and 95% confidence intervals (95% CI) were estimated using time-varying Cox proportional hazards models with age in months reported in the two-year questionnaire cycles as the time scale. The incidence rate ratio was estimated as the rate in a given quintile of micronutrient intake divided by the rate in the lowest quintile. Separate models were fit for total intake (from foods and supplements), intake from foods alone and intake from supplements alone. Both age-adjusted and multivariable models included terms for total energy intake. Multivariable models were further adjusted for the following potential confounders: race, parity, age at menarche, menstrual cycle length and body mass index (BMI). Tests for linear trend in ordinal categoric exposures were conducted by assigning the median intake value of each category to all participants in that group. Tests for heterogeneity comparing the effect estimates among cases who never reported infertility to cases with concurrent infertility were calculated with a two-sided Wald statistic referred to a chi-squared distribution with one degree of freedom. Smoking (never vs. ever) and BMI (<25 kg/m2, ≥25 kg/m2) were assessed as potential modifiers of the relation between micronutrient intake and endometriosis. Likelihood ratio tests were used to compare the fit of the main effects model to that of the model with both main effects and interaction terms to calculate tests for heterogeneity. Stepwise restricted cubic splines were used to assess non-linearity of results and produce graphs of association (16, 17).
RESULTS
A total of 1383 incident diagnoses of endometriosis were made among 70,617 women during 735,286 person years of follow-up. These cases included 1128 cases who never reported infertility and 253 cases who received an infertility assessment during the same follow-up period as laparoscopic confirmation of endometriosis. The distribution of baseline characteristics within extreme quintiles of vitamin E intake from both foods and supplements was similar to that of vitamin E intake from foods alone, with the exception of BMI (Tab. I). A smaller proportion of women reporting the highest intake of vitamin E from both foods and supplements were overweight or obese, whereas similar proportions of overweight and obese women were observed between extreme quintiles of vitamin E intake from foods alone. The only other micronutrient-specific difference observed (data not shown) was the distribution of thiamin intake from foods and supplements by parity; women in the highest quintile compared to the lowest quintile of thiamine intake from foods and supplements were more frequently nulliparous (30.8% vs. 23.9%) and, less frequently, had two or more children (48.2% vs. 58.3%).
TABLE I.
DISTRIBUTION OF POTENTIAL RISK FACTORS FOR ENDOMETRIOSIS ACCORDING TO EXTREME QUINTILES OF VITAMIN E INTAKE FROM FOODS AND SUPPLEMENTS AND FROM FOODS ALONE REPORTED IN 1991 AMONG WOMEN IN THE NURSES’ HEALTH STUDY II
| Quintile of Intake (mg/day) | ||||
|---|---|---|---|---|
|
| ||||
| Foods and Supplements | Foods Alone | |||
|
| ||||
| Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | |
| No. of women | 14346 | 13714 | 13929 | 13846 |
| Age in 1991 (y) | 35.4 | 35.7 | 34.8 | 36.3 |
| Caucasian % | 91.5 | 92.5 | 91.1 | 92.5 |
| BMI (kg/m2) % | ||||
| <19 | 5.2 | 5.5 | 5.75 | 4.6 |
| 19–24.9 | 58.8 | 57.8 | 57.6 | 57.9 |
| 25–29.9 | 18.6 | 16.0 | 17.0 | 18.9 |
| ≥30 | 13.4 | 9.5 | 12.2 | 11.6 |
| Cigarette Smoking % | ||||
| Never | 64.9 | 66.4 | 65.7 | 65.0 |
| Past | 19.2 | 23.0 | 18.8 | 24.4 |
| Current | 15.7 | 10.4 | 15.4 | 10.4 |
| Age at menarche % | ||||
| <12 years | 22.8 | 23.4 | 21.5 | 25.4 |
| 12 years | 30.0 | 30.1 | 30.3 | 30.3 |
| >12 years | 46.8 | 46.2 | 48.1 | 44.0 |
| Menstrual cycle length during ages 18 to 22 % | ||||
| <26 days | 10.6 | 11.0 | 10.3 | 11.1 |
| 26–31 days | 67.7 | 67.4 | 67.6 | 67.5 |
| 32–39 days | 15.0 | 14.7 | 15.1 | 14.7 |
| ≥40 days or too irregular | 6.7 | 6.9 | 6.9 | 6.6 |
| Oral contraceptive use % | 84.4 | 83.4 | 83.9 | 82.4 |
| Parity % | ||||
| Nulliparous | 23.3 | 33.3 | 22.6 | 32.0 |
| 1 | 15.3 | 18.4 | 16.1 | 17.3 |
| ≥2 | 60.1 | 46.5 | 59.6 | 49.1 |
BMI, body mass index; y, years.
In age-adjusted analyses, intakes of niacin, vitamin C and vitamin E were positively related to a higher risk of endometriosis (Tab. II). These associations were driven by a higher rate of endometriosis diagnosis in the highest quintile of intake compared to the bottom quintile (approximately 15%), but there was no clear dose-response relation across quintiles 2 to 4. No clear pattern of association could be revealed in the multivariable-adjusted models, although the tests for linear trend for total niacin and vitamin E intakes remained statistically significant.
TABLE II.
RATE RATIOS (RR) AND 95% CONFIDENCE INTERVALS (CIs) FOR LAPAROSCOPICALLY-CONFIRMED ENDOMETRIOSIS (N=1383) ACCORDING TO QUINTILE OF CUMULATIVE AVERAGED MICRONUTRIENT INTAKE FROM ALL SOURCES (FOODS AND SUPPLEMENTS) AMONG WOMEN IN THE NURSES’ HEALTH STUDY II, 1991–2007
| Quintile of Intake
|
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-value* | |
| Thiamine (B1) | ||||||
| Median intake, mg | 1.3 | 1.6 | 2.0 | 2.9 | 8.1 | |
| Number of cases | 288 | 255 | 278 | 274 | 287 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.85 (0.72, 1.01) | 0.91 (0.77, 1.08) | 0.89 (0.75, 1.05) | 1.01 (0.85, 1.19) | 0.21 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.89 (0.75, 1.05) | 0.95 (0.80, 1.13) | 0.92 (0.78,1.09) | 0.99 (0.84, 1.17) | 0.51 |
| Niacin (B3) | ||||||
| Median intake, mg | 20.2 | 24.7 | 29.7 | 38.1 | 59.1 | |
| Number of cases | 283 | 256 | 253 | 279 | 311 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.88 (0.74, 1.05) | 0.87 (0.73, 1.03) | 0.95 (0.81, 1.13) | 1.17 (1.00, 1.38) | 0.002 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.91 (0.76, 1.07) | 0.88 (0.74, 1.05) | 0.96 (0.81, 1.14) | 1.11 (0.94, 1.30) | 0.03 |
| Pyridoxine (B6) | ||||||
| Median intake, mg | 1.7 | 2.2 | 2.8 | 4.1 | 15.6 | |
| Number of cases | 270 | 267 | 279 | 294 | 272 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.91 (0.77, 1.08) | 0.95 (0.80, 1.13) | 1.05 (0.89, 1.24) | 0.96 (0.81, 1.14) | 0.90 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.91 (0.77, 1.08) | 0.94 (0.79, 1.11) | 1.03 (0.87, 1.21) | 0.93 (0.79, 1.10) | 0.66 |
| Folate (B9) | ||||||
| Median intake, mcg | 239.0 | 319.0 | 416.2 | 575.0 | 819.4 | |
| Number of cases | 277 | 277 | 288 | 266 | 274 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.96 (0.81, 1.13) | 1.00 (0.85, 1.18) | 0.93 (0.78, 1.10) | 0.92 (0.78, 1.09) | 0.26 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.95 (0.81, 1.13) | 0.97 (0.82, 1.15) | 0.88 (0.74, 1.04) | 0.91 (0.77, 1.08) | 0.19 |
| Cobalamine (B12) | ||||||
| Median intake, mcg | 4.0 | 6.0 | 8.0 | 11.0 | 18.5 | |
| Number of cases | 244 | 319 | 258 | 292 | 269 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 1.11 (0.94, 1.31) | 1.09 (0.92, 1.30) | 1.14 (0.96, 1.35) | 1.10 (0.92, 1.30) | 0.51 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 1.13 (0.95, 1.33) | 1.10 (0.92, 1.31) | 1.13 (0.95, 1.34) | 1.08 (0.91, 1.28) | 0.71 |
| Vitamin C | ||||||
| Median intake, mg | 84.0 | 127.0 | 172.2 | 250.5 | 604.0 | |
| Number of cases | 294 | 257 | 275 | 250 | 306 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.87 (0.74, 1.03) | 0.92 (0.78, 1.09) | 0.86 (0.73, 1.02) | 1.13 (0.96, 1.33) | 0.007 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.88 (0.74, 1.04) | 0.92 (0.78, 1.09) | 0.85 (0.71, 1.00) | 1.02 (0.87, 1.20) | 0.26 |
| Vitamin E | ||||||
| Median intake, mg | 5.1 | 6.8 | 12.2 | 25.3 | 153.0 | |
| Number of cases | 285 | 274 | 261 | 258 | 304 | |
| Age-adjusted RR (95% CI) | 1.00 (referent) | 0.96 (0.81, 1.13) | 0.89 (0.75, 1.06) | 0.90 (0.76, 1.07) | 1.17 (0.99, 1.38) | 0.005 |
| Multivariable RR (95% CI)+ | 1.00 (referent) | 0.93 (0.78, 1.09) | 0.85 (0.72, 1.01) | 0.86 (0.73, 1.02) | 1.05 (0.89, 1.23) | 0.05 |
P-value, test for linear trend calculated with median intake of the micronutrient in each quintile as a continuous variable.
Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), race (Caucasian vs. women of color), and total energy (continuous).
CI, confidence interval; RR, rate ratio.
We then examined supplement intake (Tab. III) and intake from food sources (Tab. IV) separately for the same micronutrients. Similar to the findings for total intakes, supplemental intakes of niacin, vitamin C, and vitamin E were associated with an increased rate of endometriosis diagnosis in age-adjusted analyses. The associations with these three nutrients were slightly stronger than the associations for total intake with approximately a 30% greater risk when comparing the top to bottom quintile. However, these associations did not remain in the multivariable-adjusted models.
TABLE III.
MULTIVARIABLE RATE RATIOS (RR) AND 95% CONFIDENCE INTERVALS (CIs) FOR LAPAROSCOPICALLY-CONFIRMED ENDOMETRIOSIS (N = 1383) ACCORDING TO QUINTILE OF CUMULATIVE AVERAGED MICRONUTRIENT INTAKE FROM SUPPLEMENTS ALONE AMONG WOMEN IN THE NURSES’ HEALTH STUDY II, 1991–2007
| Quintile of Intake
|
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-value* | |
| Thiamine (B1) | 1.00 (referent) | 1.17 (0.87, 1.56) | 1.13 (0.84, 1.54) | 1.20 (0.89, 1.61) | 1.22 (0.91, 1.65) | 0.34 |
| Niacin (B3) | 1.00 (referent) | 1.15 (0.85, 1.54) | 1.08 (0.79, 1.46) | 1.23 (0.91, 1.65) | 1.23 (0.91, 1.66) | 0.15 |
| Pyridoxine (B6) | 1.00 (referent) | 0.97 (0.81, 1.15) | 1.03 (0.87, 1.22) | 1.07 (0.91, 1.26) | 1.00 (0.84, 1.18) | 0.91 |
| Folate (B9) | 1.00 (referent) | 1.33 (0.98, 1.80) | 1.20 (0.87, 1.65) | 1.36 (1.00, 1.84) | 1.27 (0.94, 1.72) | 0.91 |
| Cobalamine (B12) | 1.00 (referent) | 1.00 (0.85, 1.19) | 1.08 (0.91, 1.28) | 1.16 (0.98, 1.38) | 1.00 (0.85, 1.20) | 0.87 |
| Vitamin C | 1.00 (referent) | 1.07 (0.87, 1.31) | 1.01 (0.83, 1.24) | 1.01 (0.83, 1.23) | 1.16 (0.95, 1.41) | 0.14 |
| Vitamin E | 1.00 (referent) | 1.08 (0.88, 1.34) | 1.10 (0.89, 1.35) | 1.00 (0.82, 1.23) | 1.21 (0.99, 1.48) | 0.04 |
| Frequency of Multivitamin Use | ||||||
| None | 0–2 per wk | 3–5 per wk | >5 per wk | |||
| Multivitamins | 1.00 (referent) | 0.93 (0.79, 1.11) | 0.97 (0.84, 1.13) | 1.08 (0.94, 1.23) | 0.34 | |
P-value, test for linear trend calculated with median intake of the micronutrient in each quintile as a continuous variable.
Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), race (Caucasian vs. women of color), and total energy (continuous).
CI, confidence interval; RR, rate ratio.
TABLE IV.
MULTIVARIABLE RATE RATIOS (RR) AND 95% CONFIDENCE INTERVALS (CIs) FOR LAPAROSCOPICALLY-CONFIRMED ENDOMETRIOSIS (N = 1383) ACCORDING TO QUINTILE OF CUMULATIVE AVERAGED MICRONUTRIENT INTAKE FROM FOODS ALONE AMONG WOMEN IN THE NURSES’ HEALTH STUDY II, 1991–2007
| Quintile of Intake
|
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-value* | |
| Thiamine (B1) | 1.00 (referent) | 0.88 (0.75, 1.03) | 0.88 (0.75, 1.04) | 0.85 (0.72, 1.00) | 0.84 (0.72, 0.99) | 0.04 |
| Niacin (B3) | 1.00 (referent) | 1.03 (0.87, 1.21) | 0.92 (0.78, 1.09) | 0.99 (0.84, 1.17) | 0.99 (0.84, 1.17) | 0.80 |
| Pyridoxine (B6) | 1.00 (referent) | 0.95 (0.81, 1.12) | 0.88 (0.75, 1.04) | 0.80 (0.67, 0.95) | 0.99 (0.84, 1.16) | 0.50 |
| Folate (B9) | 1.00 (referent) | 0.89 (0.75, 1.04) | 0.84 (0.72, 0.99) | 0.80 (0.68, 0.94) | 0.79 (0.66, 0.93) | 0.003 |
| Cobalamine (B12) | 1.00 (referent) | 1.11 (0.94, 1.31) | 1.20 (1.02, 1.41) | 1.03 (0.87, 1.22) | 1.04 (0.87, 1.23) | 0.86 |
| Vitamin C | 1.00 (referent) | 0.89 (0.76, 1.05) | 0.82 (0.70, 0.97) | 0.87 (0.74, 1.02) | 0.81 (0.68, 0.95) | 0.02 |
| Vitamin E | 1.00 (referent) | 0.85 (0.73, 1.00) | 0.87 (0.74, 1.03) | 0.80 (0.68, 0.94) | 0.70 (0.59, 0.83) | <.0001 |
P-value, test for linear trend calculated with median intake of the micronutrient in each quintile as a continuous variable.
Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), race (Caucasian vs. women of color), and total energy (continuous).
Analyses restricted to food sources of these nutrients, however, revealed inverse associations of thiamine (B1), folate (B9), vitamin C and vitamin E intakes with risk of laparoscopically-confirmed endometriosis (Tab. IV). Multivariate-adjusted comparisons between extreme quintiles of intakes from foods revealed a 16% lower risk for thiamin (B1), 21% lower risk for folate (B9), 19% lower risk for vitamin C. Intake of vitamin E from foods was associated with the largest risk reduction when comparing extreme quintiles. Those in the highest quintile of vitamin E intake had a 30% lower risk for endometriosis compared to those in the lowest quintile. Intakes of niacin (B3), pyroxidine (B6), and cobalamine (B6) from foods remained unassociated with endometriosis diagnosis risk.
Next, we examined whether the associations between micronutrient intake from food sources and endometriosis differed between cases with concurrent infertility and never infertile cases (i.e. those presenting solely with pain symptoms) (data not shown). The inverse associations observed between dietary intake of thiamine, folate, vitamin C, and vitamin E appeared to be stronger among never infertile women. The rate ratios (95% CI; P-value, test for linear trend) comparing top to bottom quintiles of intake from foods were 0.82 (0.68–0.98; 0.04) for thiamine, 0.73 (0.61–0.88; <0.001) for folate, 0.75 (0.62–0.88; 0.004) for vitamin C, and 0.71 (0.59–0.86; <0.001) for vitamin E. However, none of the tests for heterogeneity, which compared the associations observed among the never infertile women to those among concurrently infertile women, suggested any statistically significant differences between the two endometriosis case groups.
Lastly, we examined cigarette smoking, BMI, and parity as potential modifiers of the relation between micronutrient intake and endometriosis risk. Smoking modified the association between vitamin C intake from foods and laparoscopically-confirmed endometriosis diagnosis (P-value, test for heterogeneity <0.0001), and the stratified results showed vitamin C to exert a greater magnitude of protection among smokers (Fig. 1). Compared to smokers in the lowest quintile of vitamin C from foods, the multivariable rate ratio for smokers in the highest quintile of vitamin C was 0.58 (95% CI = 0.42–0.79), whereas the multivariable rate ratio for non-smokers in the highest quintile of vitamin C intake versus those in the lowest quintile was 0.92 (95% CI = 0.75–1.12). The magnitude of the association between pyroxidine intake from foods and endometriosis significantly differed between those with a healthy BMI (<25 kg/m2), and those who were overweight or obese (≥25 kg/m2) (P-value, test for heterogeneity = 0.02). No statistically significant interactions were observed between parity and any of the micronutrients examined.
Fig. 1.
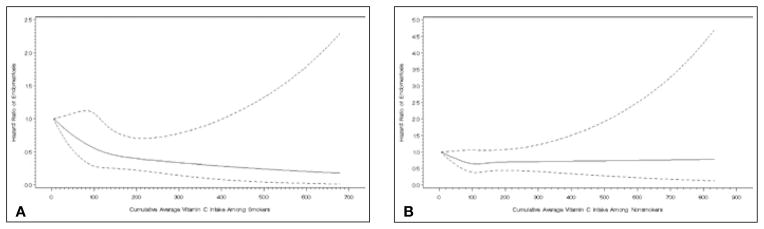
Difference in association between vitamin C and incidence of laparoscopic-confirmation of endometriosis between (A) smokers and (B) non-smokers (P-value, test for heterogeneity <0.0001).
DISCUSSION
In this large prospective cohort study, we observed inverse associations of thiamine, folate, vitamin C, and vitamin E intakes from food sources with laparoscopically confirmed endometriosis. Intake from supplements showed no significant relation to endometriosis. The disparity between the findings for intake from foods and intake from supplements could suggest that the associations observed with these antioxidant micronutrients may not be related to the nutrients themselves but rather to other factors present in dietary patterns rich in these nutrients that could not be identified in this study. Our findings of an inverse association of antioxidant vitamin intakes from food sources with endometriosis risk contrast with the results from the one previous study to examine intake of thiamine, niacin, pyroxidine, folate, cobalamine, vitamin C, and vitamin E in relation to endometriosis risk. The authors of this case-control study reported finding null associations between each nutrient and endometriosis both before and after the inclusion of supplemental sources into their calculations of intake (11).
We observed that intake of vitamin E from food sources was associated with the largest reduction in the rate of endometriosis diagnosis. The presence of mayonnaise, margarine, peanut butter, fried foods consumed away from home, and potato chips as common sources of vitamin E (Tab. V), suggests that vitamin E intake in this cohort is largely derived from foods high in fat content. As we have previously published, in this cohort total fat consumption was not significantly related to endometriosis risk (18), although consumption of long-chain omega-3 fatty acids was linked to a 22% (95% CI 0.62–0.99) decreased rate of endometriosis diagnosis. A previous case-control study found that total fat consumption was associated with a 50% (95% CI 0.2–1.0) reduced odds of endometriosis (11). We examined the possibility of confounding by fat intake by adjusting for total fat in a supplemental analysis of the association of vitamin E intake from food sources and endometriosis risk but observed no change in the rate ratio (data not shown).
TABLE V.
TOP 10 MOST COMMON SOURCES OF SELECTED VITAMINS IN 1991 AMONG THE NURSES’ HEALTH STUDY II COHORT
| Thiamine | Vitamin C | Folate | Vitamin E |
|---|---|---|---|
| 1. Cold cereal | 1. Orange juice | 1. Cold cereal | 1. Cold cereal |
| 2. Pork | 2. Broccoli | 2. Pizza | 2. Mayonnaise |
| 3. Skimmed milk | 3. Oranges | 3. Orange juice | 3. Turkey |
| 4. Pizza | 4. Other fruit juices | 4. Romaine lettuce | 4. Apple |
| 5. Pasta | 5. Other drinks | 5. Skimmed milk | 5. Peanut butter |
| 6. Ham from sandwiches | 6. Tomatoes | 6. Iceberg lettuce | 6. Fried food away from home |
| 7. Dark bread | 7. Strawberries | 7. Tea | 7. Potato chips |
| 8. English muffins | 8. Cantaloupe | 8. Broccoli | 8. Yams |
| 9. White bread | 9. Cold cereal | 9. Beans | 9. Margarine |
| 10. Mashed potatoes | 10. Mashed potatoes | 10. Dark bread | 10. Tomato sauce |
We also found that the relation between vitamin C intake and endometriosis was stronger among smokers than among non-smokers. This observation warrants further study given the contradictory findings surrounding smoking and endometriosis within the literature. Smoking itself may be “protective” against endometriosis (19–21) because of the estrogen deficiency brought about by smoking, although not all studies have observed an inverse relation (22–26). We previously observed a reduced risk of endometriosis diagnosis among smokers with concurrent infertility but an increased risk with smokers with no history of infertility in this cohort (12). Given the high levels of free radicals found in cigarette smoke (27), one hypothesis by which vitamin C is more protective against endometriosis under conditions of cigarette smoke exposure may be related to its role in minimizing oxidative stress, which could potentially be more prevalent in smokers with the disease.
It is important to consider the strengths and limitations of this study. Of greatest importance is the inability to discern the effects that micronutrient intake may exert on symptom severity that could impact the likelihood of surgical diagnosis versus effects on the establishment and progression of endometriosis itself. Indeed, this is a study of the incidence of surgical diagnosis of disease. Therefore, if vitamin C, vitamin E or B vitamins consumption affects pain symptoms, then this would manifest as the appearance of a difference in the incidence of laparoscopically-confirmed diagnosis, and not the incidence of endometriosis itself. Given the data at hand, we cannot address this concern directly. However, associations did not statistically significantly differ between women diagnosed following presentation with only pain symptoms compared to those who presented with infertility (among whom only a portion experienced pain symptoms). If the association was driven solely by an effect on pain, then we would expect to observe the relations between nutrient consumption and endometriosis diagnosis to be stronger among those with no history of infertility. Finally, it is known that endometriosis symptoms may exist for years prior to surgical confirmation (28), therefore compromising the “prospective” nature of exposure data collection. However, as we did not observe a difference between the associations observed with baseline dietary intake (as much as 16 years prior to surgical diagnosis), and with most recent intake (no more than two years prior to surgical diagnosis), there was no evidence of recall bias.
The strengths of the study include the prospective assessment of dietary information and case status thus minimizing the potential for recall bias and reverse causation. We were also able to finely control for several potential confounders. The use of previously validated dietary assessment tools is also a major strength of our study, as is the use of repeated measures of diet, which minimizes within-person variability over time leading to a reduction in misclassification of dietary exposures. The magnitude of the observed significant effects (ranging from 15% to 30% reduced rate of endometriosis diagnosis among those in the upper most quintile of nutrient intake) is sizeable for the contribution of a single micronutrient to disease risk, although, magnitude of effect is not indicative of associative “truth” (29). It should also be noted, however, that the findings were robust to detailed adjustment for other known risk factors, with little difference in point estimates between the age-adjusted and full multivariable models.
In conclusion, we observed that foods rich in thiamine, folate, vitamin C, and vitamin E obtained solely through food sources may lower the rate of laparoscopically-confirmed endometriosis. It is not clear whether these nutrients themselves, or an unidentified dietary pattern rich in these nutrients, underlies the observed associations. Further studies are required to clarify the pathways through which foods high in these micronutrients may influence endometriosis.
Acknowledgments
Financial support: This study was supported by research grants HD48544 and HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and P30 DK046200 from the National Institute of Diabetes and Digestive and Kidney Diseases. The Nurses’ Health Study II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services.
Footnotes
Conflict of interest statement: None.
References
- 1.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 2.Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 3.Trussel J, Hatcher RA, Cates W, Jr, Stewart FH, Kost K. Contraceptive failure in the United States: An update. Stud Fam Plann. 1990;21:51–4. [PubMed] [Google Scholar]
- 4.Oral E, Arici A. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:219–33. doi: 10.1016/s0889-8545(05)70301-6. [DOI] [PubMed] [Google Scholar]
- 5.Willett W. Nutritional Epidemiology. 2. New York: Oxford University Press, Inc; 1998. [Google Scholar]
- 6.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod. 2005;20:2014–20. doi: 10.1093/humrep/dei001. [DOI] [PubMed] [Google Scholar]
- 7.Biskind M. Nutritional deficiency in the etiology of menorrhagia, cystic mastitis, and premenstrual tension, treatment with vitamin B complex. J Clin Endocrinol Metab. 1943;3:277–334. [Google Scholar]
- 8.Das UN. Nutrients, essential fatty acids and prostaglandins interact to augment immune responses and prevent genetic damage and cancer. Nutrition. 1989;5:106–10. [PubMed] [Google Scholar]
- 9.Hernández Guerrero CA, Bujalil Montenegro L, de la Jara Díaz J, Mier Cabrera J, Bouchán Valencia P. Endometriosis and deficient intake of antioxidants molecules related to peripheral and peritoneal oxidative stress. Ginecol Obstet Mex. 2006;74:20–8. [PubMed] [Google Scholar]
- 10.Mier-Cabrera J, Aburto-Soto T, Burrola-Méndez S, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. 2009;7:54. doi: 10.1186/1477-7827-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabert B, Peters U, De Roos AJ, Scholes D, Holt VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. 2011;105:459–67. doi: 10.1017/S0007114510003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically-confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–96. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Agriculture. Nutrient Database for Standard Reference Release 14. US Department of Agriculture Agricultural Research Service; 2001. Available at: http://www.ars.usda.gov/ba/bhnrc/ndl. [Google Scholar]
- 15.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 16.Smith PL. Splines as a useful and convenient statistical tool. Am Stat. 1979;330:57. [Google Scholar]
- 17.Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 18.Missmer SA, Chavarro JE, Malspeis S, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25:1528–35. doi: 10.1093/humrep/deq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer DW, Wilson E, Stillman RJ, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–8. [PubMed] [Google Scholar]
- 20.Calhaz-Jorge C, Mol BW, Nunes J, Costa AP. Clinical predictive factors for endometriosis in a Portuguese infertile population. Hum Reprod. 2004;19:2126–31. doi: 10.1093/humrep/deh374. [DOI] [PubMed] [Google Scholar]
- 21.Buck Louis GM, Hediger ML, Peña JB. Intrauterine exposures and risk of endometriosis. Hum Reprod. 2007;22:3232–6. doi: 10.1093/humrep/dem338. [DOI] [PubMed] [Google Scholar]
- 22.Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7:267–74. doi: 10.1016/s1047-2797(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 23.Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res. 2007;103:121–9. doi: 10.1016/j.envres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Chapron C, Souza C, de Ziegler D, et al. Smoking habits of 411 women with histologically proven endometriosis and 567 unaffected women. Fertil Steril. 2010;94:2353–5. doi: 10.1016/j.fertnstert.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Vessey MP, Villard-Mackintosh L, Painter R. Epidemiology of endometriosis in women attending family planning clinics. BMJ. 1993;306:182–4. doi: 10.1136/bmj.306.6871.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet. 2008;277:389–93. doi: 10.1007/s00404-007-0479-1. [DOI] [PubMed] [Google Scholar]
- 27.Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–55. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nnoaham KE, Hummelshoj L, Webster P, et al. World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–73. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash T. Modern Epidemiology. 3. Lippincott Williams & Wilkins; 2008. [Google Scholar]


