Abstract
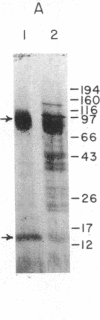
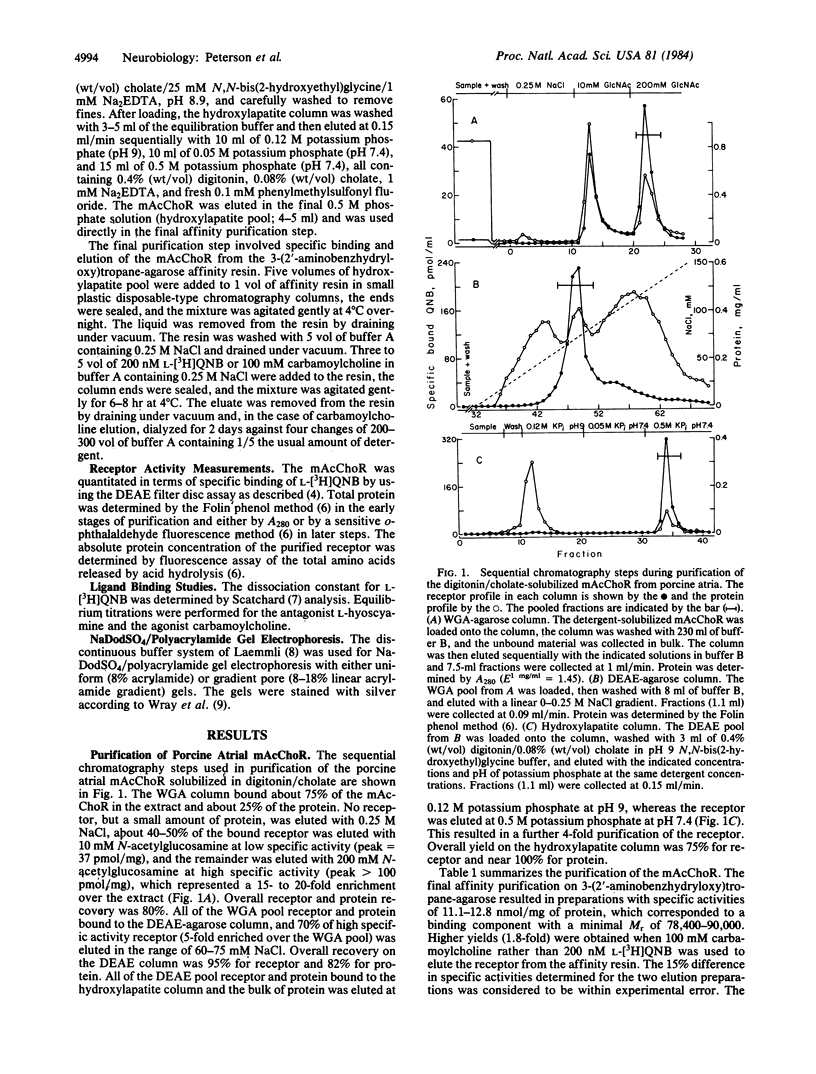
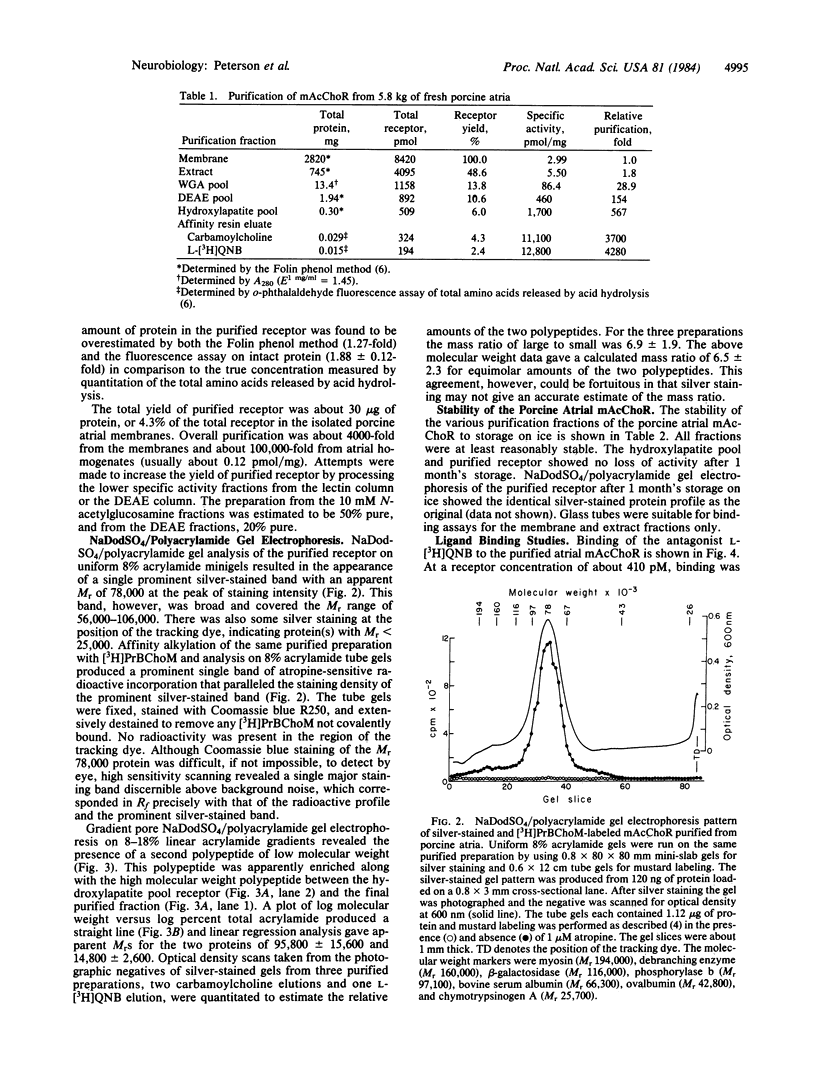
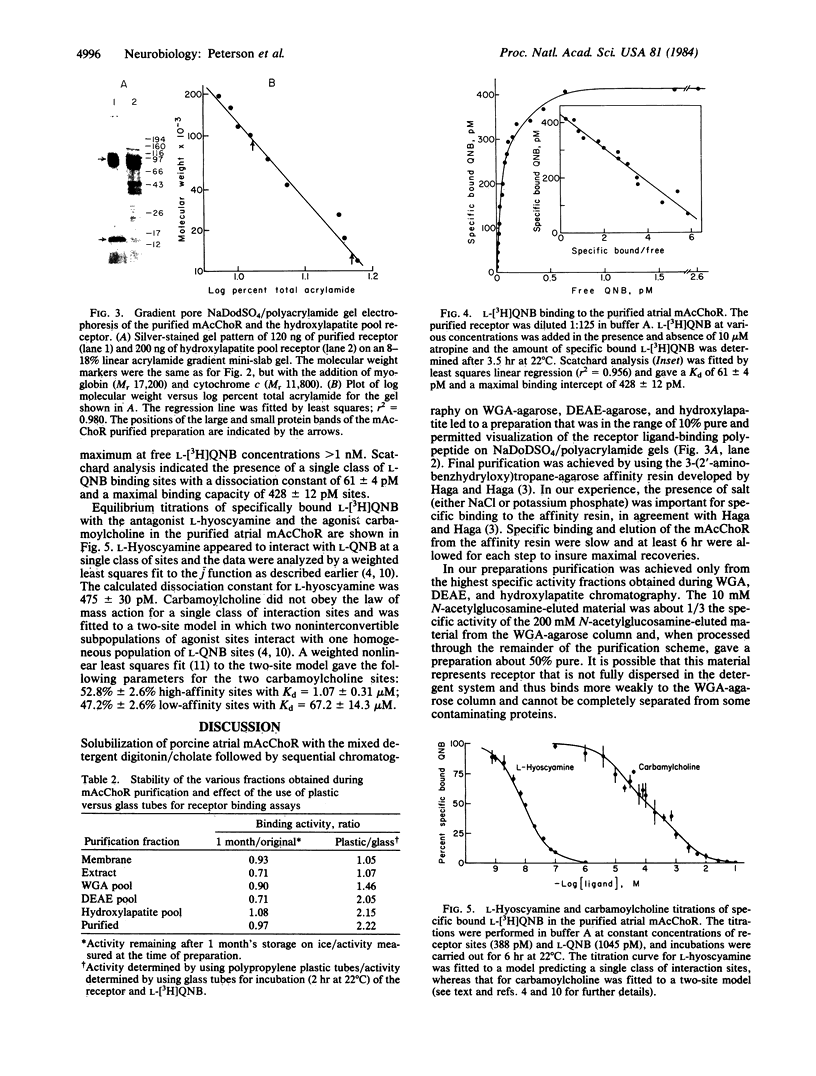
The muscarinic acetylcholine receptor from porcine atria has been purified 100,000-fold to homogeneity by solubilization in digitonin/cholate and sequential chromatography on wheat germ agglutinin-agarose, diethylaminoethylagarose, hydroxylapatite, and 3-(2'-aminobenzhydryloxy)tropane-agarose. The yield of purified receptor was 4.3% of that found in the membrane fraction, and the purified receptor bound 11.1-12.8 nmol of L-[3H]quinuclidinyl benzilate per mg of protein, corresponding to a binding component Mr of 78,400-90,000. The purified receptor preparation consisted of two polypeptides in approximately equimolar amounts when examined on silver-stained sodium dodecyl sulfate/polyacrylamide gels. The larger polypeptide (Mr 78,000 on 8% polyacrylamide gels) was specifically alkylated with [3H]propylbenzilylcholine mustard, whereas the smaller polypeptide (Mr 14,800) was not labeled. The possibility that the small polypeptide is a contaminant fortuitously appearing in equimolar amounts with the large polypeptide cannot be ruled out at this time. The purified preparation was highly stable, with no measurable change in the number of ligand binding sites or the gel pattern after 1 month's storage on ice. Scatchard analysis showed a single class of binding sites for the antagonist L-[3H]quinuclidinyl benzilate with a dissociation constant of 61 +/- 4 pM. Equilibrium titration experiments demonstrated that the antagonist L-hyoscyamine displaced L-[3H]quinuclidinyl benzilate from a single class of sites (Kd = 475 +/- 30 pM), whereas the agonist carbamoylcholine interacted at two populations of sites (53% +/- 3% high affinity, Kd = 1.1 +/- 0.3 microM; 47% +/- 3% low affinity, Kd = 67 +/- 14 microM). The ligand binding data were very similar to that for the membrane-bound receptor, suggesting that the receptor has not been altered radically during purification.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., De Backer J. P., Guillet J. C., Vanderheyden P., Vauquelin G., Strosberg A. D. Purification of muscarinic acetylcholine receptors by affinity chromatography. EMBO J. 1983;2(4):499–504. doi: 10.1002/j.1460-2075.1983.tb01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar S., Amitai G., Sokolovsky M. Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. Proc Natl Acad Sci U S A. 1983 Jan;80(1):156–159. doi: 10.1073/pnas.80.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Haga T. Affinity chromatography of the muscarinic acetylcholine receptor. J Biol Chem. 1983 Nov 25;258(22):13575–13579. [PubMed] [Google Scholar]
- Herron G. S., Schimerlik M. I. Protein composition of the atrial muscarinic acetylcholine receptor partially purified by wheat germ agglutinin affinity chromatography. Arch Biochem Biophys. 1984 May 1;230(2):533–542. doi: 10.1016/0003-9861(84)90434-x. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H., Schlegel W., Hearon J. Z. The functional unit of polyenzymes. Determination by radiation inactivation. J Biol Chem. 1980 Jul 25;255(14):6826–6831. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L., Schimerlik M. I. Large scale preparation and characterization of membrane-bound and detergent-solubilized muscarinic acetylcholine receptor from pig atria. Prep Biochem. 1984;14(1):33–74. doi: 10.1080/10826068408070612. [DOI] [PubMed] [Google Scholar]
- Schimerlik M. I., Searles R. P. Ligand interactions with membrane-bound porcine atrial muscarinic receptor(s). Biochemistry. 1980 Jul 22;19(15):3407–3413. doi: 10.1021/bi00556a001. [DOI] [PubMed] [Google Scholar]
- Venter J. C. Muscarinic cholinergic receptor structure. Receptor size, membrane orientation, and absence of major phylogenetic structural diversity. J Biol Chem. 1983 Apr 25;258(8):4842–4848. [PubMed] [Google Scholar]
- Verkman A. S., Skorecki K., Ausiello D. A. Radiation inactivation of oligomeric enzyme systems: theoretical considerations. Proc Natl Acad Sci U S A. 1984 Jan;81(1):150–154. doi: 10.1073/pnas.81.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]