Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS). The major pathological outcomes of the disease are the loss of blood-brain barrier (BBB) integrity and the development of reactive astrogliosis and MS plaque. For the disease to occur, the non-resident cells must enter into the immune-privileged CNS through a breach in the relatively impermeable BBB. It has been demonstrated that matrix metalloproteinases (MMPs) play an important role in the immunopathogenesis of MS, in part through the disruption of the BBB and the recruitment of inflammatory cells into the CNS. Moreover, MMPs can also enhance the cleavage of myelin basic protein (MBP) and the demyelination process. Regarding the growing data on the roles of MMPs and their tissue inhibitors (TIMPs) in the pathogenesis of MS, this review discusses the role of different types of MMPs, including MMP-2, -3, -7, -9, -12 and -25, in the immunopathogenesis and treatment of MS.
Keywords: Multiple Sclerosis, Blood-Brain Barrier, Matrix Metalloproteinases, Inflammation, Central Nervous System
Multiple sclerosis (MS) is the most common prototypic inflammatory disorder, characterised by inflammation, oligodendrocyte depletion, reactive astrogliosis and demyelination in the brain, optic nerve and spinal cord.1 MS plaque is the major pathological hallmark of MS. It is a unique feature of central nervous system (CNS) demyelination, which is characterised by oligodendrocyte destruction along with the loss of myelin, axonal damage and loss, and glial scar formation.2
MS usually occurs in young adults and is more common in women than men, with about 300,000 patients suffering from MS in North America alone. Literature reviews robustly imply an increased prevalence of MS in recent times.3
MS patients show a variety of clinical symptoms, including visual difficulties, muscle weakness, sensory damage and difficulties with speech and coordination. Briefly, there are four types of MS, with each having a mild, moderate or severe course.4 Most patients (∼85%) initially experience relapsing-remitting MS (RRMS); the majority is predisposed to the establishment of secondary-progressive MS (SPMS) which is characterised by an initial RRMS disease course followed by a progression with or without occasional relapses, minor remissions, and plateaus. Nearly 10% of patients display primary-progressive MS (PPMS), which indicates the disease progression from onset with occasional plateaus and temporary minor improvements. Finally, the least common form of the disease is progressive-relapsing MS (PRMS), which occurs in ∼5% of MS patients and is characterised by the progressive disease from onset, with clear acute relapses, with or without full recovery.5,6
Although the precise cause of MS is unknown, the aetiology of MS seems to be linked to a variety of genetic and environmental factors. Current opinion suggests that the activation of autoreactive, adaptive and innate immune responses which target neural antigens leads to MS.2 The self-reactive T cells are the main factors in the immunopathogenesis of MS. There is consistent evidence showing the central role of T helper 17 cell (Th17)-producing interleukin 17 and interferon γ (IFN-γ)-secreting type 1 T helper (Th1) cells in the disease’s development, in part through the secretion of inflammatory mediators and the activation of microglial cells.7
Although the microglia is involved in the phagocytosis of myelin debris and apoptotic cells during demyelination, the activation of the microglia and the release of inflammatory mediators has been suggested as a possible mechanism by which innate immunity enhances the demyelination process in MS.2,8 Therefore, microglial cells, which constitute about 10% of the CNS, are one of the first cells that cause neuronal damage and play an important role in neuroinflammatory processes.8
In MS, neural factors generated by acute stress, including myelin basic protein (MBP), substance P and corticotropin-releasing hormones, can activate brain mast cells to release inflammatory mediators and stimulate the autoreactive T cells.9
Astrocytes are involved in nearly all immunopathological processes in the brain. It has been reported that astrocytes could release factors which regulate the oligodendrocyte progenitor differentiation and myelin formation.1,10 Oligodendrocytes enhance the inflammatory cytokine production in T cells as well as chemokines that may recruit additional peripheral inflammatory leukocytes.10 The localisation of astrocytes in MS is similar to mast cells in the perivascular area.11
Two types of phagocytes (including the microglia and inflammatory macrophages) in MS and its animal model, experimental autoimmune encephalomyelitis (EAE), are derived from proliferating resident precursors and the recruitment of blood-borne progenitors, respectively.12 These phagocytes and astrocytes can recognise pathogen-associated molecular patterns (PAMPs) via Toll-like receptors (TLRs) and generate pro-inflammatory signals that trigger adaptive immune responses. Moreover, these cells enhance the autoimmune responses through the secretion of effector molecules such as nitric oxide (NO), matrix metalloproteinases (MMPs) and calpain-1 that may also play a role in the initiation of myelin and axon damage.13,14 The recruited inflammatory cells are mainly composed of cluster of differentiation 4 (CD4+) T cells, B cells, monocyte/macrophages, neutrophils and dendritic cells.15 However, these non-resident cells have to penetrate the blood-brain barrier (BBB) to reach the immunologically-privileged CNS.16 The BBB is a dynamic interface that separates the brain from the circulatory system and protects the CNS from potentially harmful chemicals. Therefore, the BBB restricts the exchange of humoral factors and cells between the blood and brain, thus playing a crucial role in maintaining cerebral homeostasis. Disruption of the BBB is considered an initial key step of the disease process in MS.17
The breakdown of the BBB usually lasts for about a month and then resolves, leaving a site of damage that can be investigated by conventional magnetic resonance imaging (MRI).18 In MS, the BBB may be impaired by MMPs attacking the basal lamina macromolecules before the formation of demyelinating foci or T-cell infiltration around the small vessels; however, once the BBB is disrupted, the massive infiltration of T cells, the augmented expression of adhesion molecules on the endothelial cell surface and the leakage of the inflammatory cytokines and antibodies aggravate the MS lesions.17 In the brains of MS patients, mast cells are placed on the perivascular area and secrete numerous vasoactive molecules and pro-inflammatory mediators that can contribute to the BBB disruption.9
The MMPs or matrixins represent a large family of zinc-dependent proteolytic enzymes that are known for their capacity to degrade extracellular matrix (ECM) components.19 MMPs are a family of proteases, classified into subfamilies based on their substrate preferences. Currently, there are 23 known MMPs, including: gelatinases (MMP-2 and -9); collagenases (MMP-1, -8, -13 and -18); stromelysins (MMP-3, -10 and -11); matrilysins (MMP-7 and -26); membrane type (MT) MMPs (including MMP-14, -15, -16, -17, -24 and -25), and a group of unnamed members (MMP-11, -12, -19, -20, -21, -23a, -23b, -27 and -28). All MMPs are secreted as proenzymes and require extracellular activation.20–23 Recent findings indicate that MMPs are involved in different physiological and pathological processes, such as placental development, morphogenesis, reproduction, wound repair, inflammation, angiogenesis, neurological disorders, and cancer cell invasion and metastasis.24
MMPs affect a variety of extracellular proteins in the CNS, including cytokines, chemokines, antimicrobial peptides and immune regulatory proteins. Using quantitative reverse transcriptase polymerase chain reaction (RT-PCR), Bar-Or et al. systematically evaluated the expression of 23 MMP members in subsets of leukocytes isolated from the blood of normal populations. They found a specific pattern of MMP expression in different cellular populations: MMP-11, -26 and -27 were plentiful in B cells, while MMP-15, -16, -24 and -28 showed up more often in T cells. The majority of MMP members are reported in monocytes: MMP-1, -3, -9, -10, -14, -19 and -25. In addition, MMP-2 and -17 were mainly represented in monocytes, although B lymphocytes had significant amounts of these MMPs.25,26
Growing evidence implies that the normal, mature CNS contains low or non-detectable levels of most MMPs; the principal cells that express these MMPs are perivascular and parenchymal microglia. On the other hand, studies on the serum, cerebrospinal fluid (CSF) and brain tissue of MS patients have shown an increase of MMP-1, -2, -3, -7, -9, -12 and -14 activity.27–29 Some data suggest that microglial-derived MMPs may mediate the turnover of the CNS’s ECM under normal conditions in microglial nodules, but in many neuroinflammatory conditions, such as encephalitis, meningitis, brain tumours, cerebral ischaemia, Guillain-Barré syndrome and MS, these enzymes are significantly upregulated.30–32
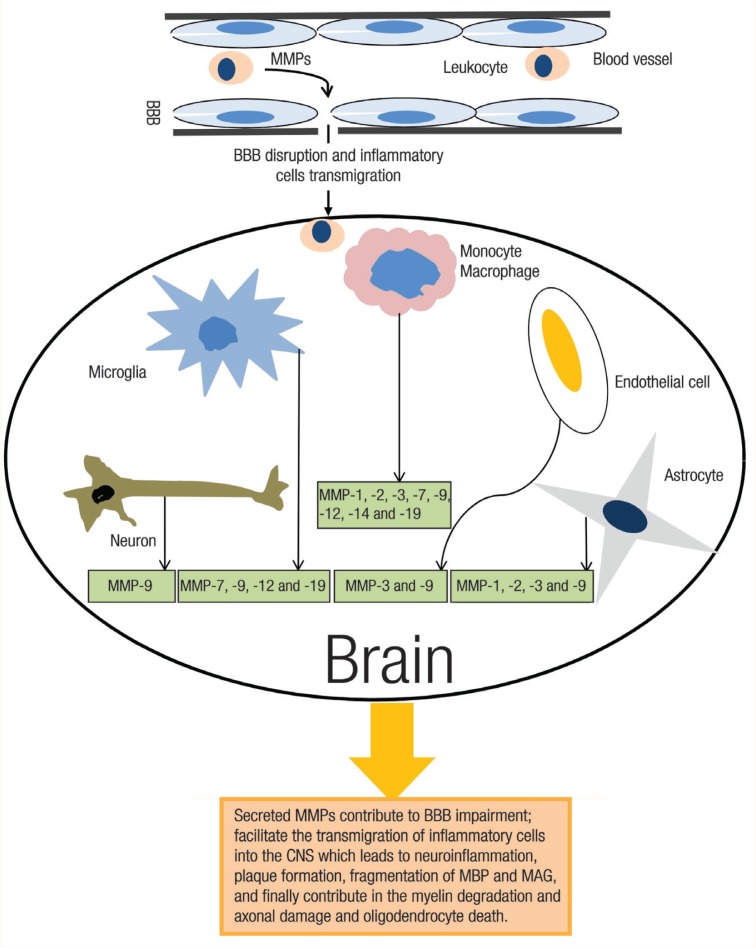
In neuroinflammatory conditions, MMPs are expressed in the CNS by a variety of cell types, including vascular endothelial cells, meninges, resident cells such as neuronal cells, astrocytes, microglial cells and accumulated inflammatory cells [Figure 1]. It has been shown that astrocytes can release MMP-1, -2, -3 and -9, whereas the microglia secretes MMP-7, -9, -12 and -19.33,34 Most microvessel endothelial cells in the CNS express MMP-3 and -9 but not -1 or -2. Also, the majority of macrophages in active MS and necrotic lesions are positive for MMP-l, -2, -3, -7, -9 and -19, whereas chronic MS lesions have fewer MMP-positive macrophages.32,35 Neurons may also release MMPs. It is reported that in normal adult rat brains, MMP-9 (but not -2) is highly expressed by neurons and localised in neuronal cell bodies and dendrites.36
Figure 1:
In multiple sclerosis, matrix metalloproteinases (MMPs) are expressed in the central nervous system (CNS) by various cell types, including vascular endothelial cells, neuron, reactive astrocytes and the microglia, and accumulated inflammatory cells. In the CNS, the high numbers of MMPs lead to the perpetuation of neuroinflammation, which contributes to myelin degradation and axonal damage.
BBB = blood-brain barrier; MBP = myelin basic protein; MAG = myelin-associated glycoprotein.
In the damaged sites of the CNS, there are complex and dynamic regulations of MMP expression by different cell types.32 The imbalance between MMP activity and the inhibitory action of tissue inhibitors of metalloproteinases (TIMPs) are implicated in MS development [Table 1], as one of the MMP roles may be to facilitate the transmigration of circulating leukocytes into the CNS.25 Therefore, it is possible that the MMPs attack the basal lamina macromolecules that line the blood vessels, disrupting the BBB’s integrity.31
Table 1:
Some important matrix metalloproteinases in the central nervous system and their relationship with the pathogenesis of multiple sclerosis
| MMP | MW in kDa | Cell source | Functions | TIMPs | |
|---|---|---|---|---|---|
| Pro-enzyme | Active form | ||||
| MMP-1 | 57 and 52 | 42 | Astrocytes, monocytes, macrophages and microglia |
|
TIMP-1 |
| MMP-2 | 72 | 63 | Astrocytes, microglia, monocytes, macrophages and endothelial cells |
|
TIMP-2 and TIMP-4 |
| MMP-3 | 57 | 54 | Astrocytes, monocytes, macrophages, endothelial cells and microglia |
|
TIMP-1 and TIMP-3 |
| MMP-7 | 29 | 20 | Microglia, monocytes and macrophages |
|
TIMP-1 |
| MMP-9 | 92 | 67 | Astrocytes, microglia, monocytes, macrophages, endothelial cells, neurons, neutrophils and CCR2+ CCR5+ T cells |
|
TIMP-1 |
| MMP-12 | ∼54 | 48 | Macrophages, microglia and astrocytes |
|
TIMP-1 |
| MMP-14 | 66 | ∼54 | Monocytes |
|
TIMP-2 and TIMP-4 |
MMP = matrix metalloproteinase; MW = molecular weight; kDa = kilodaltons; TIMP = tissue inhibitors of metalloproteinases; BBB = blood-brain barrier; CNS = central nervous system; MBP = myelin basic protein; NMDA = N-methyl-d-aspartate.
Moreover, MMPs can be involved in the fragmentation of MBP and myelin-associated glycoprotein (MAG), and injury to the myelin. In the CNS, the MBP gene is expressed in the oligodendrocytes and is named classic-MBP; in the immune cells it is named golli-MBP.37 Therefore, MMP-mediated proteolysis of the MBP isoforms is a source of immunogenic peptides in autoimmune MS.38 Consequently, through the remodelling of the blood vessels, MMPs cause hyalinosis and gliosis, and they attack the myelin, disrupting the myelin sheath and axons. Excessive proteolytic activity is detected in the CSF and blood of patients with acute MS. Moreover, MMPs are induced in immunological and non-immunological forms of demyelination.31
Regarding the high expression of many MMPs by monocytes which facilitate the transmigration of the leukocytes, Bar-Or et al. showed that monocytes migrate more rapidly through the BBB than T or B lymphocytes, in vitro.25 Although data about the role of MMPs in monocyte trafficking are limited, Lucchinetti et al.18 suggested that the frequency of macrophages/microglia in MS is approximately 10 times higher compared to lymphocytes. Also, in active MS lesions, macrophages were found to be positive for MMP-2, -7, -9, -12 and -19.39,40 Since monocytes constitute a major cell population in acute MS lesions, it may be possible that MMP secretion facilitates their entrance into the CNS. Findings on MMP secretion by blood monocytes can be useful in improving our understanding of the immunopathogenesis of MS.41
To summarise, it seems that monocytes are key contributors to the neuroinflammatory process in MS through a mechanism that involves the high expression of different MMPs, such as MMP-1, -2, -3, -7, -9, -14 and decreased expression of TIMP-1 and TIMP-2.25,42
Matrix Metalloproteinase-2
Matrix metallopeptidase-2 (also known as gelatinase A and a 72 kilodalton [kDa] type II collagenase) plays an important role in inflammation and immunity in addition to its physiological function in degrading and remodelling the ECM. The expression of MMP-2 is upregulated in many human diseases as well as in animal models of inflammatory and immune diseases. Bar-Or et al., following analyses of all MMPs in leukocytes, implicated monocytes as major inflammatory cells in MS. They found higher levels of monocyte-expressed MMP-2 and -14 in MS patients compared to normal subjects.25 Moreover, another report suggested that MMP-2 can be expressed not only in monocytes but also in the astrocytes, microglia and macrophages.43
Although the correlation between the frequency of macrophages and reactive glial cells with axon injuries in the acute plaques is well-established, little is known about the precise role of MMP-2 in the immunopathogenesis of MS.44,45 Anthony et al.46 and Maeda et al.32 showed that MMP-2 was upregulated not only in plaques but also in the seemingly normal white matter adjacent to the acute plaques. In these acute plaques, the myelin was degraded and engulfed by the reactive glial cells and macrophages, leading to demyelination.35 In addition, Newman et al. showed that a microinjection of MMP-2 into rat subcortical white matter led to axonal injury.47
It seems that MMP-2 plays a key role in the BBB disruption, which facilitates immune cell transmigration into the CNS and the development of MS.38,43,48 Therefore, the downregulation of MMP-2 may inhibit the BBB disruption and migration of the inflammatory cells to the CNS. Although little is known about the involvement of MMP-2 and its tissue inhibitor TIMP-2 in MS, it has been shown that TIMP-2 is elevated in the monocytes of MS patients. However, it should be noted that there are no data regarding the functionality of TIMP-2 in MS patients.
TIMP-2 is an inhibitor of activated MMPs; it inhibits the cell surface activation of pro-MMP-2 by MMP-14.25,49 MMP-14 acts as a receptor for TIMP-2 (but not TIMP-1) and the pro-MMP-2/TIMP-2 complex, thereby facilitating the activation of pro-MMP-2. In addition, the serum MMP-2:TIMP-2 ratio may represent a useful indicator for monitoring the MS patient during a recovery phase.45 Benesová et al. observed in 2009 that there was a significant elevation in MMP-2 serum levels and the MMP-2:TIMP-2 ratio in the PPMS and SPMS groups compared to the RRMS group. This increase was also associated with the level of disability in the patient and the severity of the disease.43 Thus, it seems that MMP-2, -14 and TIMP-2 can be considered interesting targets for potential therapeutic interventions to inhibit the entry of monocytes into the CNS, and to alleviate injuries of the CNS in MS patients.25
Matrix Metalloproteinase-3
Also known as stromelysin-1, MMP-3 is an enzyme involved in ECM remodelling. MMP-3 is an activator of other MMPs, including MMP-1, -7 and -9.50 It has been demonstrated that MMP-3 contributes to different pathological conditions, such as rheumatoid arthritis (RA), asthma, cancer and neurological disorders, including MS, Parkinson’s disease (PD) and Alzheimer’s disease (AD).51 In MS, the increased circulatory level of MMP-3 correlates with disease activity in RRMS. This may contribute to the breakdown of the BBB at the time of relapse.52 Moreover, the release of MMP-3 into the ECM activates microglial or brain cells in the white matter.53 Some evidence strongly suggests that the distinctive signal of neuronal apoptosis is the release of the active form of MMP-3 that activates the microglia and subsequently exacerbates neuronal degeneration.54 It has been shown consistently that the inhibition of MMP-3 leads to the suppression of inducible nitric oxide synthase, (iNOS), proinflammatory cytokines and the inflammatory transcription factors nuclear factor kappa B (NF-κB), activator protein 1 (AP1) and mitogen-activated protein kinases (MAPKs) in the microglia.53
Interestingly, it has been reported that MMP-3 can be involved in normal CNS-remodelling and in the remyelination process, as shown by the strict spatiotemporal MMP-3 upregulation in the injured CNS, which may contribute to stem cell migration, neuroprotection and remyelination in the injured sites.51 An involvement of MMP-3 in remyelination during the regenerative period after a CNS injury was demonstrated for the first time in the murine cuprizone-induced demyelination model. It was reported that not only during the early demyelination stage, but also during the stage of remyelination, MMP-3 was highly expressed in astrocytes of the corpus callosum. Although the exact mechanism is unknown, MMP-3 might be involved in remyelination through enhancing insulin growth factor (IGF) secretion, which is essential for the proliferation and differentiation of myelin-forming cells.55–57 In addition, MMP-3 might stimulate remyelination by removing and cleaving the myelin debris, which inhibits the oligodendrocyte precursor cell differentiation.51,58
Matrix Metalloproteinase-7
Also known as matrilysin, MMP-7 is increased in the serum, CSF and brain tissues of MS patients.27 It has been shown that the messenger ribonucleic acid (mRNA) levels of MMP-7 and -9 are elevated in all stages of lesion formation in MS patients.59 Moreover, MMP-7 expression was found to be upregulated in microglia/macrophages within acute MS lesions. In chronic MS lesions, the expression of MMP-7 was confined to the macrophages within the perivascular cuffs, whereas only a low level of MMP-7 expression was detected in normal brain tissue.46 In a study by Cossins et al., MMP-7 immunoreactivity was weakly detected in microglial-like cells in normal brain tissue sections, and was very strongly detected in the parenchymal macrophages in active demyelinating MS lesions. MMP-7 immunoreactivity was not detected in macrophages in the spleen or tonsils, indicating that it is specifically induced in infiltrating macrophages in active demyelinating MS lesions.35 Elevated levels of MMP-7 and -9 have also been detected in cases of EAE. In one study of adoptive transfer EAE, mRNA for MMP-7 was increased, with maximum levels at the peak of the disease.60
Matrix Metalloproteinase-9
Also known as gelatinase B and type IV collagenase, MMP-9 is secreted from neutrophils, macrophages and a number of transformed cells in zymogen form.50 Upon activation, MMP-9 acts on many inflammatory processes and is involved in the progression of cardiovascular disease, RA, chronic obstructive pulmonary disease and MS.24 MMP-9 is also important in cytokine and protease modulation; it degrades the serine protease inhibitor α1-antitrypsin, which may lead to the destruction of the lungs.24 Different studies have indicated the increased expression of MMP-9 in MS.61 In one study, zymography methods showed that MMP-9 levels were high in the CSF of MS patients and in patients with infections of the CNS and other inflammatory diseases.28 In patients with MS, high MMP-9 levels were associated with the immunoglobulin G (IgG) index. Hence, MMP-9 is an unspecific laboratory marker of inflammation. For instance, the expression of MMP-9 as well as other MMPs (for instance, MMP-2, -7 and -12) is increased in MS brain sections as measured by immunohistochemical analysis.32,35,46,62 In addition, high MMP-9 levels in serum or leucocytes were detected by immunochemical methods.63 Long-term follow-up studies have enabled patterns to be recorded with MRI; additionally, enhanced MMP-9 steady-state mRNA levels were measured in MS.59
In the CNS, MMP-9 can be expressed in the vascular endothelial cells, meninges, microglia, astrocytes and accumulated inflammatory cells, as well as in inflamed MS plaques and in the seemingly normal white matter or cerebral-infarction tissue.64 It has been also observed that MMP-9 was strongly expressed by the neutrophils in patients up to one week after an infarction—at that point a large number of macrophages were expressing MMP-9.46 In Wistar rat EAE models, an elevated expression of MMP-9 may play a role in some pathological changes, similar to MMP-2.65 For example, it increases the permeability of the BBB, facilitates the infiltration of leukocytes into the CNS and causes myelin sheath degradation and neuronal damage.24 Also, in MMP-9-deficient mice, the chemotaxis of neutrophils to intradermally-injected granulocyte chemotactic protein-2 was decreased.66 It was found that young MMP-9 knockout mice are partially resistant to the development of EAE.61,67 However, when both gelatinases (MMP-2 and -9) were genetically knocked out, a complete resistance against myelin oligodendrocyte glycoprotein peptide-induced EAE was observed.68 The latter study is important as it shows that many proteinases act in these cascades or networks.69
Indeed, investigations of MMP-2 indicate that it is able to activate MMP-9 in vitro.70 Both enzymes thus reinforce each other and they also share a number of substrates, including denatured collagen or gelatin.71 In addition, decreased serum MMP-8 and -9 levels were correlated with a decreased number of contrast-enhanced T2-weighted MRI lesions in MS patients.72 In the same study, MS patients treated with interferon β 1b (IFN-β1b) showed a reduction in serum MMP-8 and -9 in parallel with the disease stabilisation. The authors concluded that the serial measurement of MMPs and other inflammatory mediators may serve as sensitive markers for measuring therapeutic response to IFN-β1b during the first year of treatment.
As mentioned earlier, it is suggested that MMP-9 has a role in MS by mediating T-cell migration through the BBB. During a relapse course, the CCR2+CCR5+ T cells are abundant in the CSF of MS patients. These T cells have the potent ability to produce osteopontin and MMP-9, both of which have an important role in the MS pathology.73 Also, in MS patients in the relapse phase, these subtypes of T cells are reactive to MBP, as this ability can be evaluated by IFN-γ production. Other findings suggested that the CCR6- subtype (but not the CCR6+ subtype within CCR2+CCR5+ T cells) is very abundant in the CSF during a MS relapse and can produce higher levels of MMP-9 and IFN-γ.73,74
As TIMP-1 is a tissue inhibitor of MMP-9, a considerable elevation in the MMP-9:TIMP-1 ratio and in MMP-9 serum levels was observed in MS patients in the RRMS and SPMS groups.43 Fainardi et al. have suggested that a shift in the MMP-9:TIMP-1 ratio towards MMP-9 proteolytic activity can be the consequence of MS immune downregulation.75 Moreover, using immunohistochemistry, TIMP-1 was found to be upregulated in chronic plaques.62
The concentrations of these metalloproteinases inhibitors in the CSF and plasma were low in patients with MS, whereas during treatment with interferon β (IFN-β), their concentrations increased.48,76,77 Moreover, the levels of active MMP-9 in the serum and CSF of MS patients may represent indicators for the monitoring of disease activity. In particular, the serum active MMP-9:TIMP-1 ratio seems to be a very suitable and easily measurable biomarker of the continuous inflammation in MS.75 Furthermore, in a MS clinical trial, erythropoietin induced the expression of TIMP-1 in the endothelial cells, which helped to maintain the BBB integrity. The protective effects of erythropoietin were associated with an increase in the number of astrocytes expressing TIMP-1 in the brain and spinal cord in cases of EAE.78 It has also been demonstrated that there is a significant association between the gene polymorphism of MMP-9 and MS susceptibility and severity.74,79,80
Matrix Metalloproteinase-12
MMP-12 is a macrophage-specific MMP with a broad substrate specificity and is expressed in MS lesions at various stages.39 Moreover, the transient expression of MMP-12 has also been reported in the microglial cells and astrocytes of MS patients.39 It has also been demonstrated that in active demyelinating lesions, phagocytic macrophages express MMP-12. Moreover, in inactive lesions and chronic active demyelinating lesions, lower ratios of phagocytic cells were MMP-12-positive.39
Out of all of the MMPs that could be measured in the spinal cord tissue at the peak of the disease, MMP-12 is significantly upregulated.81 In contrast to previous data, Weaver et al. demonstrated that an elevation in MMP-12 expression was related to protection against EAE. In this study, MMP-12-null mice had significantly severe EAE when compared to the control mice. In addition, in vitro findings showed that the lymph node and spleen cells of the MMP-12-null mice had a significantly higher Th1 to type 2 T helper cell (Th2) cytokine proportion compared with similar cells in the control mice. Evaluations of the main transcription factors of T cell polarisation also showed that MMP-12-null cells had reduced GATA-3 and increased the T-bet expression, a situation that is in favor of Th1 bias.82
Matrix Metalloproteinase-25
MMP-25 is a member of the MT MMPs, which is expressed almost exclusively in peripheral blood leukocytes and in anaplastic astrocytomas and glioblastomas, but not in meningiomas.83
It was previously shown that the gene expression of the majority of MMPs was upregulated in the spinal cords of Swiss/Jackson laboratory (SJL) mice with severe EAE. Here, four of the six MT MMPs (MMP-15, -16, -17 and -24) were downregulated and the two remaining MT MMPs (MMP-14 and -25) were upregulated in whole tissue.84 MMP-25 proteolysis can inactivate a MS regulator known as αB-crystallin. Therefore, MMP-25 functions and their restricted cell/tissue expression patterns play an important role in demyelinating diseases such as MS.37,85 MMP-25 cleaves golli-MBP isoforms in the immune cells, therefore stimulating specific clones of the autoreactive T cells.37 It is possible that the transmission of these autoreactive T cells, through the disrupted BBB, and the appearance of MBP in the neuronal cells can lead to inflammation and self-reactive responses. The activation of different elevated MMPs and the dysregulation of TIMPs enhances inflammation, autoreactive responses, MBP cleavage and demyelination and, consequently, the development of MS.37,85
Matrix Metalloproteinase-based Therapeutic Approaches to Multiple Sclerosis
In view of the above, it seems that the direct inhibition of proteolysis or the induction of a balance between the endogenous proteinases and their natural inhibitors could be possible approaches to MS treatment. However, further proof is needed to demonstrate the pathogenic role of extracellular proteolysis.86 Generally, the role of MMP involvement in pathology is that of matrix degradation, so MMP inhibition may be of therapeutic benefit.
The first disease targeted with MMPs was RA but the range of potential applications has broadened to include the treatment of cancer.87 However, the multiple roles of MMPs in the CNS make them a therapeutic target for the treatment of neurological disorders. Further complications are that the various MMP members induce or compensate for one another, and most MMP inhibitors are non-selective for particular MMP members. There is also the likelihood that, while MMP inhibitors may protect against certain of the detrimental effects of some MMPs, they will also block the useful actions of these MMPs, thus slowing disease recovery.88 The first synthetic MMP inhibitor was developed in the early 1980s as a pseudopeptide derivative based on the structure of the collagen molecule at the site of the initial cleavage by interstitial collagenase.89 A number of synthetic inhibitors of MMP activity have been developed and have been shown to decrease the incidence and severity of EAE. These inhibitors include GM6001,90,91 Ro-31-9790,92 BB-1101,93 UK221 and D-penicillamine.94
A semi-synthetic tetracycline derivative, minocycline, also has a MMP inhibitory function.95 In EAE mice models, it has been shown that minocycline decreases the expression and function of MMP-9 in T cells and attenuates the disease severity and neuropathology.96 In the clinical trial of minocycline in RRMS, it significantly decreased gadolinium-enhanced MRI activity within the first two months of treatment; this effect was associated with the role of MMPs in reducing disruption of the BBB.97 However, the decreased function of MMP-9 during treatment would also support this mechanism.98 The beneficial effect of IFN-β, which is the current drug in MS therapy, is also associated with the reduced production of MMP-9 by T cells, the decreased ability of T cells to transmigrate a basement membrane barrier and the reduced gadolinium-enhanced MRI activity.99–101 These results imply that IFN-β exerts alleviating effects in part through the suppression of the MMPs’ functions. Clinical studies support the possibility that IFN-β regulates MMP and TIMP-1 levels in patients with MS.48,102,103 However, there is no justified data regarding the anti-MMP effects of glatiramer acetate, another current drug in MS therapy.101 Interestingly, it has been shown that MMP-9 could disrupt IFN-β and that this was inhibited by minocycline through antagonism of the MMP-9 functioning.104 Thus, it seems that the combination of IFN-β and minocycline leads to promising results in MS therapy.105 It has been shown consistently that the combination of IFN-β and minocycline in the treatment of EAE leads to a greater alleviation of the EAE severity score and histological outcomes, compared to either medication alone.106 However, because several MMPs are elaborated, it seems that a non-selective MMP inhibitor might be more efficient than those that target only specific MMPs or subclasses of the MMP family. It is suggested that current MMP inhibitors should only be used in short treatment courses, so that they do not inhibit the MMP-mediated repair processes that subsequently ensue after an injury. Thus, careful selection of the time frames and doses of MMP inhibitors is recommended and patients can benefit from their utilisation in neurological conditions.88
Another strategy to treat MS is targeting the upstream molecules which regulate the expression of MMPs and TIMPs. For instance, the ECM metalloproteinase inducer EMMPRIN (also known as CD147) induces MMPs and this constitutes a new therapeutic target.107 It is reported that EMMPRIN is upregulated on the peripheral leukocytes before the onset of EAE. In brain sections of EAE cases, EMMPRIN expression was localised with MMP-9 protein and activity. The increased EMMPRIN levels were also characteristic of brain samples from MS subjects, particularly in plaque-containing areas. As EMMPRIN regulates leukocyte trafficking by increasing MMP activity, it may be a novel therapeutic target in the treatment of MS.107 Additionally, in keeping with this concept to interfere with the proteinase/inhibitor balances in MS, statins have also been studied as they have been shown to reduce the expression level of MMP-9.108,109
The effect of intravenous IgG in MS is disputed; however, it has been reported in animal models that intravenous IgG might be effective as a prophylaxis, preventing disease development, and exerting its function by suppressing the activation of MMPs-2 and -9 by blocking MMP activities at the interface between the blood stream and CNS.110
Another interesting strategy is the control of MMP regulator cytokines and chemokines; by using neutralising antibodies or pharmacological antagonists against cytokines, chemokines and/or their receptors may bring considerable change in the therapeutic landscape for MS.86,111
Conclusion
In MS disease, increased MMP activity and reduced TIMP levels contribute to a loss of the BBB integrity and infiltration of inflammatory immune cells to the CNS. Therefore, MMPs and their TIMPs play a key role in the immunopathogenesis of MS, and are suggested as potential targets to treatments. Hence, more research in MMPs/TIMPs domain and their roles in immunopathogenesis of disease might be recommended as a therapeutic toll for controlling MS.
References
- 1.John GR. Investigation of astrocyte - Oligodendrocyte interactions in human cultures. Methods Mol Biol. 2012;814:401–14. doi: 10.1007/978-1-61779-452-0_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sriram S. Role of glial cells in innate immunity and their role in CNS demyelination. J Neuroimmunol. 2011;239:13–20. doi: 10.1016/j.jneuroim.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–32. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 4.Frohman EM, Racke MK, Raine CS. Multiple sclerosis: The plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 5.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 6.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–51. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60:306–21. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 9.Theoharides TC, Kempuraj D, Kourelis T, Manola A. Human mast cells stimulate activated T cells: Implications for multiple sclerosis. Ann N Y Acad Sci. 2008;1144:74–82. doi: 10.1196/annals.1418.029. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers JM, Zhou L, Miller SD. Act 1, scene brain: Astrocytes play a lead role. Immunity. 2010;32:302–4. doi: 10.1016/j.immuni.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation. 2011;8:25. doi: 10.1186/1742-2094-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–9. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Harada C, Namekata K, Matsuzawa A, Camps M, Ji H, et al. Regulation of the severity of neuroinflammation and demyelination by TLR-ASK1-p38 pathway. EMBO Mol Med. 2010;2:504–15. doi: 10.1002/emmm.201000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batoulis H, Addicks K, Kuerten S. Emerging concepts in autoimmune encephalomyelitis beyond the CD4/T(H)1 paradigm. Ann Anat. 2010;192:179–93. doi: 10.1016/j.aanat.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, et al. Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J Immunol. 2011;187:274–82. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol. 2010;184:6891–900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu F, Kanda T. [Disruption of the blood-brain barrier in inflammatory neurological diseases] Brain Nerve. 2013;65:165–76. [PubMed] [Google Scholar]
- 18.Morales Y, Parisi JE, Lucchinetti CF. The pathology of multiple sclerosis: Evidence for heterogeneity. Adv Neurol. 2006;98:27–45. [PubMed] [Google Scholar]
- 19.van Horssen J, Vos CM, Admiraal L, van Haastert ES, Montagne L, van der Valk P, et al. Matrix metalloproteinase-19 is highly expressed in active multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2006;32:585–93. doi: 10.1111/j.1365-2990.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 20.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 21.Birkedal-Hansen H. Matrix metalloproteinases. Adv Dent Res. 1995;9:16. doi: 10.1177/0895937495009003S0701. [DOI] [PubMed] [Google Scholar]
- 22.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Muroski ME, Roycik MD, Newcomer RG, Van den Steen PE, Opdenakker G, Monroe HR, et al. Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr Pharm Biotechnol. 2008;9:34–46. doi: 10.2174/138920108783497631. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–49. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-alpha. J Neurochem. 2007;102:900–12. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurzepa J, Bartosik-Psujek H, Suchozebrska-Jesionek D, Rejdak K, Stryjecka-Zimmer M, Stelmasiak Z. [Role of matrix metalloproteinases in the pathogenesis of multiple sclerosis] Neurol Neurochir Pol. 2005;39:63–7. [PubMed] [Google Scholar]
- 28.Gijbels K, Masure S, Carton H, Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992;41:29–34. doi: 10.1016/0165-5728(92)90192-n. [DOI] [PubMed] [Google Scholar]
- 29.Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36:432–40. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- 30.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–11. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg GA. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8:586–95. doi: 10.1177/1073858402238517. [DOI] [PubMed] [Google Scholar]
- 32.Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–9. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–8. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Vos CM, Sjulson L, Nath A, McArthur JC, Pardo CA, Rothstein J, et al. Cytotoxicity by matrix metalloprotease-1 in organotypic spinal cord and dissociated neuronal cultures. Exp Neurol. 2000;163:324–30. doi: 10.1006/exnr.2000.7388. [DOI] [PubMed] [Google Scholar]
- 35.Cossins JA, Clements JM, Ford J, Miller KM, Pigott R, Vos W, et al. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997;94:590–8. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- 36.Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–30. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiryaev SA, Remacle AG, Savinov AY, Chernov AV, Cieplak P, Radichev IA, et al. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J Biol Chem. 2009;284:30615–26. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiryaev SA, Savinov AY, Cieplak P, Ratnikov BI, Motamedchaboki K, Smith JW, et al. Matrix metalloproteinase proteolysis of the myelin basic protein isoforms is a source of immunogenic peptides in autoimmune multiple sclerosis. PLoS One. 2009;4:e4952. doi: 10.1371/journal.pone.0004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol. 2003;138:106–14. doi: 10.1016/s0165-5728(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 40.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–8. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 41.Kouwenhoven M, Ozenci V, Gomes A, Yarilin D, Giedraitis V, Press R, et al. Multiple sclerosis: Elevated expression of matrix metalloproteinases in blood monocytes. J Autoimmun. 2001;16:463–70. doi: 10.1006/jaut.2001.0505. [DOI] [PubMed] [Google Scholar]
- 42.Altimiras J, Borras JM, Mendez E, Pastor E, Bassons T. Knowledge of medication in hospitalized chronic respiratory patients. Pharm Weekbl Sci. 1992;14:174–9. doi: 10.1007/BF01962534. [DOI] [PubMed] [Google Scholar]
- 43.Benesová Y, Vasku A, Novotná H, Litzman J, Stourac P, Beránek M, et al. Matrix metalloproteinase-9 and matrix metalloproteinase-2 as biomarkers of various courses in multiple sclerosis. Mult Scler. 2009;15:316–22. doi: 10.1177/1352458508099482. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006;111:289–99. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 45.Fainardi E, Castellazzi M, Tamborino C, Trentini A, Manfrinato MC, Baldi E, et al. Potential relevance of cerebrospinal fluid and serum levels and intrathecal synthesis of active matrix metalloproteinase-2 (MMP-2) as markers of disease remission in patients with multiple sclerosis. Mult Scler. 2009;15:547–54. doi: 10.1177/1352458509102372. [DOI] [PubMed] [Google Scholar]
- 46.Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–15. [PubMed] [Google Scholar]
- 47.Newman TA, Woolley ST, Hughes PM, Sibson NR, Anthony DC, Perry VH. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: Involvement of metalloproteinases. Brain. 2001;124:2203–14. doi: 10.1093/brain/124.11.2203. [DOI] [PubMed] [Google Scholar]
- 48.Galboiz Y, Shapiro S, Lahat N, Rawashdeh H, Miller A. Matrix metalloproteinases and their tissue inhibitors as markers of disease subtype and response to interferon-beta therapy in relapsing and secondary-progressive multiple sclerosis patients. Ann Neurol. 2001;50:443–51. doi: 10.1002/ana.1218. [DOI] [PubMed] [Google Scholar]
- 49.Emmert-Buck MR, Emonard HP, Corcoran ML, Krutzsch HC, Foidart JM, Stetler-Stevenson WG. Cell surface binding of TIMP-2 and pro-MMP-2/TIMP-2 complex. FEBS Lett. 1995;364:28–32. doi: 10.1016/0014-5793(95)00345-a. [DOI] [PubMed] [Google Scholar]
- 50.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–4. [PubMed] [Google Scholar]
- 51.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system: A look on the bright side. J Neurochem. 2012;123:203–16. doi: 10.1111/j.1471-4159.2012.07900.x. [DOI] [PubMed] [Google Scholar]
- 52.Kanesaka T, Mori M, Hattori T, Oki T, Kuwabara S. Serum matrix metalloproteinase-3 levels correlate with disease activity in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77:185–8. doi: 10.1136/jnnp.2005.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–80. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O, Shin DH, et al. Matrix metalloproteinase-3: A novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–11. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology? Pathol Biol (Paris) 2000;48:80–6. [PubMed] [Google Scholar]
- 56.Fowlkes JL, Serra DM, Bunn RC, Thrailkill KM, Enghild JJ, Nagase H. Regulation of insulin-like growth factor (IGF)-I action by matrix metalloproteinase-3 involves selective disruption of IGF-I/IGF-binding protein-3 complexes. Endocrinology. 2004;145:620–6. doi: 10.1210/en.2003-0636. [DOI] [PubMed] [Google Scholar]
- 57.McCawley LJ, Matrisian LM. Matrix metalloproteinases: They’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–40. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 58.Skuljec J, Gudi V, Ulrich R, Frichert K, Yildiz O, Pul R, et al. Matrix metalloproteinases and their tissue inhibitors in cuprizone-induced demyelination and remyelination of brain white and gray matter. J Neuropathol Exp Neurol. 2011;70:758–69. doi: 10.1097/NEN.0b013e3182294fad. [DOI] [PubMed] [Google Scholar]
- 59.Lindberg RL, De Groot CJ, Montagne L, Freitag P, van der Valk P, Kappos L, et al. The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain. 2001;124:1743–53. doi: 10.1093/brain/124.9.1743. [DOI] [PubMed] [Google Scholar]
- 60.Kieseier BC, Kiefer R, Clements JM, Miller K, Wells GM, Schweitzer T, et al. Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain. 1998;121:159–66. doi: 10.1093/brain/121.1.159. [DOI] [PubMed] [Google Scholar]
- 61.Opdenakker G, Nelissen I, Van Damme J. Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol. 2003;2:747–56. doi: 10.1016/s1474-4422(03)00587-8. [DOI] [PubMed] [Google Scholar]
- 62.Cuzner ML, Gveric D, Strand C, Loughlin AJ, Paemen L, Opdenakker G, et al. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: Comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996;55:1194–204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Sellebjerg F, Madsen HO, Jensen CV, Jensen J, Garred P. CCR5 delta32, matrix metalloproteinase-9 and disease activity in multiple sclerosis. J Neuroimmunol. 2000;102:98–106. doi: 10.1016/s0165-5728(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 64.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong M, Liu R, Guo L, Li C, Tan G. Pathological findings in rats with experimental allergic encephalomyelitis. APMIS. 2008;116:972–84. doi: 10.1111/j.1600-0463.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 66.D’Haese A, Wuyts A, Dillen C, Dubois B, Billiau A, Heremans H, et al. In vivo neutrophil recruitment by granulocyte chemotactic protein-2 is assisted by gelatinase B/MMP-9 in the mouse. J Interferon Cytokine Res. 2000;20:667–74. doi: 10.1089/107999000414853. [DOI] [PubMed] [Google Scholar]
- 67.Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–15. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–19. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- 70.Fridman R, Toth M, Peña D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2) Cancer Res. 1995;55:2548–55. [PubMed] [Google Scholar]
- 71.Van den Steen PE, Proost P, Grillet B, Brand DD, Kang AH, Van Damme J, et al. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. 2002;16:379–89. doi: 10.1096/fj.01-0688com. [DOI] [PubMed] [Google Scholar]
- 72.Alexander JS, Harris MK, Wells SR, Mills G, Chalamidas K, Ganta VC, et al. Alterations in serum MMP-8, MMP-9, IL-12p40 and IL-23 in multiple sclerosis patients treated with interferon-beta1b. Mult Scler. 2010;16:801–9. doi: 10.1177/1352458510370791. [DOI] [PubMed] [Google Scholar]
- 73.Sato W, Tomita A, Ichikawa D, Lin Y, Kishida H, Miyake S, et al. CCR2(+)CCR5(+) T cells produce matrix metalloproteinase-9 and osteopontin in the pathogenesis of multiple sclerosis. J Immunol. 2012;189:5057–65. doi: 10.4049/jimmunol.1202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zivković M, Djurić T, Dincić E, Raicević R, Alavantić D, Stanković A. Matrix metalloproteinase-9 -1562 C/T gene polymorphism in Serbian patients with multiple sclerosis. J Neuroimmunol. 2007;189:147–50. doi: 10.1016/j.jneuroim.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 75.Fainardi E, Castellazzi M, Bellini T, Manfrinato MC, Baldi E, Casetta I, et al. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler. 2006;12:294–301. doi: 10.1191/135248506ms1274oa. [DOI] [PubMed] [Google Scholar]
- 76.Lee MA, Palace J, Stabler G, Ford J, Gearing A, Miller K. Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain. 1999;122:191–7. doi: 10.1093/brain/122.2.191. [DOI] [PubMed] [Google Scholar]
- 77.Ozenci V, Rinaldi L, Teleshova N, Matusevicius D, Kivisäkk P, Kouwenhoven M, et al. Metalloproteinases and their tissue inhibitors in multiple sclerosis. J Autoimmun. 1999;12:297–303. doi: 10.1006/jaut.1999.0285. [DOI] [PubMed] [Google Scholar]
- 78.Thorne M, Moore CS, Robertson GS. Lack of TIMP-1 increases severity of experimental autoimmune encephalomyelitis: Effects of darbepoetin alfa on TIMP-1 null and wild-type mice. J Neuroimmunol. 2009;211:92–100. doi: 10.1016/j.jneuroim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Benesová Y, Vasků A, Stourac P, Hladiková M, Beránek M, Kadanka Z, et al. Matrix metalloproteinase-9 and matrix metalloproteinase-2 gene polymorphisms in multiple sclerosis. J Neuroimmunol. 2008;205:105–9. doi: 10.1016/j.jneuroim.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Fernandes KS, Brum DG, Sandrim VC, Guerreiro CT, Barreira AA, Tanus-Santos JE. Matrix metalloproteinase-9 genotypes and haplotypes are associated with multiple sclerosis and with the degree of disability of the disease. J Neuroimmunol. 2009;214:128–31. doi: 10.1016/j.jneuroim.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Ulrich R, Gerhauser I, Seeliger F, Baumgärtner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: A reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27:408–18. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- 82.Weaver A, Goncalves da Silva A, Nuttall RK, Edwards DR, Shapiro SD, Rivest S, et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19:1668–70. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- 83.Pei D. Leukolysin/MMP25/MT6-MMP: A novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9:291–303. doi: 10.1038/sj.cr.7290028. [DOI] [PubMed] [Google Scholar]
- 84.Toft-Hansen H, Babcock AA, Millward JM, Owens T. Downregulation of membrane type-matrix metalloproteinases in the inflamed or injured central nervous system. J Neuroinflammation. 2007;4:24. doi: 10.1186/1742-2094-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Devy L, Dransfield DT. New strategies for the next generation of matrix-metalloproteinase inhibitors: Selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem Res Int. 2011;2011:191670. doi: 10.1155/2011/191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Opdenakker G, Van Damme J. Probing cytokines, chemokines and matrix metalloproteinases towards better immunotherapies of multiple sclerosis. Cytokine Growth Factor Rev. 2011;22:359–365. doi: 10.1016/j.cytogfr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Brown PD. Matrix metalloproteinase inhibitors. Breast Cancer Res Treat. 1998;52:125–36. doi: 10.1023/a:1006119319695. [DOI] [PubMed] [Google Scholar]
- 88.Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: Where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Brown PD, Giavazzi R. Matrix metalloproteinase inhibition: A review of anti-tumour activity. Ann Oncol. 1995;6:967–74. doi: 10.1093/oxfordjournals.annonc.a059091. [DOI] [PubMed] [Google Scholar]
- 90.Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994;94:2177–82. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graesser D, Mahooti S, Madri JA. Distinct roles for matrix metalloproteinase-2 and alpha4 integrin in autoimmune T cell extravasation and residency in brain parenchyma during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;109:121–31. doi: 10.1016/s0165-5728(00)00275-7. [DOI] [PubMed] [Google Scholar]
- 92.Hewson AK, Smith T, Leonard JP, Cuzner ML. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res. 1995;44:345–9. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- 93.Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, et al. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: An overview. J Neuroimmunol. 1997;72:155–61. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 94.Norga K, Paemen L, Masure S, Dillen C, Heremans H, Billiau A, et al. Prevention of acute autoimmune encephalomyelitis and abrogation of relapses in murine models of multiple sclerosis by the protease inhibitor D-penicillamine. Inflamm Res. 1995;44:529–34. doi: 10.1007/BF01757357. [DOI] [PubMed] [Google Scholar]
- 95.Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–51. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- 96.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: Minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 97.Metz LM, Zhang Y, Yeung M, Patry DG, Bell RB, Stoian CA, et al. Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2004;55:756. doi: 10.1002/ana.20111. [DOI] [PubMed] [Google Scholar]
- 98.Zabad RK, Metz LM, Todoruk TR, Zhang Y, Mitchell JR, Yeung M, et al. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: A pilot study. Mult Scler. 2007;13:517–26. doi: 10.1177/1352458506070319. [DOI] [PubMed] [Google Scholar]
- 99.Stüve O, Dooley NP, Uhm JH, Antel JP, Francis GS, Williams G, et al. Interferon beta-1b decreases the migration of T lymphocytes in vitro: Effects on matrix metalloproteinase-9. Ann Neurol. 1996;40:853–63. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]
- 100.Leppert D, Waubant E, Bürk MR, Oksenberg JR, Hauser SL. Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: A possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol. 1996;40:846–52. doi: 10.1002/ana.410400606. [DOI] [PubMed] [Google Scholar]
- 101.Yong VW. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology. 2002;59:802–8. doi: 10.1212/wnl.59.6.802. [DOI] [PubMed] [Google Scholar]
- 102.Fawcett HA, Wansbrough-Jones MH, Clark AE, Leigh IM. Prophylactic topical acyclovir for frequent recurrent herpes simplex infection with and without erythema multiforme. Br Med J (Clin Res Ed) 1983;287:798–9. doi: 10.1136/bmj.287.6395.798-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trojano M, Avolio C, Liuzzi GM, Ruggieri M, Defazio G, Liguori M, et al. Changes of serum sICAM-1 and MMP-9 induced by rIFNbeta-1b treatment in relapsing-remitting MS. Neurology. 1999;53:1402–8. doi: 10.1212/wnl.53.7.1402. [DOI] [PubMed] [Google Scholar]
- 104.Nelissen I, Martens E, Van den Steen PE, Proost P, Ronsse I, Opdenakker G. Gelatinase B/matrix metalloproteinase-9 cleaves interferon-beta and is a target for immunotherapy. Brain. 2003;126:1371–81. doi: 10.1093/brain/awg129. [DOI] [PubMed] [Google Scholar]
- 105.Yong VW, Zabad RK, Agrawal S, Goncalves Dasilva A, Metz LM. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J Neurol Sci. 2007;259:79–84. doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 106.Giuliani F, Fu SA, Metz LM, Yong VW. Effective combination of minocycline and interferon-beta in a model of multiple sclerosis. J Neuroimmunol. 2005;165:83–91. doi: 10.1016/j.jneuroim.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 107.Agrawal SM, Silva C, Tourtellotte WW, Yong VW. EMMPRIN: A novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:669–77. doi: 10.1523/JNEUROSCI.3659-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stüve O, Prod’homme T, Slavin A, Youssef S, Dunn S, Steinman L, et al. Statins and their potential targets in multiple sclerosis therapy. Expert Opin Ther Targets. 2003;7:613–22. doi: 10.1517/14728222.7.5.613. [DOI] [PubMed] [Google Scholar]
- 109.Antel JP, Miron VE. Central nervous system effects of current and emerging multiple sclerosis-directed immuno-therapies. Clin Neurol Neurosurg. 2008;110:951–7. doi: 10.1016/j.clineuro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 110.Niimi N, Kohyama K, Kamei S, Matsumoto Y. Intravenous immunoglobulin therapy prevents development of autoimmune encephalomyelitis and suppresses activation of matrix metalloproteinases. Neuropathology. 2011;31:392–400. doi: 10.1111/j.1440-1789.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 111.Opdenakker G, Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol Today. 1994;15:103–7. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]