Abstract
Objectives:
This study was carried out to determine the effects of tocotrienol-rich fraction (TRF) (200 mg/Kg) on biomarkers of oxidative stress on erythrocyte membranes and leukocyte deoxyribonucleic acid (DNA) damage in streptozotocin (STZ)-induced diabetic rats.
Methods:
Male rats (n = 40) were divided randomly into four groups of 10: a normal group; a normal group with TRF; a diabetic group, and a diabetic group with TRF. Following four weeks of treatment, fasting blood glucose (FBG) levels, oxidative stress markers and the antioxidant status of the erythrocytes were measured.
Results:
FBG levels for the STZ-induced diabetic rats were significantly increased (P <0.001) when compared to the normal group and erythrocyte malondialdehyde levels were also significantly higher (P <0.0001) in this group. Decreased levels of reduced glutathione and increased levels of oxidised glutathione (P <0.001) were observed in STZ-induced diabetic rats when compared to the control group and diabetic group with TRF. The results of the superoxide dismutase and glutathione peroxidase activities were significantly lower in the STZ-induced diabetic rats than in the normal group (P <0.001). The levels of DNA damage, measured by the tail length and tail moment of the leukocyte, were significantly higher in STZ-induced diabetic (P <0.0001). TRF supplementation managed to normalise the level of DNA damage in diabetic rats treated with TRF.
Conclusion:
Daily supplementation with 200 mg/Kg of TRF for four weeks was found to reduce levels of oxidative stress markers by inhibiting lipid peroxidation and increasing the levels of antioxidant status in a prevention trial for STZ-induced diabetic rats.
Keywords: Antioxidants, Diabetes Mellitus, Oxidative Stress, Tocotrienols
Advances in Knowledge
- The antioxidant potential of tocotrienols has been a focus of scientific interest in recent years. Recent findings have shown that tocotrienols are responsible for the majority of the activity of vitamin E. This study shows that a tocotrienol-rich fraction (TRF) from palm oil at 200 mg/Kg of body weight for four weeks provides sufficient antioxidant activity to reduce oxidative stress, by inhibiting lipid peroxidation and reducing levels of deribonucleic acid (DNA) damage, as well as increasing the status of the antioxidant system in a prevention trial for diabetic rats.
Application to Patient Care
- In terms of using tocotrienols for antioxidant agent activity in diabetic humans, the finding of this study suggests that TRF at 200 mg/Kg for four weeks may play an important role in reducing the complications of oxidative stress-mediated diabetes mellitus.
According to the World Health Organization (WHO), a total of 171 million people in all age groups worldwide (2.8% of the world’s population) have diabetes; this number is expected to rise to 366 million (4.4% of the world’s population) by 2030.1 A balance between the rate of free radical generation and the rate of radical elimination is important so that cellular radical generation is not harmful.2 However, if there is a significant increase in radical generation or a decrease in radical elimination of the cells, this balance is disrupted, resulting in the formation of oxidative stress.3 Free radicals and oxidative stress are among the factors involved in the pathogenesis of diabetes; in particular, they appear to play a major role in the development of chronic complications from diabetes.4
Vitamin E is widely accepted as the first line of defense against lipid peroxidation, protecting the polyunsaturated fatty acids in the cell membranes through its free radical-quenching activity in the biomembranes in the early stages of the free radical attack.5 Palm oil contains a larger concentration of tocotrienols (70%) than any other natural source, except annatto (100%).6 Tocotrienols and tocopherols act as potent antioxidants, serving to protect the cellular membranes from free radical-catalysed lipid peroxidation.7 A number of health-related biological properties of tocotrienols have been identified, including anti-cancer, anti-cholesterolemic, antihypertensive, antioxidant, immunomodulatory and neuro-protective properties.6
Tocotrienol-rich fraction (TRF) has unique biological properties that make it a potential neuroprotective dietary factor. In addition to its antioxidant activity, TRF at micromolar concentrations displays cholesterol-lowering activities in cells, animal models and some, but not all, human subjects by means of the inhibition of the activity of the rate-limiting enzyme in cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase.8 Furthermore, TRF at lower concentrations (∼10 nmol/L) modulates signalling pathways involved in neuronal cell death in cell culture experiments.8 Currently, TRF is addressed as an anti-apoptotic and anti-inflammatory agent, and emerging findings suggest a role for nuclear factor kappa-B cells (NF-κB) as a mediator of the effects of dietary factors on neuronal plasticity.9
Our previous study showed that the TRF of palm oil (200 mg/Kg of body weight) has some protective effects as it reduces lipid peroxidation and protein oxidation, and prevents the physical and morphological alterations of erythrocytes after four weeks.10 Another study reported that the supplementation of TRF reduced oxidative stress and blood glucose levels and had protective effects on diabetes-induced pancreatic damage in diabetic rats.11 The purpose of this study was to investigate the effects of TRF on oxidative stress and antioxidant status on erythrocytes in streptozotocin (STZ)-induced diabetic rats.
Methods
For this study, male Sprague-Dawley rats were provided by the Laboratory Animal Resources Unit, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur. The animals were 8–10 weeks old and weighed 200–250 g. During the study period, the rats had unrestricted access to food and tap water with a standard diet containing 22% crude protein, 5% crude fibre, 3% fat, 13% moisture, 8% ash, 0.85–1.2% calcium, 0.6–1% phosphorus and 49% nitrogen-free extract in the form of mouse pellet 702-P (Gold Coin Holdings, Kuala Lumpur, Malaysia). They were housed in pairs in plastic cages with wood chips for bedding (changed weekly for the normal groups and daily for the diabetic groups). The animals were acclimatised to standard laboratory conditions, with a temperature of 25 °C with standard light-darkness cycles of 12 hours for one week before experimentation. The study was approved by the Universiti Kebangsaan Malaysia Animal Ethics Committee.
The experiment was designed with 40 rats, divided randomly into four groups of 10 rats: a normal group; a normal group supplemented with TRF; a diabetic group, and a diabetic group supplemented with TRF. Initially, all experimental groups had similar body weights (200–250 g). At the end of the study period, both diabetic groups (STZ-induced diabetic rats and diabetic rats with TRF) showed a significant loss of body weight (average weight 190 g) when compared to the normal groups (average weight 319 g). Diabetes was induced after an overnight fast by a single intravenous injection of STZ via the tail vein (45 mg/ Kg of body weight), which was freshly dissolved in a normal saline solution (9 g/L of sodium chloride). Three days later, blood was collected via the tail vein and the glucose concentration was measured by a strip-operated blood glucose sensor (Companion 2, Medisense Ltd., Birmingham, UK). Rats with blood glucose levels of >7.0 mmol/L were included in the study.
TRF was obtained from the Sime Darby Group (Kuala Lumpur, Malaysia) as follows: α-tocopherol at 171.1 mg/g, α-tocotrienol at 190.4 mg/g, β-tocotrienol at 36.0 mg/g, γ-tocotrienol at 211.2 mg/g and δ-tocotrienol at 150 mg/g. It was administered orally at a dose of 200 mg/Kg of body weight per day throughout the four-week feeding period, and supplementation was begun the same day. After four weeks of supplementation, all the rats underwent overnight fasting and were then euthanised by a cardiac puncture while under deep anaesthesia with diethyl ether.
The biochemical investigation commenced while the rats were under deep anaesthesia. A blood sample was taken from each of the rats via a cardiac puncture using a 21 gauge needle with a 10 ml syringe. Samples were then transferred into tubes containing a sodium fluoride plastic tube (fluoride oxalate tube, #X2230, Teklab Ltd., UK) for fasting blood glucose (FBG) analysis and ethylene diamine tetra acetic acid (EDTA) tubes (BD Vacutainer® Plus Plastic K2EDTA Tube #368589, Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) and kept on ice. Plasma glucose levels were analysed on the same day using enzymatic glucose-oxidase kits (Glucose Oxidase Reagent, # TR15103, Fisher Scientific Ltd., Loughborough, UK). Erythrocyte pellets were separated from the plasma and the leukocyte layer by centrifugation at 4,000 rpm for 10 mins at 4 °C. The supernatant plasma was pipetted into 1.5 ml Eppendorf tubes (Eppendorf AG, Hamburg, Germany) and frozen at −80 °C until use. After the removal of the buffy coat, the erythrocyte pellets were prepared by being washed three times with a cold (4 °C) normal saline (9%) solution. These pellets were then stored at −80 °C for biochemical analyses. The amount of blood taken from each rat varied from 6–10 ml.
Erythrocyte malondialdehyde (MDA) samples were measured using the high performance liquid chromatography (HPLC) method with some modifications as described by Pilz et al., based on the derivation with 2, 4-dinitrophenylhydrazine (DNPH).12 The total MDA was prepared from the erythrocytes by perchloric acid deproteinisation whereas an alkaline hydrolysation step for 30 mins at 60 °C was introduced prior to protein precipitation for the determination of the total (free and bound) MDA. The standard and samples were processed under the same conditions as described in this section.
The MDA standard was prepared by dissolving 25 μl of 1,1, 3,3-tetraethoxypropane (TEP) in 100 ml of 1% sulfuric acid to give a 1 mM stock solution and was kept at 4 °C overnight. The working standard was prepared by the hydrolysis of the 1 ml of TEP stock solution in 50 ml of distilled water (dH2O) and incubated for two hours at room temperature.13 The concentration of 1, 2, 4, 6, 8, and 10 μmol was prepared from the working standard to get the standard curve for the estimation of total MDA.
A solution of 200 μl of sodium hydroxide (NaOH) of 1.3 M was added to the 50 μl standard or sample. For the protein-bound alkaline hydrolysis step, the mixture was incubated in a 60 °C water bath for 30 mins and then cooled in ice for five mins. The hydrolysis samples were acidified with 100 μl of 35% (volume/volume [v/v]) perchloric acid. After centrifugation at 1,000 g for 10 mins, 300 μl of the supernatant were mixed with 12.5 μl of the DNPH solution and incubated in the dark at room temperature for 30 mins. Finally, 40 μl was injected into the HPLC system.
Analytical HPLC separations were performed with a 655A-12 liquid chromatography instrument (SCL-10AVP system controller, Shimadzu Scientific Instruments, Kyoto, Japan) equipped with an auto injector (655A-40) and a variable-wavelength ultraviolet (UV) detector (655A) operated at 310 nm on a 125 × 3 mm Nucleosil C18 column of 5 μm particle size on reversed-phase with an integrated precolumn (SepServ HPCL, Separation Service Berlin Ohg, Berlin, Germany). The column was kept at 30 °C in a column oven with a mobile phase. The HPLC mobile phase was prepared by mixing 380 ml acetonitrile with 620 ml of dH2O acidified with 0.2% (v/v) acetic acid and degassed under reduced pressure with a flow-rate of 1 ml/min.
Superoxide dismutase (SOD) activity was determined according to Fridovich and Beyer’s method, based on the inhibition or reduction of nitroblue tetrazolium (NBT).14 A reaction mixture of a substrate solution containing L-methionine (30 mg/ml), tritone X-100 (1%) and NBT (1.14 mg/ml) was added in a phosphate buffer saline (PBS) solution (pH 7.8). Lyses erythrocytes (20 μl) were added to 0.1 ml of cold dH2O and were kept at 4 °C for 15 mins. The mixture (1 ml) was added to 20 μl of lyses erythrocyte or a PBS solution (8 g of sodium chloride [NaCl], 0.2 g of potassium chloride, 1.44 g of disodium hydrogen phosphate and 0.24 g of monopotassium phosphate) (control), and 10 μl of riboflavin was then added. The samples were incubated for seven mins. The absorbance of the mixture at 560 nm was determined using a spectrophotometer UV-160A (Shimadzu Scientific Instruments). The enzyme activities were calculated in U/g haemoglobin (Hb).
Glutathione peroxidase (GPx) activity was determined according to the Lawrence method, based on the principle that GPx catalyses the oxidation of glutaredoxin (GRx) and nicotinamide adenine dinucleotide phosphate (NADPH).15 The oxidised glutathione was immediately converted to the reduced form with a concomitant oxidation, NADPH to NADP+. A 100 μl sample of lysed erythrocyte and 0.8 ml of a buffered solution (1 mM of sodium azide, 1 mM of EDTA, 0.2 mM of NADPH, 50 units of GRx1 mM of GSH and 50 mM of PBS; pH 7) were incubated at room temperature for 5 mins, then 100 μl (0.25 mM) of hydrogen peroxide was added. The absorbance of the mixture at 340 nm was determined using the Shimadzu Scientific Instruments spectrophotometer. Activities of enzymes were calculated in U/g Hb. A commercial glutathione detection kit (BioVision, Inc., Milpitas, California, USA) was used to measure GSH, GSSG and total glutathione. The readings for samples and the standard were determined using a fluorescence plate reader equipped with an excitation wavelength/emission wavelength (Ex/Em) of 340/420 nm (SkanIt Software, Thermo Fisher Scientific Inc.).
The concentration of vitamin E in the rat erythrocyte samples was measured by the HPLC method with some modification of the procedures described by Brandt et al.16 Briefly, α-tocopherol and α-tocopheryl-acetate (the internal standard) in the samples were extracted into hexane. The hexane extract was dried under nitrogen and dissolved in the mobile phase for injection into the HPLC for separation with detection by UV. The mobile phase used was composed of methanol and toluene of HPLC grade (BDH Merck Ltd., Poole, UK) in the ratio of 80:20.
A stock standard of 10 mmol/L of α-tocopherol (BDH Merck Ltd.) was prepared by dissolving 0.0216 g of vitamin E in 5 ml of absolute ethanol. Different standards concentrations of α-tocopherol (10, 20, 30, 40 and 50 umol/L) were prepared. The internal standard, α-tocopheryl acetate, was prepared by dissolving 0.0236 g of α-tocopheryl acetate in 5 ml of absolute ethanol and then pipetting 0.75 ml of this into 50 ml of absolute ethanol. The final concentration of α-tocopheryl acetate in every standard and sample was 150 µmol/L.
Lyphochek 1 and Lyphochek 2 (Bio-Rad Laboratories, Hercules, California, USA) were used as the low and high precision controls, respectively, and treated as plasma samples. A 100 µl of sample or standard was added to 100 μl of ethanol together with 100 µl of α-tocopheryl acetate. Hexane (200 µl) was added and centrifuged at 2,500 g (for 10 mins at 4 °C). Then 120 µl of the upper layer (hexane extract) was evaporated. A total of 60 μl of the mobile phase were added to dissolve the extract, and this was immediately loaded for injection into the HPLC system.
Analytical HPLC separations were performed as previously described in this section. The running condition of HPLC was seven mins per sample. The flow-rate was 1 ml/min. The injection volume was 20 μl and the detection wavelength was at 292 nm. The retention time was ∼4.4 mins for vitamin E and ∼5.3 mins for α-tocopheryl acetate.
A calibration curve was constructed by plotting the peak area ratio of vitamin E and α-tocopheryl-acetate against the vitamin E standard values. The slope obtained from the calibration curve was used to determine the α-tocopherol concentration in the sample by the following calculation:
The deoxyribonucleic acid (DNA) oxida-tive damage was then measured as follows. The alkaline single-cell gel electrophoresis assay procedure was performed according to the method described by Singh et al.17 For slide preparation, 100 μl (0.5%) of normal melting agarose at about 121 °C in calcium ion- and magnesium ion-free butylphthalate plasticised styrene, was immediately covered with a cover slip and kept on ice for 5 mins to allow the
agarose to solidify. Around 10 μl of whole blood was suspended in 80 μl (0.5%) of low melting agarose. The cell suspension was rapidly pipetted onto the first agarose layer and spread using a cover slip, and the slides were immersed in a lysis buffer solution (2.5 M of NaCl, 100 mM of sodium EDTA, 10 mM of trisaminomethane hydrochloride (Tris-HCl) and 1% of Triton X-100), and 10% dimethyl sulfoxide was added just before use at 4 °C for a minimum of one hour. The slides were placed in a horizontal gel electrophoresis tank and filled with fresh cold electrophoresis solution (10 N of sodium hydroxide and 200 mM of sodium (in the form of Na2) EDTA) and were left in the solution for 20 mins to allow the DNA to unwind. Electrophoresis was conducted at 4 °C for 20 mins. The slides were neutralised with a Tris-HCl buffer (pH 7.5) three times for five mins and stained with ethidium-bromide (100 μg/ml) for 10 mins. Slides were examined at x 20 magnification using a fluorescence microscope with 100 cells per slide analysed using image analysing software (Comet Assay Software Project (CaspLab), Version 1.2.2XCAP™ Image Processing Software (Version 1.1.1), CaspLabEPIX, Inc., Swietokrzyska Academy, Kielce, Poland).
All results were expressed as mean ± standard error of mean (SEM). The data were analysed using the Statistical Package for the Social Sciences (SPSS), Version 20.0 (IBM, Corp., Chicago, Illinois, USA), and Microsoft Office Excel 2007 (Microsoft, Inc., Redmond, Washington, USA) was used to draw the graphs. The Shapiro-Wilk test was used to check the normality of the variables. Accordingly, a oneway analysis of variance (ANOVA) test followed by a post hoc least significant difference (LSD) multiple comparison test to estimate the significance of difference between groups, and the Kruskal-Wallis one-way ANOVA test was used to analyse the data that followed non-normal behaviour of distribution patterns, respectively. The difference between groups was considered significant when P <0.05.
Results
As far as plasma glucose levels are concerned, Figure 1 shows that the levels of FBG were significantly increased (P <0.0001) in the STZ-induced diabetic rats compared to the normal group. On the other hand, FBG levels were significantly lower in the diabetic group (P <0.001), which had been supplemented with TRF, in comparison with the STZ-induced diabetic rats; however, this improvement was not sufficient to have restored the blood glucose levels to their normal state.
Figure 1:
The effect of treatment with tocotrienol-rich fraction (TRF) on blood glucose in the experimental groups of rats.
a = Significantly different compared to the normal group (P <0.05); b = Significantly different from the normal with TRF group (P <0.05); c = Significantly different from the diabetic group (P <0.05).
As to the MDA erythrocyte membrane, the erythrocyte MDA levels were statistically significantly higher in the STZ-induced diabetic rats (76.69 ± 6.8) compared with the normal rats (1.9 ± 0.1; P <0.0001) [Table 1]. On the other hand, the diabetic rats treated with TRF showed significantly decreased (13.11 ± 0.3; P <0.001) MDA levels compared with STZ-induced diabetic rats.
Table 1:
Effects of treatment with tocotrienol-rich fraction on antioxidant status and oxidative stress
| Group | SOD in U/gHb | GPx in U/gHb | GSH in μmol/gHb | GSSG in nmol/gHb | MDA in nmol/gHb |
|---|---|---|---|---|---|
| Normal | 3673 ± 182 | 71.6 ± 1.8 | 8.32 ± 0.66 | 73.34 ± 2.1 | 1.9 ± 0.13 |
| Normal with TRF | 4028 ± 161† | 65.3 ± 4.7† | 18.70 ± 0.7* | 79.24 ± 1.9† | 7.5 ± 0.6 |
| Diabetic | 2916 ± 126* | 39.3 ± 2.6* | 4.91 ± 0.3* | 92.75 ± 4.6* | 76.6 ± 6.8* |
| Diabetic with TRF | 3364 ± 137† | 46.5 ± 3.4 | 8.84 ± 0.4† | 77.76 ± 1.4† | 13.1 ± 0.3*† |
SOD = superoxide dismutase; GPx = glutathione peroxidase; GSH = reduced glutathione; GSSG = oxydised glutathione; MDA = malondialdehyde; TRF = tocotrienol-rich fraction.
Results are expressed as mean ± standard error of mean;
Significantly different as compared to the control group (P <0.05);
Significantly different as compared to the diabetic group (P <0.05).
The results for the erythrocyte antioxidants status were as follows. In the diabetic group there was significantly decreased activity of SOD, GPx and levels of GSH (P values were 0.006, 0.0001 and 0.001, respectively). On the other hand, levels of GSSG were significantly increased (P = 0.002) compared with the normal group. However, TRF supplementation produced a significant increase in SOD activity and levels of GSH, as well as a decreased level of GSSG in the diabetic with TRF group when compared to the STZ-induced diabetic rats group [Table 1 and Figure 1].
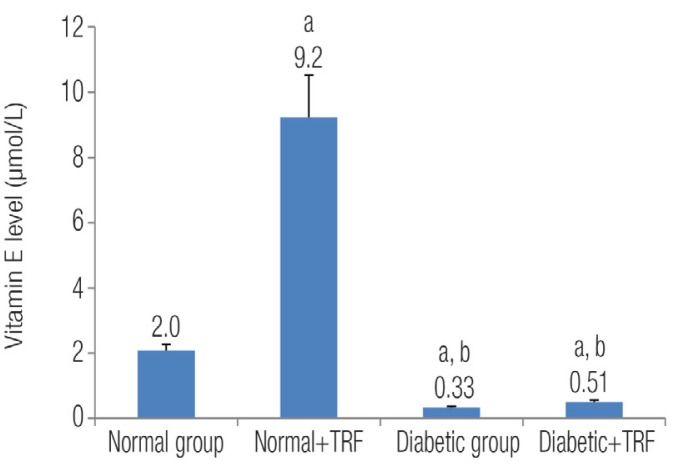
As far as the vitamin E erythrocyte membrane was concerned, the concentration of vitamin E was significantly lower in the diabetic groups (STZ-induced diabetic rats and diabetic rats with TRF) compared with the normal group (0.33 ± 0.03 and 0.55 ± 0.05 versus 2.07 ± 0.29). P values were 0.01 and 0.04, respectively. On the other hand, vitamin E levels in the normal group supplemented with TRF were significantly increased when compared to other groups (P = 0.0001) [Figure 2].
Figure 2:
The effect of treatment with tocotrienol-rich fraction (TRF) on the concentration of vitamin E in the experimental groups of rats.
a = Significantly different from the normal group (P <0.05); b = Significantly different from the normal with TRF group (P <0.05).
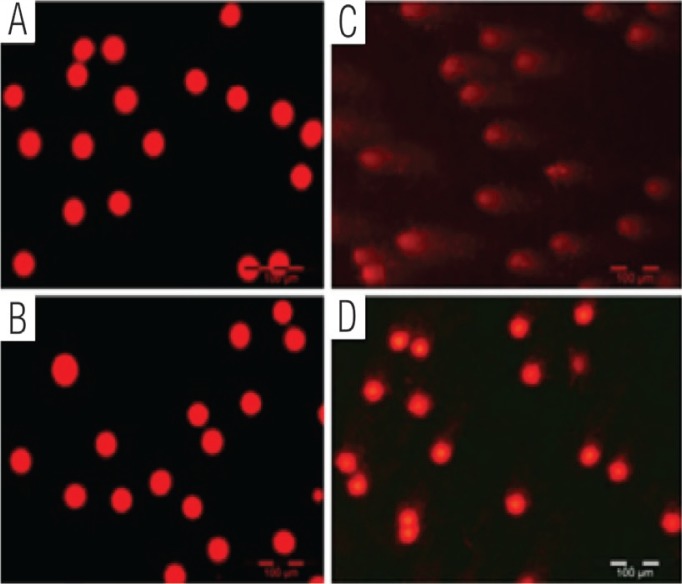
The levels of DNA damage were significantly higher in the STZ-induced diabetic rats compared to normal rats [Table 2]. TRF supplementation was found to minimise the DNA damage parameters in diabetic rats with TRF. Figure 3 shows the normal and damaged cells with long tails of DNA migration.
Table 2:
Effect of treatment with tocotrienol-rich fraction on the level of deoxyribonucleic acid (DNA) damage (determined by tail length, tail moment and percentage of tail DNA) of the leukocytes
| TL in μm | TM in arbitrary units | Tail DNA in % | |
|---|---|---|---|
| Normal group | 4.57 ± 0.23 | 0.015 ± 0.002 | 0.114 ± 0.016 |
| Normal with TRF group | 4.03 ± 0.15 | 0.0086 ± 0.001 | 0.084 ± 0.012 |
| Diabetic group | 127.38 ± 3.17* | 39.009 ± 1.45* | 24.93 ± 0.66* |
| Diabetic with TRF group | 45.28 ± 0.75*† | 3.93 ± 0.12† | 7.93 ± 0.16*† |
TL = tail length; TM = tail moment; DNA = deoxyribonucleic acid; TRF = tocotrienol rich fraction.
Results are expressed as mean ± standard error of mean;
Significantly different as compared to the control group (P <0.05);
Significantly different as compared to the diabetic group (P <0.05).
Figure 3:
Single-cell gel electrophoresis assay image of the deoxyribonucleic acid (DNA) using rat whole blood (10 μl) directly for the assay. Single-cell gel electrophoresis image of the DNA in the (A) normal group; (B) normal with tocotrienol-rich fraction (TRF) group; (C) diabetic group, and (D) diabetic with TRF group.
Discussion
This study shows that a daily supplementation of TRF (200 mg/Kg) reduced the levels of oxidative stress markers in STZ-induced diabetic rats. The results of the FBG tests indicated increased plasma glucose levels in STZ-induced diabetic rats, which could be due to the inhibition of insulin secretion resulting from the injection of STZ. Previous studies have demonstrated that injections of STZ in experimental diabetic rats led to the degeneration of beta cells of the islets of Langerhans, thereby increasing FBG levels.18 In this study, the supplementation of TRF at 200 mg/Kg for four weeks improved blood glucose levels. However, the improvement was not enough to restore blood glucose levels to a normal state.
The mechanism through which tocotrienols reduce blood glucose levels in patients and in pre-clinical animal models was investigated by Fang et al.19 They proposed that tocotrienols function as peroxisome proliferator-activated receptor (PPAR) modulators. PPARs are ligand-regulated transcription factors that play essential roles in energy metabolism. Synthetic PPARα and PPARγ ligands have been used recently in the treatment of hyperlipidaemia and diabetes. Both α- and γ-tocotrienol activated PPARα, while δ-tocotrienol alone activated PPARα, PPARγ and PPARδ in reporter-based assays.
Several studies have shown an increased lipid peroxidation in clinical and experimental diabetes.20 In the first part of this study, we confirmed that oxidative stress is enhanced in STZ-induced diabetic rats as demonstrated by the significant augmentation in diabetic erythrocyte MDA. MDA is a late-stage lipid oxidation by-product that can be formed non-enzymatically, or is a by-product of cyclooxygenase activity.21
Previous studies have reported elevated levels of lipid peroxidation products in the erythrocytes, plasma and retinas of diabetic patients and animals.22 The findings of the present study showed elevated levels of lipid peroxidation in erythrocyte STZ-induced diabetic rats, and this finding was in agreement with the reports documented in the literature.22–24 Increased levels of lipid peroxide may cause oxidative injury to the erythrocytes and cross-linking in the membrane proteins and lipids.25 Moreover, the levels of MDA were slightly increased in the normal group treated with TRF compared to the control group, although the difference was not statistically significant. This might be related to having exposed the rats to stressors during the force-feeding of the TRF supplementation.
In this study, four weeks of TRF supplementation showed significantly lower erythrocyte lipid peroxidation in the diabetic with TRF group compared to the STZ-induced diabetic group. Moreover, the normalisation of lipid peroxides in the STZ-induced diabetic rats treated with TRF was in agreement with Sung et al., who found that vitamin E inhibited the thiobarbituric acid reactive substances in the erythrocyte membrane.26 Also, the formation of lipid peroxidation products such as MDA was prevented by vitamin E treatment. Evidence from the present study suggests that TRF supplementation may help prevent and protect against free radical production in diabetes.
Increased oxidative damage and changes in the antioxidant defense system have been reported in experimental diabetes and have been associated with the development of diabetic complications.27 Previous studies have shown that the presence of oxidative stress in diabetes mellitus, and antioxidant enzymes such as SOD, catalase (CAT) and GPx as well as molecules such as GSH, vitamins E and C, and beta carotene, increase the response of the antioxidant defense system to oxidative stress in the body.28,29 In the present study, the levels of erythrocyte antioxidant enzymes, SOD and GPx, were decreased in both the STZ-induced diabetic rats and the diabetic with TRF group in comparison to the normal group. These findings agree with other research showing that the presence of oxidative stress in rat erythrocytes was caused by STZ-induced diabetes.28,30 In this study, supplementation with TRF was found to augment the antioxidative system and increase the levels of antioxidant enzymes, such as SOD, which catalytically converts superoxide anion radicals to hydrogen peroxide; this was especially remarkable in the diabetic with TRF group. While GPx activity was not significantly different in the diabetic with TRF group compared with the STZ-induced diabetic group, these results agree with some previous studies which showed that GPx appears to be a relatively stable enzyme;31,32 thus, it may only be inactivated under conditions of severe oxidative stress.33 In addition, other previous experimental animal studies have reported that high levels of glucose have no effect on GPx activity and its gene expression.34
Decreased erythrocyte GSH together with significantly increased GSSG was observed in STZ-induced diabetic rats when compared with the normal rats. These findings are consistent with other studies.32 The current study found that the supplementation of TRF significantly increased the levels of GSH and decreased GSSG in the diabetic group treated with TRF, and may indicate that high concentrations of TRF can significantly reduce the levels of lipid peroxidation and the reduction of oxidative agents, thereby increasing levels of antioxidant GSH.
The increased production of reactive oxygen species (ROS) under diabetic conditions underlines the increased amount of oxidatively-damaged DNA in different tissues. The presence of ROS may lead to increased DNA damage in peripheral blood lymphocytes that may be revealed by the single-cell gel electrophoresis assay. Significant differences were detected between the control and STZ-induced diabetic rats in terms of the frequency of damaged cells. The tail moment (TM) and tail length (TL) parameters for DNA damage were significantly higher in the STZ-induced diabetic rats than in the control rats, which might indicate that these cells are handling more oxidative damage on a regular basis. Also, clear differences between the STZ-induced diabetic rats and diabetic rats with four weeks of TRF supplementation were observed on the basis of the extent of DNA migration during single-cell gel electrophoresis, supporting the hypothesis that antioxidants protect cells from DNA damage. A previous study showed that antioxidants such as vitamin E exhibited a protective effect against oxidative DNA damage.35
Conclusion
This study showed that a daily oral supplementation of 200 mg/Kg of TRF of palm oil had the beneficial effect of reducing levels of oxidative stress markers by an inhibition of lipid peroxidation and an increase in the levels of antioxidant defense system. This was demonstrated through a significant increase in the SOD activity, and the GSH levels in diabetic rats supplemented with TRF. In contrast, GPx activity and vitamin E levels were not significantly affected following four weeks of TRF supplementation. Furthermore, the antioxidant properties of TRF showed some protective effects in the reduction of the percentage of damaged DNA in the tail. The DNA TL and TM of the leukocyte single-cell gel electrophoresis assay were used as indicators of oxidative DNA damage. Additionally, TRF was also found to have a beneficial effect in improving hyperglycaemic status, which resulted in a lowering of the plasma glucose levels after four weeks of supplementation. This study suggests that TRF supplementation plays an important role in reducing oxidative stress-induced diabetes mellitus.
Acknowledgments
The study was financed by the Government of Libya (Grant #NN-026-2010).
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Moussa SA. Oxidative stress in diabetes mellitus. Romanian J Biophys. 2008;18:225–36. [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 5.Fukuzawa K. Dynamics of lipid peroxidation and antioxidion of alpha-tocopherol in membranes. J Nutr Sci Vitaminol (Tokyo) 2008;54:273–85. doi: 10.3177/jnsv.54.273. [DOI] [PubMed] [Google Scholar]
- 6.Tan B, Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E Beyond Tocopherols. 2nd ed. Florida: CRC Press, Taylor & Francis Group; 2013. [Google Scholar]
- 7.Gapor AB, Ong ASH, Kato A, Watanabe H, Kawada T. Antioxidant activities of palm vitamin E with special reference to tocotrienols. J Oil Palm Res. 1989;1:63–7. [Google Scholar]
- 8.Frank J, Chin XW, Schrader C, Eckert GP, Rimbach G. Do tocotrienols have potential as neuroprotective dietary factors? Ageing Res Rev. 2012;11:163–80. doi: 10.1016/j.arr.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Wilankar C, Sharma D, Checker R, Khan NM, Patwardhan R, Patil A, et al. Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienols. Free Radic Biol Med. 2011;51:129–43. doi: 10.1016/j.freeradbiomed.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Matough FA, Budin SB, Hamid ZA, Louis SR, Alwahaibi N, Mohamed J. Palm vitamin E reduces oxidative stress, and physical and morphological alterations of erythrocyte membranes in streptozotocin-induced diabetic rats. Oxi Antioxid Med Sci. 2012;1:59–68. [Google Scholar]
- 11.Budin SB, Yusof KM, Idris MHM, Abd Hamid Z, Mohamed J. Tocotrienol-rich fraction of palm oil reduced pancreatic damage and oxidative stress in streptozotocin-induced diabetic rats. Australian J Basic Appl Sci. 2011;5:2367–74. [Google Scholar]
- 12.Pilz J, Meineke I, Gleiter CH. Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl. 2000;742:315–25. doi: 10.1016/s0378-4347(00)00174-2. [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Lang J, Zadravec S, Slater TF. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–28. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- 14.Beyer WF, Jr, Fridovich I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–66. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–8. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 16.Brandt RB, Kaugars GE, Riley WT, Bei RA, Silverman S, Jr, Lovas JG, et al. Evaluation of serum and tissue levels of alpha-tocopherol. Biochem Mol Med. 1996;57:64–6. doi: 10.1006/bmme.1996.0009. [DOI] [PubMed] [Google Scholar]
- 17.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 18.Ikebukuro K, Adachi Y, Yamada Y, Fujimoto S, Seino Y, Oyaizu H, et al. Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats. Transplantation. 2002;73:512–8. doi: 10.1097/00007890-200202270-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fang F, Kang Z, Wong C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol Nutr Food Res. 2010;54:345–52. doi: 10.1002/mnfr.200900119. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci (Lond) 1998;94:623–32. doi: 10.1042/cs0940623. [DOI] [PubMed] [Google Scholar]
- 21.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2002;43:550–7. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- 22.Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–43. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe PC, Liu WH, Yue KK, McMaster D, Catherwood MA, McGinty AM, et al. Glucose-induced oxidative stress in vascular contractile cells: Comparison of aortic smooth muscle cells and retinal pericytes. Diabetes. 1998;47:801–9. doi: 10.2337/diabetes.47.5.801. [DOI] [PubMed] [Google Scholar]
- 24.Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, Tritschler H, Rosen P, Halliwell B, et al. Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia. 1997;40:647–53. doi: 10.1007/s001250050729. [DOI] [PubMed] [Google Scholar]
- 25.Parthibhan A, Vijaylingam S, Shanmughsundaram K, Mohan R. Oxidative stress and the development of diabetic complications: Antioxidants and lipid peroxidation in erythrocytes and cell membrane. Cell Bio Int. 1995;19:987–93. doi: 10.1006/cbir.1995.1040. [DOI] [PubMed] [Google Scholar]
- 26.Ihm SH, Yoo HJ, Park SW, Ihm J. Effect of aminoguanidine on lipid peroxidation in streptozotocin-induced diabetic rats. Metabolism. 1999;48:1141–5. doi: 10.1016/s0026-0495(99)90128-2. [DOI] [PubMed] [Google Scholar]
- 27.Thompson KH, Godin DV. Micronutrient and antioxidants in the progression of diabetes. Nutr Res. 1995;15:1377–410. [Google Scholar]
- 28.Vural H, Sabuncu T, Arslan SO, Aksoy N. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J Pineal Res. 2001;31:193–8. doi: 10.1034/j.1600-079x.2001.310301.x. [DOI] [PubMed] [Google Scholar]
- 29.Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34:407–13. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 30.Aksoy N, Vural H, Sabuncu T, Aksoy S. Effects of melatonin on oxidative-antioxidative status of tissues in streptozotocin-induced diabetic rats. Cell Biochem Funct. 2003;21:121–5. doi: 10.1002/cbf.1006. [DOI] [PubMed] [Google Scholar]
- 31.Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26:163–76. [PubMed] [Google Scholar]
- 32.Firoozrai M, Nourbakhsh M, Razzaghy-Azar M. Erythrocyte susceptibility to oxidative stress and antioxidant status in patients with type 1 diabetes. Diabetes Res Clin Pract. 2007;77:427–32. doi: 10.1016/j.diabres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Condell RA, Tappel AL. Evidence for suitability of glutathione peroxidase as a protective enzyme: Studies of oxidative damage, renaturation, and proteolysis. Arch Biochem Biophys. 1983;223:407–16. doi: 10.1016/0003-9861(83)90604-5. [DOI] [PubMed] [Google Scholar]
- 34.Forsberg H, Borg LA, Cagliero E, Eriksson UJ. Altered levels of scavenging enzymes in embryos subjected to a diabetic environment. Free Radic Res. 1996;24:451–9. doi: 10.3109/10715769609088044. [DOI] [PubMed] [Google Scholar]
- 35.Claycombe KJ, Meydani SN. Vitamin E and genome stability. Mutat Res. 2001;475:37–44. doi: 10.1016/s0027-5107(01)00077-x. [DOI] [PubMed] [Google Scholar]