Abstract
Apolipoprotein (apo) A-IV is a 46,000 Mr protein that is a major component of rat high density lipoproteins and chylomicrons. We have isolated, from a rat intestinal cDNA library, a full-length apo A-IV clone of 1423 base pairs and determined its nucleotide sequence. The 1173-nucleotide coding region specifies a protein of 391 amino acids, which includes a 20-amino acid signal peptide. The portion of the cDNA sequence representing the mature plasma protein contains a 66-nucleotide subsequence that is repeated at least 13 times. Although each repeated unit shows some sequence variation, base changes in comparably positioned codons generally conserve the chemical type, if not the identity, of the corresponding amino acids. Nine of the thirteen 22-amino acid repeat units in the derived protein sequence begin with proline and most of these docosapeptides are predicted to have a high content of alpha-helix according to Chou-Fasman rules. When hydration potentials of individual residues are considered, many of the helices are shown to be amphipathic and may thus constitute lipid-binding domains with the ability to activate lecithin:cholesterol acyltransferase. The structure and organization of the repeat units of rat apo A-IV bear a striking similarity to a corresponding repeated sequence block in human apo A-I. This finding raises the possibility of close tandem linkage of the apo A-I and A-IV genes and suggests unequal crossing-over as a mechanism for the evolution of these genes.
Full text
PDF


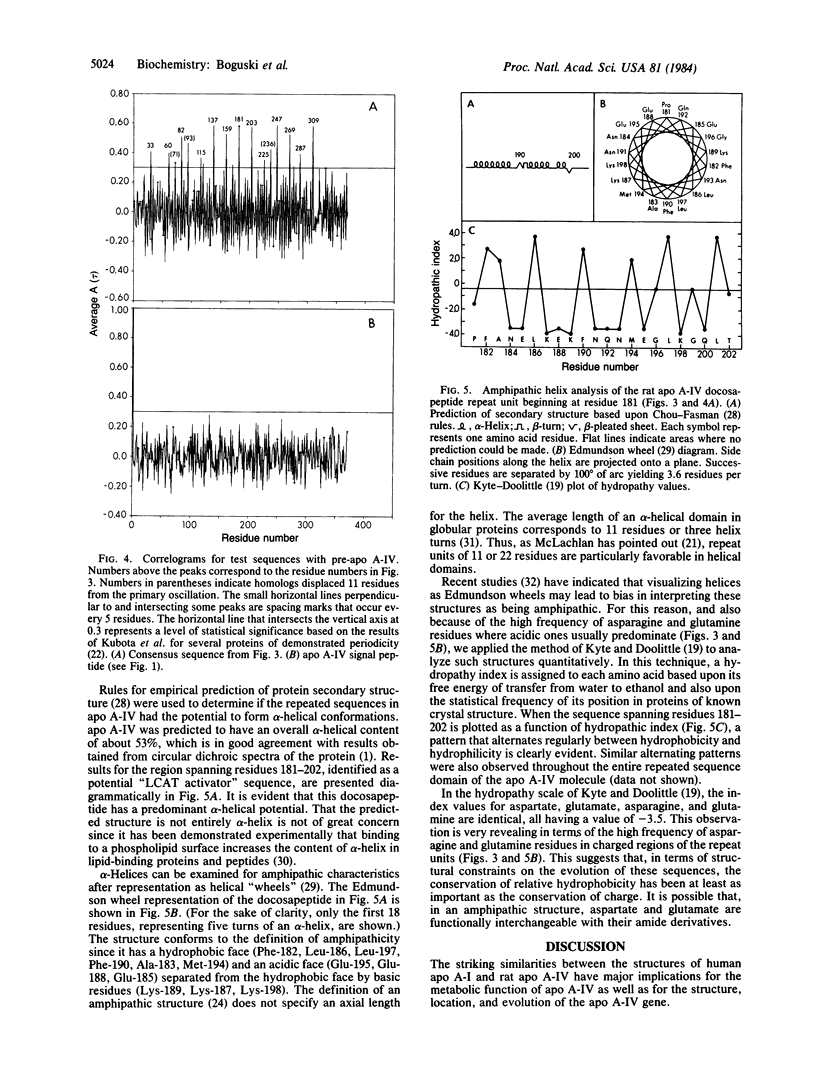

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. C., Dayhoff M. O. Evolution of lipoproteins deduced from protein sequence data. Comp Biochem Physiol B. 1977;57(4):309–315. doi: 10.1016/0305-0491(77)90060-8. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M. Kilo-sequencing: an ordered strategy for rapid DNA sequence data acquisition. Nucleic Acids Res. 1983 Jan 25;11(2):349–368. doi: 10.1093/nar/11.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisiegel U., Utermann G. An apolipoprotein homolog of rat apolipoprotein A-IV in human plasma. Isolation and partial characterisation. Eur J Biochem. 1979 Feb 1;93(3):601–608. doi: 10.1111/j.1432-1033.1979.tb12860.x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- DeLamatre J. G., Hoffmeier C. A., Lacko A. G., Roheim P. S. Distribution of apolipoprotein A-IV between the lipoprotein and the lipoprotein-free fractions of rat plasma: possible role of lecithin:cholesterol acyltransferase. J Lipid Res. 1983 Dec;24(12):1578–1585. [PubMed] [Google Scholar]
- Fidge N. H. The redistribution and metabolism of iodinated apolipoprotein A-IV in rats. Biochim Biophys Acta. 1980 Jul 14;619(1):129–141. doi: 10.1016/0005-2760(80)90249-0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Phylogenies constrained by the crossover process as illustrated by human hemoglobins and a thirteen-cycle, eleven-amino-acid repeat in human apolipoprotein A-I. Genetics. 1977 Jul;86(3):623–644. doi: 10.1093/genetics/86.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinta C., von Heijne G., Johansson J. Helical sidedness and the distribution of polar residues in trans-membrane helices. J Mol Biol. 1983 Jul 25;168(1):193–196. doi: 10.1016/s0022-2836(83)80330-1. [DOI] [PubMed] [Google Scholar]
- Fukushima D., Yokoyama S., Kroon D. J., Kézdy F. J., Kaiser E. T. Chain length-function correlation of amphiphilic peptides. Synthesis and surface properties of a tetratetracontapeptide segment of apolipoprotein A-I. J Biol Chem. 1980 Nov 25;255(22):10651–10657. [PubMed] [Google Scholar]
- Gordon J. I., Budelier K. A., Sims H. F., Edelstein C., Scanu A. M., Strauss A. W. Biosynthesis of human preproapolipoprotein A-II. J Biol Chem. 1983 Nov 25;258(22):14054–14059. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Alpers D. H., Strauss A. W. Cloning of a complementary deoxyribonucleic acid encoding a portion of rat intestinal preapolipoprotein AIV messenger ribonucleic acid. Biochemistry. 1982 Oct 26;21(22):5424–5431. doi: 10.1021/bi00265a007. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Alpers D. H., Strauss A. W. Proteolytic processing of the primary translation product of rat intestinal apolipoprotein A-IV mRNA. Comparison with preproapolipoprotein A-I processing. J Biol Chem. 1982 Jul 25;257(14):8418–8423. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Andy R., Alpers D. H., Schonfeld G., Strauss A. W. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem. 1982 Jan 25;257(2):971–978. [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Secondary structures of proteins and peptides in amphiphilic environments. (A review). Proc Natl Acad Sci U S A. 1983 Feb;80(4):1137–1143. doi: 10.1073/pnas.80.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis S. K., McPherson J., Zannis V. I., Breslow J. L. Linkage of human apolipoproteins A-I and C-III genes. 1983 Jul 28-Aug 3Nature. 304(5924):371–373. doi: 10.1038/304371a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Zannis V. I., Breslow J. L. Isolation and characterization of the human apolipoprotein A-I gene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6147–6151. doi: 10.1073/pnas.80.20.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Takahashi S., Nishikawa K., Ooi T. Homology in protein sequences expressed by correlation coefficients. J Theor Biol. 1981 Jul 21;91(2):347–361. doi: 10.1016/0022-5193(81)90237-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Repeated helical pattern in apolipoprotein-A-I. Nature. 1977 Jun 2;267(5610):465–466. doi: 10.1038/267465a0. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipid-protein interactions in the plasma lipoproteins. Biochim Biophys Acta. 1977 Aug 9;472(2):93–133. doi: 10.1016/0304-4157(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Garner C. W., Baker H. N., Sparrow J. T., Jackson R. L., Gotto A. M., Smith L. C. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry. 1975 Jul 15;14(14):3057–3064. doi: 10.1021/bi00685a003. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Krüger E., Deutzmann R. Cell-free translation of human liver apolipoprotein AI and AII mRNA. Processing of primary translation products. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):227–237. doi: 10.1515/bchm2.1983.364.1.227. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Braithwaite F., Eder H. A. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977 Jan 25;16(2):271–278. doi: 10.1021/bi00621a018. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Bersot T. P., Mahley R. W. Isolation and characterization of an apoprotein from the d less than 1.006 lipoproteins of human and canine lymph homologous with the rat A-IV apoprotein. Biochem Biophys Res Commun. 1978 Nov 14;85(1):287–292. doi: 10.1016/s0006-291x(78)80041-2. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]