Abstract
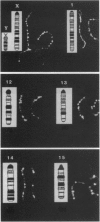
Eukaryotic homologs of Escherichia coli Rec-A protein have been shown to form nucleoprotein filaments with single-stranded DNA that recognize homologous sequences in duplex DNA. Several recent reports in four widely diverse species have demonstrated the association of RecA homologs with meiotic prophase chromatin. The current immunocytological study on mouse spermatocytes and oocytes shows that a eukaryotic homolog, Rad5l, associates with a subset of chromatin sites as early as premeiotic S phase, hours before either the appearance of precursors of synaptonemal complexes or the initiation of synapsis. When homologous chromosomes do begin to pair, the Rad5l-associated sequences are sites of initial contact between homologues and of localized DNA synthesis. Distribution of Rad5l foci on the chromatin of fully synapsed bivalents at early pachynema corresponds to an R-band pattern of mitotic chromosomes. R-bands are known to be preferred sites of both synaptic initiation and recombination. The time course of appearance of Rad51 association with chromatin, its distribution, and its interaction with other Rad5l-associated sequences suggests that it plays an important role preselection of sequences and synaptic initiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley T. G-band position effects on meiotic synapsis and crossing over. Genetics. 1988 Feb;118(2):307–317. doi: 10.1093/genetics/118.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley T., Plug A. W., Xu J., Solari A. J., Reddy G., Golub E. I., Ward D. C. Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma. 1995 Oct;104(1):19–28. doi: 10.1007/BF00352222. [DOI] [PubMed] [Google Scholar]
- Bishop D. K. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994 Dec 16;79(6):1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Boyle A. L., Feltquite D. M., Dracopoli N. C., Housman D. E., Ward D. C. Rapid physical mapping of cloned DNA on banded mouse chromosomes by fluorescence in situ hybridization. Genomics. 1992 Jan;12(1):106–115. doi: 10.1016/0888-7543(92)90412-l. [DOI] [PubMed] [Google Scholar]
- Cao L., Alani E., Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990 Jun 15;61(6):1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Carpenter A. T. Gene conversion, recombination nodules, and the initiation of meiotic synapsis. Bioessays. 1987 May;6(5):232–236. doi: 10.1002/bies.950060510. [DOI] [PubMed] [Google Scholar]
- Dobson M. J., Pearlman R. E., Karaiskakis A., Spyropoulos B., Moens P. B. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci. 1994 Oct;107(Pt 10):2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Itoh T., Tomizawa J. Antisense RNA. Annu Rev Biochem. 1991;60:631–652. doi: 10.1146/annurev.bi.60.070191.003215. [DOI] [PubMed] [Google Scholar]
- Haaf T., Golub E. I., Reddy G., Radding C. M., Ward D. C. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2298–2302. doi: 10.1073/pnas.92.6.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Chromosome bands, their chromatin flavors, and their functional features. Am J Hum Genet. 1992 Jul;51(1):17–37. [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Ito M., Stern H. Synthesis of DNA during meiosis. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1184–1191. doi: 10.1073/pnas.56.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Stern H. Analysis of DNA synthesis during meiotic prophase in Lilium. J Mol Biol. 1971 Feb 14;55(3):337–355. doi: 10.1016/0022-2836(71)90322-6. [DOI] [PubMed] [Google Scholar]
- John B. Myths and mechanisms of meiosis. Chromosoma. 1976 Mar 10;54(4):295–325. doi: 10.1007/BF00292812. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Padmore R., Bishop D. K. Meiotic chromosome metabolism: one view. Cold Spring Harb Symp Quant Biol. 1991;56:729–743. doi: 10.1101/sqb.1991.056.01.082. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Weiner B. M. Potential advantages of unstable interactions for pairing of chromosomes in meiotic, somatic, and premeiotic cells. Cold Spring Harb Symp Quant Biol. 1993;58:553–565. doi: 10.1101/sqb.1993.058.01.062. [DOI] [PubMed] [Google Scholar]
- Kofman-Alfaro S., Chandley A. C. Meiosis in the male mouse. An autoradiographic investigation. Chromosoma. 1970;31(4):404–420. doi: 10.1007/BF00285832. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Dixon D. A., Eggleston A. K., Lauder S. D., Rehrauer W. M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994 Sep;58(3):401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Luciani J. M., Guichaoua M. R., Cau P., Devictor B., Salagnon N. Differential elongation of autosomal pachytene bivalents related to their DNA content in human spermatocytes. Chromosoma. 1988;97(1):19–25. doi: 10.1007/BF00331791. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., John B. Heterochromatin and satellite DNA in man: properties and prospects. Am J Hum Genet. 1979 May;31(3):264–280. [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Heyting C., Dietrich A. J., van Raamsdonk W., Chen Q. Synaptonemal complex antigen location and conservation. J Cell Biol. 1987 Jul;105(1):93–103. doi: 10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polani P. E., Crolla J. A. A test of the production line hypothesis of mammalian oogenesis. Hum Genet. 1991 Nov;88(1):64–70. doi: 10.1007/BF00204931. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Rasmussen S. W., Holm P. B. Mechanics of meiosis. Hereditas. 1980;93(2):187–216. doi: 10.1111/j.1601-5223.1980.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Roeder G. S. Chromosome synapsis and genetic recombination: their roles in meiotic chromosome segregation. Trends Genet. 1990 Dec;6(12):385–389. doi: 10.1016/0168-9525(90)90297-j. [DOI] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Matsuda Y., Ushio N., Ikeo K., Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993 Jul;4(3):239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- Smithies O., Powers P. A. Gene conversions and their relation to homologous chromosome pairing. Philos Trans R Soc Lond B Biol Sci. 1986 Jan 29;312(1154):291–302. doi: 10.1098/rstb.1986.0008. [DOI] [PubMed] [Google Scholar]
- Solari A. J. Synaptosomal complexes and associated structures in microspread human spermatocytes. Chromosoma. 1980;81(3):315–337. doi: 10.1007/BF00368145. [DOI] [PubMed] [Google Scholar]
- Speed R. M. Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma. 1982;85(3):427–437. doi: 10.1007/BF00330366. [DOI] [PubMed] [Google Scholar]
- Stern H., Hotta Y. Biochemical controls of meiosis. Annu Rev Genet. 1973;7:37–66. doi: 10.1146/annurev.ge.07.120173.000345. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989 Mar 2;338(6210):87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Sung P., Robberson D. L. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell. 1995 Aug 11;82(3):453–461. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- Terasawa M., Shinohara A., Hotta Y., Ogawa H., Ogawa T. Localization of RecA-like recombination proteins on chromosomes of the lily at various meiotic stages. Genes Dev. 1995 Apr 15;9(8):925–934. doi: 10.1101/gad.9.8.925. [DOI] [PubMed] [Google Scholar]
- Vourc'h C., Taruscio D., Boyle A. L., Ward D. C. Cell cycle-dependent distribution of telomeres, centromeres, and chromosome-specific subsatellite domains in the interphase nucleus of mouse lymphocytes. Exp Cell Res. 1993 Mar;205(1):142–151. doi: 10.1006/excr.1993.1068. [DOI] [PubMed] [Google Scholar]
- Zickler D., Moreau P. J., Huynh A. D., Slezec A. M. Correlation between pairing initiation sites, recombination nodules and meiotic recombination in Sordaria macrospora. Genetics. 1992 Sep;132(1):135–148. doi: 10.1093/genetics/132.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]