Abstract
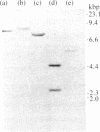
We characterized a clone carrying the guinea pig preproinsulin gene, which, in contrast to other mammalian preproinsulin genes, is highly divergent in its regions encoding the B and A chains of mature insulin. Blot hybridization analysis indicates that this gene is present in only one copy in the guinea pig genome and that other normal or mutated preproinsulin genes do not exist in this animal. Moreover, the position of introns in this gene and the homology of its 3' flanking region to the corresponding regions of other sequenced mammalian genes show that it has been derived from the common mammalian stock. The rapid evolution of the region encoding the B and A chains can be interpreted, according to our sequence-divergence analysis, as due to the fixation of both neutral and adaptive mutations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980 Mar 6;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R. Sequence of a cDNA encoding Syrian hamster preproinsulin. Diabetes. 1984 Mar;33(3):297–300. doi: 10.2337/diab.33.3.297. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Wood S. P. Is the evolution of insulin Darwinian or due to selectively neutral mutation? Nature. 1975 Sep 18;257(5523):197–203. doi: 10.1038/257197a0. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Emdin S. O., Kwok S. C., Kramer J. M., Falkmer S., Steiner D. F. Messenger RNA sequence and primary structure of preproinsulin in a primitive vertebrate, the Atlantic hagfish. J Biol Chem. 1981 Jul 25;256(14):7595–7602. [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Hahn V., Winkler J., Rapoport T. A., Liebscher D. H., Coutelle C., Rosenthal S. Carp preproinsulin cDNA sequence and evolution of insulin genes. Nucleic Acids Res. 1983 Jul 11;11(13):4541–4552. doi: 10.1093/nar/11.13.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda M., Polonsky K. S., Bergenstal R. M., Jaspan J. B., Shoelson S. E., Blix P. M., Chan S. J., Kwok S. C., Wishner W. B., Zeidler A. Familial hyperinsulinemia due to a structurally abnormal insulin. Definition of an emerging new clinical syndrome. N Engl J Med. 1984 May 17;310(20):1288–1294. doi: 10.1056/NEJM198405173102004. [DOI] [PubMed] [Google Scholar]
- Hobart P. M., Shen L. P., Crawford R., Pictet R. L., Rutter W. J. Comparison of the nucleic acid sequence of anglerfish and mammalian insulin mRNA's from cloned cDNA's. Science. 1980 Dec 19;210(4476):1360–1363. doi: 10.1126/science.7001633. [DOI] [PubMed] [Google Scholar]
- Horuk R., Blundell T. L., Lazarus N. R., Neville R. W., Stone D., Wollmer A. A monomeric insulin from the porcupine (Hystrix cristata), an Old World hystricomorph. Nature. 1980 Aug 21;286(5775):822–824. doi: 10.1038/286822a0. [DOI] [PubMed] [Google Scholar]
- Horuk R., Goodwin P., O'connor K., Neville R. W., Lazarus N. R., Stone D. Evolutionary change in the insulin receptors of hystricomorph rodents. Nature. 1979 May 31;279(5712):439–440. doi: 10.1038/279439a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Efstratiadis A., Forget B. G., Weissman S. M. Molecular evolution of human and rabbit beta-globin mRNAs. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5618–5622. doi: 10.1073/pnas.74.12.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Ohta T. On some principles governing molecular evolution. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2848–2852. doi: 10.1073/pnas.71.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Heldin C. H. Sharing of biological effect and receptors between guinea pig insulin and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1308–1312. doi: 10.1073/pnas.80.5.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Kahn C. R. Non-parallel evolution of metabolic and growth-promoting functions of insulin. Nature. 1981 Aug 13;292(5824):644–646. doi: 10.1038/292644a0. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Rubenstein A. H., Steiner D. F., Faber O. K., Binder C. Heterogeneity of circulating human C-peptide. Diabetes. 1978;27 (Suppl 1):184–191. doi: 10.2337/diab.27.1.s184. [DOI] [PubMed] [Google Scholar]
- Kwok S. C., Chan S. J., Steiner D. F. Cloning and nucleotide sequence analysis of the dog insulin gene. Coded amino acid sequence of canine preproinsulin predicts an additional C-peptide fragment. J Biol Chem. 1983 Feb 25;258(4):2357–2363. [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Mann G. V., Crofford O. B. Insulin levels in primates by immunoassay. Science. 1970 Sep 25;169(3952):1312–1313. doi: 10.1126/science.169.3952.1312. [DOI] [PubMed] [Google Scholar]
- Markussen J., Volund A. New method of calculating evolutionary rates of proteins applied to insulin and C-peptides. Int J Pept Protein Res. 1974;6(2):79–86. doi: 10.1111/j.1399-3011.1974.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Rosenzweig J. L., Le Roith D., Lesniak M. A., MacIntyre I., Sawyer W. H., Roth J. Two distinct insulins in the guinea pig: the broad relevance of these findings to evolution of peptide hormones. Fed Proc. 1983 Jun;42(9):2608–2614. [PubMed] [Google Scholar]
- Rosenzweig J. L., Lesniak M. A., Samuels B. E., Yip C. C., Zimmerman A. E., Roth J. Insulin in the extrapancreatic tissues of guinea pigs differs markedly from the insulin in their pancreas and plasma. Trans Assoc Am Physicians. 1980;93:263–278. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. F. Species variation in the amino acid sequence of insulin. Am J Med. 1966 May;40(5):662–666. doi: 10.1016/0002-9343(66)90145-8. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Markussen J., Sundby F. The amino acid sequence of guinea pig C-peptide. Nature. 1974 Mar 8;248(5444):151–152. doi: 10.1038/248151a0. [DOI] [PubMed] [Google Scholar]
- Sorokin A. V., Petrenko O. I., Kavsan V. M., Kozlov Y. I., Debabov V. G., Zlochevskij M. L. Nucleotide sequence analysis of the cloned salmon preproinsulin cDNA. Gene. 1982 Dec;20(3):367–376. doi: 10.1016/0378-1119(82)90205-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. On the role of the proinsulin C-peptide. Diabetes. 1978;27 (Suppl 1):145–148. doi: 10.2337/diab.27.1.s145. [DOI] [PubMed] [Google Scholar]
- Sundby F. Species variations in the primary structure of glucagon. Metabolism. 1976 Nov;25(11 Suppl 1):1319–1321. doi: 10.1016/s0026-0495(76)80132-1. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Emdin S. O., Clark J. L., Steiner D. F. Studies on the conversion of proinsulin to insulin. II. Evidence for a chymotrypsin-like cleavage in the connecting peptide region of insulin precursors in the rat. J Biol Chem. 1973 May 25;248(10):3476–3482. [PubMed] [Google Scholar]
- Ullrich A., Dull T. J., Gray A., Brosius J., Sures I. Genetic variation in the human insulin gene. Science. 1980 Aug 1;209(4456):612–615. doi: 10.1126/science.6248962. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Dull T. J., Gray A., Philips J. A., 3rd, Peter S. Variation in the sequence and modification state of the human insulin gene flanking regions. Nucleic Acids Res. 1982 Apr 10;10(7):2225–2240. doi: 10.1093/nar/10.7.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med. 1981 Jun 18;304(25):1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med. 1981 Jun 25;304(26):1575–1580. doi: 10.1056/NEJM198106253042604. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Groneberg J., Leineweber M., Wengenmayer F., Winnacker E. L. The nucleotide sequence of cDNA coding for preproinsulin from the primate Macaca fascicularis. Gene. 1982 Sep;19(2):179–183. doi: 10.1016/0378-1119(82)90004-x. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Blundell T. L., Wollmer A., Lazarus N. R., Neville R. W. The relation of conformation and association of insulin to receptor binding; x-ray and circular-dichroism studies on bovine and hystricomorph insulins. Eur J Biochem. 1975 Jul 15;55(3):531–542. doi: 10.1111/j.1432-1033.1975.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. E., Moule M. L., Yip C. C. Guinea pig insulin. II. Biological activity. J Biol Chem. 1974 Jul 10;249(13):4026–4029. [PubMed] [Google Scholar]