Abstract
The endothelium forms a selective semi-permeable barrier controlling bidirectional transfer between blood vessel and irrigated tissues. This crucial function relies on the dynamic architecture of endothelial cell–cell junctions, and in particular, VE-cadherin-mediated contacts. VE-cadherin indeed chiefly organizes the opening and closing of the endothelial barrier, and is central in permeability changes. In this review, the way VE-cadherin-based contacts are formed and maintained is first presented, including molecular traits of its expression, partners, and signaling. In a second part, the mechanisms by which VE-cadherin adhesion can be disrupted, leading to cell–cell junction weakening and endothelial permeability increase, are described. Overall, the molecular basis for VE-cadherin control of the endothelial barrier function is of high interest for biomedical research, as vascular leakage is observed in many pathological conditions and human diseases.
Keywords: VE-cadherin, permeability, VEGF, catenins, internalization, phosphorylation, vascular barrier, endothelial cells
Cell–cell interactions are dynamic structures allowing cohesion and plasticity of organs. In vascular endothelial cells, endothelial junctions have to maintain homeostasis of blood vessels, while they retain their ability to rearrange during angiogenesis. Both adherens (AJs) and tight junctions (TJs) join neighboring cells together, and can adapt quickly to changes in the perivascular microenvironment, such as angiogenic/antiangiogenic cues, blood flow, shear stress, inflammatory conditions, etc. The AJ protein, VE-cadherin (vascular endothelial cadherin, also known as cadherin 5 and CD144) is specifically responsible for endothelial AJ assembly and barrier architecture. In this review, VE-cadherin properties will be presented, along with the mechanisms involved in endothelial cell–cell junction remodeling.
Building VE-Cadherin Junctions and the Endothelial Barrier
The vascular wall compartmentalizes blood circulation from surrounding tissues, while allowing finely tuned exchanges of metabolites, fluids, and cells. Blood vessels are constructed with endothelial cells, pericytes, and smooth muscle cells, embedded within a specific basal membrane. From a molecular standpoint, the endothelial barrier is sealed by cell–cell adhesion molecules, among which VE-cadherin serves as a cornerstone.
VE-cadherin expression
VE-cadherin belongs to the super-family of classical cadherins, and as such, mediates homotypic calcium-dependent cell–cell interactions.1 VE-cadherin expression is tissue-specific and exclusive to endothelial cells, in a way that its promoter is repressed in other cell types and can be used to target the endothelial compartment in transgenic mice.2,3 Importantly, VE-cadherin gene knockout is lethal in mouse embryos that exhibit severe angiogenic defects, attributed to endothelial apoptosis and abnormal VEGF (vascular endothelial growth factor) signaling.4,5 Moreover, interfering with VE-cadherin in embryos and adult mice affects vascular integrity.6,7 Besides, silencing VE-cadherin expression and blocking its adhesive function in vitro provided evidence that this adhesion molecule is essential for AJ formation and endothelial barrier maintenance.8-11 Accordingly, VE-cadherin emerges as the mastermind of endothelial cell–cell junctions, as it dictates the level of expression and/or the localization of other junctional molecules, including claudin-5 and N-cadherin.9,12,13
VE-cadherin and its partners
Similarly to classical cadherins such as E- or N-cadherins, VE-cadherin recruits catenins through its cytosolic tail. These accessory molecules, mainly β-and p120-catenins, bridge cadherin multimers to the actin cytoskeleton via actin binding proteins, among which are α-catenin, vinculin, and eplin.14-18 Interestingly, it has been recently observed that β-catenin dephosphorylation, together with VE-cadherin mobility, contribute to endothelial cell–cell junction stabilization.19 However, the role of β-catenin in the endothelial barrier remains complex, as this multifaceted protein is also an essential mediator of the Wnt signaling cascade, operating as a transcription factor in the nucleus. Thus, β-catenin may exert broader effects on gene expression and vascular plasticity, including barrier function.20-22 Additionally, γ-catenin (also known as plakoglobin) can directly interact with VE-cadherin. Although β- and γ-catenins appear somehow interchangeable, only γ-catenin conveys VE-PTP adhesive function.23
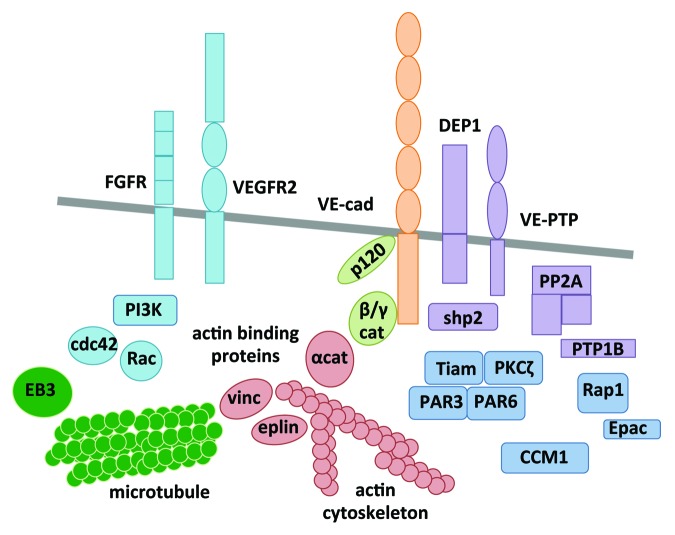
On the other hand, p120-catenin, which interacts with VE-cadherin on its juxtamembrane domain, is a key regulator of VE-cadherin expression, trafficking, and stability at the plasma membrane.24-27 Nonetheless, VE-cadherin rescue is not sufficient to maintain the endothelial barrier integrity in p120-depleted cells.27 This suggests a more complex role of p120-catenin in regulating cell–cell junction assembly in endothelial cells. In addition to catenins, a myriad of molecules are found in the vicinity of VE-cadherin, and could impact on the assembly and/or stability of VE-cadherin-mediated cellular interactions (Fig. 1). For instance, polarity complex proteins, including PAR3 and PAR6, accumulate at cell–cell junctions and interact with VE-cadherin.28 They were shown to modulate the overall architecture of endothelial cell–cell contacts, including AJs and TJs. Although their mode of action in endothelial AJs is not fully elucidated and probably differs from what is known in the context of epithelial cells, the polarity complex might engage additional signaling components required for the assembly of VE-cadherin junctions. This includes CCM1 (cerebral cavernous malformation protein 1), the GTPase Rap1, the atypical PKCζ (protein kinase C), and the G-protein exchange factor (GEF) Tiam (T-cell lymphoma invasion and metastasis).29 It is noteworthy that Rap1, together with its GEF, namely Epac, have extensively been demonstrated to favor the formation of VE-cadherin-based junctions. 17,30-34
Figure 1. Assembly of VE-cadherin-mediated cell–cell contacts. Different classes of molecules interact with VE-cadherin (orange) and modulate its adhesive function: catenins (light green), actin binding proteins (red), phosphatases (purple), polarity complex (dark blue), signaling molecules and growth factor receptors (light blue), and microtubule (dark green). cat, catenin; cdc42, cell division control protein 42 homolog; CCM1, cerebral cavernous malformation protein 1; DEP1, density enhanced protein tyrosine phosphatase 1; EB3, end binding protein 3; FGFR, fibroblast growth factor receptor; PP2A, protein phosphatase 2A; PAR3/6, partition defect protein 3/6; PI3K, phosphoinositide 3 kinase; PKC, protein kinase C; PTP1B, protein tyrosine phosphatase 1B; Shp2, Src homology protein tyrosine phosphatase; Tiam, T-cell lymphoma invasion and metastasis; VE-cad, VE-cadherin; VEGFR2, vascular endothelial growth factor receptor 2; VE-PTP, vascular endothelial protein tyrosine phosphatase; vinc, vinculin.
Finally, serine/threonine and tyrosine phosphatases are part of the VE-cadherin interactome. Indeed, VE-PTP (vascular endothelial protein tyrosine phosphatase), shp2 (src homology protein tyrosine phosphatase2), PP2A (protein phosphatase 2A), DEP-1/CD148 (density enhanced protein tyrosine phosphatase 1), and PTP1B (protein tyrosine phosphatase 1B) were all found to directly stabilize VE-cadherin-based adhesion.19,23,35-39 These enzymes are suspected to maintain VE-cadherin and associated catenins in a non-phosphorylated status, which in turn strengthens cell–cell adhesion.
VE-cadherin signaling
In addition to being associated with molecules that control its adhesive behavior, VE-cadherin coalesces signaling platforms at cell–cell contacts, involved in the endothelial biology, and in particular, barrier integrity.40 First, it has been early described that VE-cadherin function combines with VEGF-R2 (vegf receptor 2, also known as Flk1). They biochemically and functionally interact in quiescent endothelial cells, where VE-cadherin regulates VEGF-R2 antiapoptotic action.4,37,41 Interestingly, signaling pathways, which affect the endothelial plasticity in the course of angiogenesis, such as cell sprouting, migration, and permeability, frequently converge on the modulation of VE-cadherin/VEGF-R2 interaction.42-47 In addition, VE-cadherin contributes to convey the proper activity of other receptors in endothelial cells, such as angiopoietin 1 receptor Tie2, TGFβ-R2 (transforming growth factor receptor), and FGF (fibroblast growth factor) signaling.46,48-52 Besides membrane receptors, VE-cadherin triggers the activation of intracellular molecules, such as small GTPases from the Ras superfamily, which can loop on cell–cell adhesion. Notably, VE-cadherin induces Rac activation through Tiam.53-55 In addition, VE-cadherin is able to increase PI3K (phosphoinositide 3 kinase) catalytic activity, which can signal through the nucleus via FoxO1 and β-catenin transcription factors, to maintain endothelial features.4,9 Finally, it has been elegantly demonstrated that VE-cadherin could stabilize cell–cell contacts and organize the endothelial barrier through an original outside-in signaling mechanism, involving calcium signaling and microtubule dynamics.56
There are a growing number of molecules detected at endothelial AJs that can adjust VE-cadherin expression, cell surface availability, phosphorylation status, turnover, and signaling. Targeting each of these properties could be employed to tune endothelial permeability and vascular leakage.
Breaking Down VE-Cadherin Junctions and the Endothelial Barrier
VE-cadherin is instrumental for vascular integrity, and therefore, is the target of a plethora of signaling events, which can provoke endothelial barrier disruption and vascular permeability increase (Fig. 2).
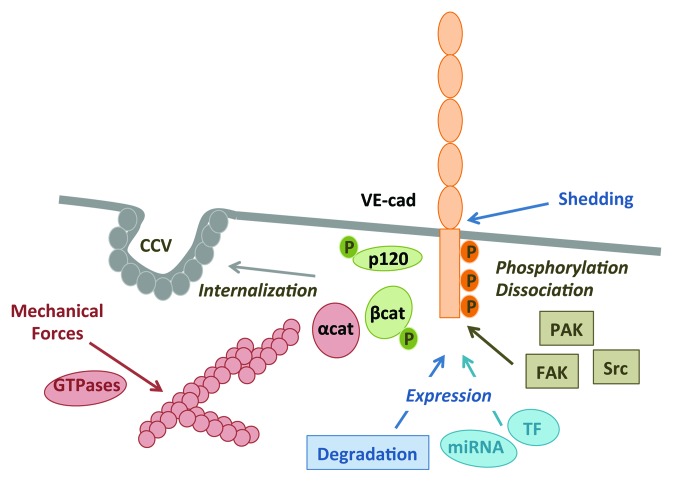
Figure 2. Disassembly of VE-cadherin-mediated cell–cell contacts. Four main routes modulate VE-cadherin disassembly and vascular permeability: (1) phosphorylation within VE-cadherin (VE-cad) C-tail and associated catenins on Y and S. At least, PAK (p21-activated kinase), Src, and FAK (focal adhesion kinase) were shown to catalyze VE-cadherin phosphorylation. This post-translational modification can ultimately lead to VE-cadherin/catenin dissociation; (2) internalization into clathrin-coated vesicles (CCV), which depends on p120-catenin (cat) and VE-cad phosphorylation; (3) mechanical forces and tension exerted on cell adhesion, notably through the actin cytoskeleton and Rho GTPases; and (4) expression regulated either at the transcriptional level through transcription factors (TF) and miRNA (miR), or at the protein level through extracellular shedding or intracellular cleavage, ubiquitin-, and lysosomal-driven degradation. P is for phosphorylation.
VE-cadherin phosphorylation
The cytosolic tail of VE-cadherin harbors nine putative phospho-tyrosine sites, among which Y645, Y658, Y685, Y731, and Y733 that could be implicated in barrier integrity.57-60 In addition, VE-cadherin serine phosphorylation on the S665 residue has been shown to modulate AJ assembly.8,38 Indeed, many vascular-promoting agents, such as VEGF and histamine, were extensively documented to increase the phosphorylation of VE-cadherin and of associated catenins; resulting in the loosening of VE-cadherin/catenin binding.1 Conversely, the artificial stabilization of VE-cadherin/catenin complexes hampers vascular permeability and leukocyte extravasation.61 Several kinases were proposed to catalyze VE-cadherin phosphorylation, while the exact molecular mechanisms linking phosphorylation to vascular leakage remains to be elucidated. To this regard, the non-receptor tyrosine kinase Src was demonstrated to contribute to the phosphorylation of VE-cadherin and catenins, and to AJ disassembly.8,38,47,62-64 However, Src-directed VE-cadherin phosphorylation appears insufficient to drive endothelial barrier opening, even when its activity was upregulated through the expression of a Src dominant active mutant or a Csk (C-terminal Src kinase) dominant negative form.65 Besides Src, FAK (focal adhesion kinase) emerges as a prominent mediator of VE-cadherin-mediated AJ disorganization and permeability elevation.66,67 PAK (p21-activated kinase) could also phosphorylate VE-cadherin on serine, causing its internalization and cell–cell junction weakening.8 An alternate mechanism for VE-cadherin/catenin dissociation and enhanced permeability relies on caveolin phosphorylation, which could, in its phosphorylated form, trap catenins away from VE-cadherin.68
VE-cadherin and mechanical forces
It is now widely accepted that mechanical tension exerted on endothelial cells, such as shear stress, can be sensed and integrated through VE-cadherin and VEGF-R2 complexes, although an additional adhesion molecule, namely PECAM (also referred to as CD31), is essential to transduce tensile forces.69-71 Multiple signaling pathways have been further demonstrated to convey and adapt the endothelial cellular responses to environmental forces.70 The most studied one corresponds to the modulation of the acto-myosin contractility apparatus via the activation of small GTPases, including RhoA, Rac, and cdc42, and myosin light chain (MLC) phosphorylation.72 However, the individual contribution of RhoA, Rac, and cdc42 to the endothelial barrier function might depend on cellular context and extracellular cues.72 For instance, thrombin primarily operates through RhoA that induces stress fiber formation and endothelial barrier permeability increase, while histamine mainly uses cdc42.73-75 Likewise, Rac mediates VEGF-induced permeability, while it is essential for cell–cell contact formation upon VE-cadherin transactivation.8,75 Conversely, VE-cadherin can also impact cellular tension through direct signaling to GTPases, as mentioned earlier.53-55,76
VE-cadherin internalization
Availability of VE-cadherin at the plasma membrane is instrumental in regulating cell–cell adhesion and endothelial barrier function.42,58,77 Indeed, it is recognized that VE-cadherin is a highly dynamic adhesion molecule, whose endosomal trafficking through clathrin-coated vesicles is tightly controlled.8,11,24 As cadherin internalization is associated with cell–cell junction loosening, this mechanism could contribute to endothelial behavior, such as cell migration and permeability.8,26,78-81 How VE-cadherin trafficking is dynamically modulated in endothelial cells has been the focus of intense investigation. In particular, it has been shown that p120-catenin could inhibit constitutive and induced VE-cadherin endocytosis.24,82 A cluster of three amino acids has been further identified within the core p120-binding region to the VE-cadherin cytosolic tail. This sequence is responsible for VE-cadherin uptake.26 In addition, VE-cadherin phosphorylation on serine and tyrosine residues has been shown to provoke its internalization both in vitro and in vivo, leading to increase in vascular permeability.8,35,38,58,62,81,83-85 Again, several signaling pathways have been shown to impact the entry of VE-cadherin in clathrin-coated pits. For instance, PI3K signaling is involved in VE-cadherin internalization and loss of vascular barrier, although the exact molecular mechanisms are not fully elucidated.81,84-86 Depending on the signal input, such as VEGF, chemokines, and inflammation, different PI3K isoforms can be engaged in VE-cadherin trafficking. For instance, class I PI3Kα transduces TNFα (tumor necrosis factor α) signaling to VE-cadherin destabilization, while PI3Kγ conveys chemokine’s one.84-86 On the other hand, class II PI3K-C2α deletion specifically impairs vascular sprouting and maturation in mice, most likely in response to VEGF.81 Additionally, Src kinase also triggers VE-cadherin internalization, directly through its phosphorylation or of its associated molecules, or through the activation of other kinases, such as PAK.8,35,58,80 Conversely, anti-permeability agents could operate through the blockade of VE-cadherin internalization.42,62,87
VE-cadherin availability
During physiological and pathological blood vessel formation and in response to vascular permeability-promoting agents, the endothelial barrier function could be directly altered through the level of expression of VE-cadherin. This could be achieved by playing on either transcription of VE-cadherin mRNA or on the protein turnover. As mentioned above, VE-cadherin promoter is repressed in non-endothelial cells, and can be activated in response to angiogenic signals, such as FGF.2 Interestingly, several transcription factors have been identified for their positive action on VE-cadherin expression, notably during developmental angiogenesis and inflammation. For instance, the Kruppel-like factor 4 (KLF4) binds VE-cadherin promoter and enhances its transcription, most likely downstream Wnt3a/β-catenin signaling.88 Interestingly, KLF4 depletion leads to VE-cadherin reduction, loss of AJ and of barrier integrity. This might ultimately aggravated lung vascular leakage in bacteria lipopolysaccharide (LPS) challenge. Likewise, Wnt3a/β-catenin signaling controls claudin-3 expression and barrier maturation, albeit β-catenin cooperates with FoxO1 to repress claudin-5 expression.9,21 Thus, the respective action of Wnt signaling and β-catenin in VE-cadherin transcription remains far from being understood. Keeping with this, the catenin p120 could also play an essential role on VE-cadherin transcription, as its depletion in mice leads to VE-cadherin downregulation, microvasculature disorganization, and loss of vascular integrity.89 Additional nuclear factors were discovered to control the endothelial barrier through their binding to the VE-cadherin promoter, including the ETS-related gene Erg, the hypoxia-inducing factor HIF2α, the basic helix-loop-helix TAL-1/SCL, the scaffold protein β-arrestin, the Sex-determining region Y (SRY)-related HMG box transcription factor Sox7, and the serum response element SRF.11, 90-95
Additionally, several miRNA, which have emerged as endogenous non-coding RNA molecules able to downregulate gene expression, have been found implicated in the regulation of VE-cadherin expression. For instance, miR27a can control VE-cadherin expression, angiogenesis, and vascular permeability. Interestingly, blocking specifically miR27a-dependent VE-cadherin repression precludes edema formation in vivo.96 Likewise, miR101, miR125b, and miR142a can transcriptionally hamper on VE-cadherin expression, and impact on angiogenesis and permeability in vivo.97-99 Conversely, the combination of miR99b and miR181a/b enhances endothelial differentiation, and notably, the expression of VE-cadherin in human embryonic stem cells.100 Finally, VE-cadherin protein expression can be regulated at the post-translational level through ubiquitination-driven processing, lysosomal degradation, and proteolytic cleavage by a desintegrin and metalloprotease ADAM10 and m-calpain.58,101-104 This latter can directly cleave VE-cadherin, leading to AJ disassembly and increased vascular leakage, associated with atherosclerosis lesions.104 However, the molecular mechanisms that specifically control VE-cadherin turnover remain elusive. For instance, neither the ubiquitin ligases, nor the lysine residues involved in VE-cadherin ubiquitination have been identified so far.
Our knowledge on the molecular mechanisms involved in vascular permeability elevation has been improved. To this regard, any deregulation of this central function takes part in the initiation and progression of many pathological conditions and human diseases.77,105 It is now accepted that VE-cadherin-mediated cell–cell adhesion organizes the endothelial junctions and maintains the barrier function. Now that many partners of VE-cadherin involved in assembly/disassembly of endothelial junctions have been identified, efforts have to be done to uncover the mechanistic differences that may exist between physiological and pathological permeability increase. In addition, restoring endothelial cell–cell junctions could normalize vascular barrier function and therefore be of high interest in medicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research in JG laboratory is funded by: Canceropole Ile-de-France, Centre National pour la Recherche Scientifique, Fondation ARC, Fondation pour la Recherche Medicale, Institut National du Cancer, Ligue nationale contre le cancer comite de Paris, and a Marie Curie International Reintegration Grant within The Seventh Framework Program.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/27330
References
- 1.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583:1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Prandini MH, Dreher I, Bouillot S, Benkerri S, Moll T, Huber P. The human VE-cadherin promoter is subjected to organ-specific regulation and is activated in tumour angiogenesis. Oncogene. 2005;24:2992–3001. doi: 10.1038/sj.onc.1208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–92. [PubMed] [Google Scholar]
- 4.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–57. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 5.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–8. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosby CV, Fleming PA, Argraves WS, Corada M, Zanetta L, Dejana E, Drake CJ. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood. 2005;105:2771–6. doi: 10.1182/blood-2004-06-2244. [DOI] [PubMed] [Google Scholar]
- 7.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 9.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–34. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 10.Heupel WM, Efthymiadis A, Schlegel N, Müller T, Baumer Y, Baumgartner W, Drenckhahn D, Waschke J. Endothelial barrier stabilization by a cyclic tandem peptide targeting VE-cadherin transinteraction in vitro and in vivo. J Cell Sci. 2009;122:1616–25. doi: 10.1242/jcs.040212. [DOI] [PubMed] [Google Scholar]
- 11.Hebda JK, Leclair HM, Azzi S, Roussel C, Scott MG, Bidère N, Gavard J. The C-terminus region of β-arrestin1 modulates VE-cadherin expression and endothelial cell permeability. Cell Commun Signal. 2013;11:37. doi: 10.1186/1478-811X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavard J, Gutkind JS. VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol. 2008;10:883–5. doi: 10.1038/ncb0808-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giampietro C, Taddei A, Corada M, Sarra-Ferraris GM, Alcalay M, Cavallaro U, Orsenigo F, Lampugnani MG, Dejana E. Overlapping and divergent signaling pathways of N-cadherin and VE-cadherin in endothelial cells. Blood. 2012;119:2159–70. doi: 10.1182/blood-2011-09-381012. [DOI] [PubMed] [Google Scholar]
- 14.Broman MT, Kouklis P, Gao X, Ramchandran R, Neamu RF, Minshall RD, Malik AB. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res. 2006;98:73–80. doi: 10.1161/01.RES.0000198387.44395.e9. [DOI] [PubMed] [Google Scholar]
- 15.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–52. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, Prandini MH, Huber P, Gulino-Debrac D. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem. 2012;287:7556–72. doi: 10.1074/jbc.M111.328682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through alpha- and beta-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Mol Biol Cell. 2010;21:584–96. doi: 10.1091/mbc.E09-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, van Buul JD. The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol Biol Cell. 2012;23:4212–25. doi: 10.1091/mbc.E12-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–49. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–17. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paolinelli R, Corada M, Ferrarini L, Devraj K, Artus C, Czupalla CJ, Rudini N, Maddaluno L, Papa E, Engelhardt B, et al. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS One. 2013;8:e70233. doi: 10.1371/journal.pone.0070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–45. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–51. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199:365–80. doi: 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herron CR, Lowery AM, Hollister PR, Reynolds AB, Vincent PA. p120 regulates endothelial permeability independently of its NH2 terminus and Rho binding. Am J Physiol Heart Circ Physiol. 2011;300:H36–48. doi: 10.1152/ajpheart.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, Ohno S, Behrens J, Vestweber D, Ebnet K. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 2006;7:1239–46. doi: 10.1038/sj.embor.7400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–80. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 30.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 32.Pannekoek WJ, van Dijk JJ, Chan OY, Huveneers S, Linnemann JR, Spanjaard E, Brouwer PM, van der Meer AJ, Zwartkruis FJ, Rehmann H, et al. Epac1 and PDZ-GEF cooperate in Rap1 mediated endothelial junction control. Cell Signal. 2011;23:2056–64. doi: 10.1016/j.cellsig.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Wimmer R, Cseh B, Maier B, Scherrer K, Baccarini M. Angiogenic sprouting requires the fine tuning of endothelial cell cohesion by the Raf-1/Rok-α complex. Dev Cell. 2012;22:158–71. doi: 10.1016/j.devcel.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–76. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Guelte A, Galan-Moya EM, Dwyer J, Treps L, Kettler G, Hebda JK, Dubois S, Auffray C, Chneiweiss H, Bidere N, et al. Semaphorin 3A elevates endothelial cell permeability through PP2A inactivation. J Cell Sci. 2012;125:4137–46. doi: 10.1242/jcs.108282. [DOI] [PubMed] [Google Scholar]
- 36.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–95. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spring K, Chabot C, Langlois S, Lapointe L, Trinh NT, Caron C, Hebda JK, Gavard J, Elchebly M, Royal I. Tyrosine phosphorylation of DEP-1/CD148 as a mechanism controlling Src kinase activation, endothelial cell permeability, invasion, and capillary formation. Blood. 2012;120:2745–56. doi: 10.1182/blood-2011-12-398040. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, et al. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–91. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010;22:651–8. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaengel K, Niaudet C, Hagikura K, Laviña B, Muhl L, Hofmann JJ, Ebarasi L, Nyström S, Rymo S, Chen LL, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–99. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–74. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 44.Cook BD, Ferrari G, Pintucci G, Mignatti P. TGF-beta1 induces rearrangement of FLK-1-VE-cadherin-beta-catenin complex at the adherens junction through VEGF-mediated signaling. J Cell Biochem. 2008;105:1367–73. doi: 10.1002/jcb.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesh D, Fredette N, Rostama B, Tang Y, Vary CP, Liaw L, Urs S. RhoA-mediated signaling in Notch-induced senescence-like growth arrest and endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2011;31:876–82. doi: 10.1161/ATVBAHA.110.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat Commun. 2013;4:1672. doi: 10.1038/ncomms2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113:885–94. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 49.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–26. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 50.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garrè M, Liebner S, Letarte M, ten Dijke P, et al. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, Deutsch U, Nottebaum AF, Vestweber D. VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol. 2009;185:657–71. doi: 10.1083/jcb.200811159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–89. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, Birukov KG. VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost. J Cell Physiol. 2012;227:3405–16. doi: 10.1002/jcp.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komarova YA, Huang F, Geyer M, Daneshjou N, Garcia A, Idalino L, Kreutz B, Mehta D, Malik AB. VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol Cell. 2012;48:914–25. doi: 10.1016/j.molcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 58.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–77. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 60.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–70. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/S1097-2765(00)80221-X. [DOI] [PubMed] [Google Scholar]
- 65.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem. 2010;285:7045–55. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189:955–65. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–57. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kronstein R, Seebach J, Grossklaus S, Minten C, Engelhardt B, Drab M, Liebner S, Arsenijevic Y, Taha AA, Afanasieva T, et al. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res. 2012;93:130–40. doi: 10.1093/cvr/cvr256. [DOI] [PubMed] [Google Scholar]
- 69.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 70.Conway DE, Schwartz MA. Flow-dependent cellular mechanotransduction in atherosclerosis. J Cell Sci. 2013;126:5101–9. doi: 10.1242/jcs.138313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–30. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/S1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 73.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–40. doi: 10.1161/01.RES.87.4.335. [DOI] [PubMed] [Google Scholar]
- 74.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 75.Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 76.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–53. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Guelte A, Dwyer J, Gavard J. Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol Cell. 2011;103:593–605. doi: 10.1042/BC20110069. [DOI] [PubMed] [Google Scholar]
- 78.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res. 2012;111:739–49. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Troyanovsky RB, Sokolov EP, Troyanovsky SM. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol Biol Cell. 2006;17:3484–93. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li R, Ren M, Chen N, Luo M, Zhang Z, Wu J. Vitronectin increases vascular permeability by promoting VE-cadherin internalization at cell junctions. PLoS One. 2012;7:e37195. doi: 10.1371/journal.pone.0037195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med. 2012;18:1560–9. doi: 10.1038/nm.2928. [DOI] [PubMed] [Google Scholar]
- 82.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–80. doi: 10.1091/mbc.E08-07-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dwyer J, Hebda JK, Le Guelte A, Galan-Moya EM, Smith SS, Azzi S, Bidere N, Gavard J. Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS One. 2012;7:e45562. doi: 10.1371/journal.pone.0045562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dwyer J, Le Guelte A, Galan Moya EM, Sumbal M, Carlotti A, Douguet L, Gutkind JS, Grange PA, Dupin N, Gavard J. Remodeling of VE-cadherin junctions by the human herpes virus 8 G-protein coupled receptor. Oncogene. 2011;30:190–200. doi: 10.1038/onc.2010.411. [DOI] [PubMed] [Google Scholar]
- 85.Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29:2469–80. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188:863–76. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–53. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107:959–66. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. p120-Catenin is required for mouse vascular development. Circ Res. 2010;106:941–51. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan L, Le Bras A, Sacharidou A, Itagaki K, Zhan Y, Kondo M, Carman CV, Davis GE, Aird WC, Oettgen P. ETS-related gene (ERG) controls endothelial cell permeability via transcriptional regulation of the claudin 5 (CLDN5) gene. J Biol Chem. 2012;287:6582–91. doi: 10.1074/jbc.M111.300236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Le Bras A, Lionneton F, Mattot V, Lelièvre E, Caetano B, Spruyt N, Soncin F. HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–9. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 92.Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deleuze V, Chalhoub E, El-Hajj R, Dohet C, Le Clech M, Couraud PO, Huber P, Mathieu D. TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells. Mol Cell Biol. 2007;27:2687–97. doi: 10.1128/MCB.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E, Li Z. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 2008;15:448–61. doi: 10.1016/j.devcel.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 95.Costa G, Mazan A, Gandillet A, Pearson S, Lacaud G, Kouskoff V. SOX7 regulates the expression of VE-cadherin in the haemogenic endothelium at the onset of haematopoietic development. Development. 2012;139:1587–98. doi: 10.1242/dev.071282. [DOI] [PubMed] [Google Scholar]
- 96.Young JA, Ting KK, Li J, Moller T, Dunn L, Lu Y, Moses J, Prado-Lourenço L, Khachigian LM, Ng M, et al. Regulation of vascular leak and recovery from ischemic injury by general and VE-cadherin-restricted miRNA antagonists of miR-27. Blood. 2013;122:2911–9. doi: 10.1182/blood-2012-12-473017. [DOI] [PubMed] [Google Scholar]
- 97.Mishra R, Singh SK. HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. The Journal of neuroscience: the official journal of the Society for Neuroscience 2013; 33:5992-6000. [DOI] [PMC free article] [PubMed]
- 98.Muramatsu F, Kidoya H, Naito H, Sakimoto S, Takakura N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene. 2013;32:414–21. doi: 10.1038/onc.2012.68. [DOI] [PubMed] [Google Scholar]
- 99.Lalwani MK, Sharma M, Singh AR, Chauhan RK, Patowary A, Singh N, Scaria V, Sivasubbu S. Reverse genetics screen in zebrafish identifies a role of miR-142a-3p in vascular development and integrity. PLoS One. 2012;7:e52588. doi: 10.1371/journal.pone.0052588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kane NM, Howard L, Descamps B, Meloni M, McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, et al. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012;30:643–54. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- 102.Mansouri M, Rose PP, Moses AV, Früh K. Remodeling of endothelial adherens junctions by Kaposi’s sarcoma-associated herpesvirus. J Virol. 2008;82:9615–28. doi: 10.1128/JVI.02633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102:1192–201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M, et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–32. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 105.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]