Abstract
Cell migration is fundamental to many biological processes, including development, normal tissue remodeling, wound healing, and many pathologies. However, cell migration is a complex process, and understanding its regulation in health and disease requires the ability to manipulate and measure this process quantitatively under controlled conditions. This report describes a simple in vitro assay for quantitative analysis of cell migration in two-dimensional cultures that is an inexpensive alternative to the classic “scratch” assay. The method described utilizes flexible silicone masks fabricated in the lab according to the research demands of the specific experiment to create a cell-free area for cells to invade, followed by quantitative analysis based on widely available microscopic imaging tools. This experimental approach has the important advantage of visualizing cell migration in the absence of the cellular damage and disruption of the substrate that occurs when the “wound” is created in the scratch assay. This approach allows the researcher to study the intrinsic migratory characteristics of cells in the absence of potentially confounding contributions from cellular responses to injury and disruption of cell–substrate interactions. This assay has been used with vascular smooth muscle cells, fibroblasts, and epithelial cell types, but should be applicable to the study of practically any type of cultured cell. Furthermore, this method can be easily adapted for use with fluorescence microscopy, molecular biological, or pharmacological manipulations to explore the molecular mechanisms of cell migration, live cell imaging, fluorescence microscopy, and correlative immunolabeling.
Keywords: sylgard elastomer, culture mask, migration, microscope, ImageJ, image analysis, scratch assay
Introduction
Cell migration is a complex process that is fundamental to embryogenesis, development, wound healing, normal tissue remodeling, and many pathologies. To investigate the mechanisms regulating cell migration it is essential to measure cell migration under carefully controlled conditions. Cell culture systems are ideally suited to this task because they permit the direct visualization and measurement of cell migration and are amenable to pharmacological and molecular manipulations.
For quantitative studies of cell migration, it is often necessary to create a defined area within a culture for cells to invade, so that migratory cells can be identified unequivocally in the microscope. A classic and widely used approach to creating a defined cell-free area is the “scratch assay.” In this assay, cultured cells are grown to confluence, and a sharp object such as a pipette tip is then scraped across the culture dish to create a wound that is then invaded by surviving cells along the wound margin.1,2 This approach has the advantages of being extremely simple and inexpensive, and has been a very useful experimental tool. However, the scratch assay also has significant limitations. First, the wound produces extensive cell injury and death and perturbs the underlying substrate, which potentially can affect the migratory behaviors of the surviving cells. Second, repeatedly creating equivalent wounds across experiments can be difficult.
For many studies it is advantageous for the researcher to create a cell-free area for invasion without inducing cell damage or perturbing the migratory substrate. One approach to this challenge is to physically mask an area of a desired size and shape within a culture to prevent cells from colonizing that region when they are plated.3,4 The cells are allowed to grow to confluence and the mask is then removed to create a cell-free area without damaging cells or perturbing the substrate. Custom-fabricated masks of this type have been applied very effectively to study cell migration.3,4 However, the methods used to create these masks can require custom fabrication, materials, and techniques that increase expense or are not readily available to many researchers.
Silicone masks have been used effectively in this context (for example, see ref. 3) and can provide a very useful and adaptable experimental tool for the study of cell migration. This report describes one simple method to make circular or rectangular silicone elastomer masks for creating cell-free areas within a culture without inducing cell damage or disturbing the substrate, and their application for microscopic quantification of cell migration. This approach uses inexpensive components and laboratory equipment that is widely available to many researchers. For the purposes of this report, we focus on migration of cultured vascular smooth muscle cells (VSMCs) stimulated with platelet derived growth factor-BB (PDGF),5,6 using the S31 VSMC cell line derived from rat cardiac microvessels.7-9 However, this approach is broadly applicable and also has been used successfully to examine migration of fibroblasts and epithelial cells. This approach also can be adapted for use with live cell imaging, imaging of fluorescent dyes, probes, and reporters in migrating cells, and subsequent correlative immunolabeling or electron microscopy.
Results
A flowchart describing the assay method is provided in Figure 1. Briefly, masks of desired size and shape are made from Sylgard184 silicone elastomer. Examples shown are derived from experiments using cylindrical masks to produce circular cell-free areas within a culture for cells to invade. Masks are positioned on the floor of a sterile well in a multi-well plate, petri dish, or on a glass coverslip using sterile forceps. Cells are then plated into the prepared culture well and allowed to grow in serum-containing medium. When cells reach confluence, the elastomer mask is removed carefully using sterile forceps to create a cell-free area. After an initial microscopic image is obtained, the assay is started by adding a migratory stimulator via the medium. In some cases, a period of serum starvation is performed; in the case of S31 VSMCs, we typically serum-starve for 48 h. However, serum starvation is not necessary to achieve migration. PDGF in fresh serum-free medium was used as the migratory stimulus for VSMCs in the examples shown. Migration of cells into the cell-free area is imaged in the microscope at time intervals appropriate to the speed of migration by the cells of interest. The magnitude of the migratory response is then determined from the image sequence using image analysis software.
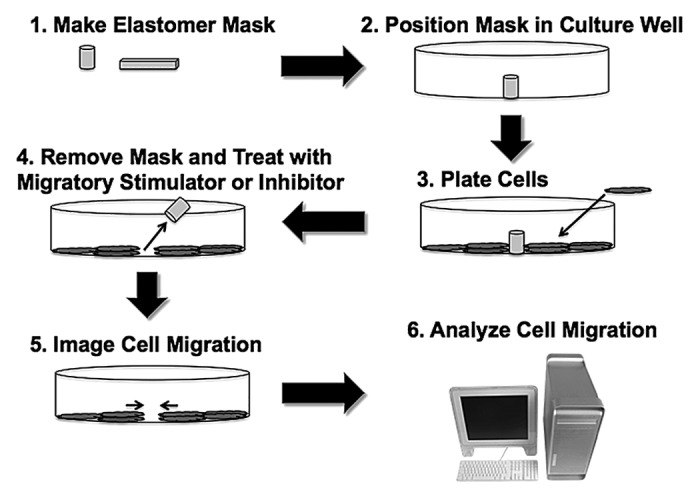
Figure 1. Flowchart for performing migration assay using elastomer masks.
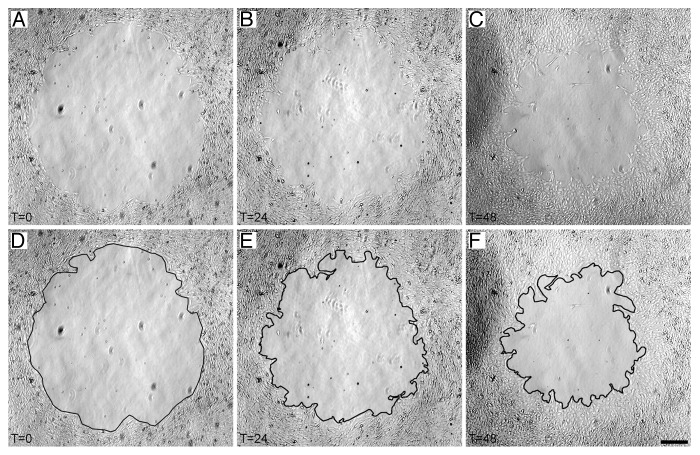
A typical sequence of images showing PDGF-induced VSMC migration into a circular cell-free area created with a 2 mm cylindrical elastomer mask is shown in Figure 2. Images were captured at the time that the mask was removed (T0), at 24 h after the assay was initiated (T24), and at 48 h after the assay was initiated (T48) (Fig. 2A–C). Cell migration into the cell-free area was clearly visible by 24 h, consistent with the known role of PDGF in inducing VSMC migration.5,6 Cell migration was analyzed quantitatively by tracing the leading edge of cells invading the cell-free area at T0, T24, and T48 (Fig. 2D–F). The proportion of the original cell-free area invaded was analyzed quantitatively using ImageJ software to determine the time course and magnitude of VSMC migration under control and PDGF-treated conditions (Fig. 3). Untreated control VSMCs display very little migration over the entire assay period, while PDGF-treated VSMCs show a distinct migratory response by 24 h that continues out to 48 h.
Figure 2. Elastomer mask cell migration assay. (A–C) Sequence of images of one cell-free area in a PDGF-treated culture showing migration of VSMCs at T0, T24, and T48. (D–F) Image sequence from panels A-C with the leading edge of migratory cells traced for analysis of the proportion of the cell-free area invaded. Scale bar = 250 µm for all panels.
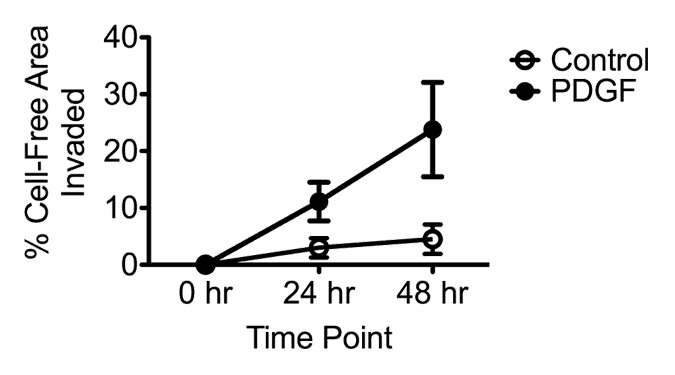
Figure 3. Comparison of VSMC migration using elastomer masks in untreated control and PDGF-treated cultures. Time-course analysis over 48 h is shown.
The low magnification brightfield imaging approach described here has inherently low contrast. Adjustment of the manual condenser on the microscope was used to enhance the contrast of the image. First, the condenser was aligned and the field and aperture iris diaphragms were adjusted to produce the best brightfield image. To further enhance contrast, an empty slot in the condenser ring holding the phase contrast inserts was moved into the light path. While viewing the specimen, the condenser ring was then rotated slightly out of position to produce a contrasting shadow along the margin of the cell-free area. This intentional mis-adjustment was a standard part of the acquisition procedure for capturing images of this assay using the 2X objective lens. Image contrast and brightness were further adjusted digitally if needed during image analysis using the “enhance contrast” function of ImageJ. Manual mis-adjustment of the condenser is not recommended for use with motorized condensers, as damage could result. Digital enhancement of contrast is recommended in these cases.
The mask migration assay is also amenable to analyses of details of individual cells or cell ensembles with other imaging modalities (Fig. 4), as removal of the mask does not affect the optical properties of the culture. For example, to visualize the organization of cell ensembles and individual cells at the migratory edge created by mask removal, phase contrast optics in combination with 10X, 20X, or 40X long working distance objective lenses were used (10X shown in Fig. 4). Similarly, cells expressing fluorescent reporter proteins can be imaged by fluorescence microscopy (not shown).
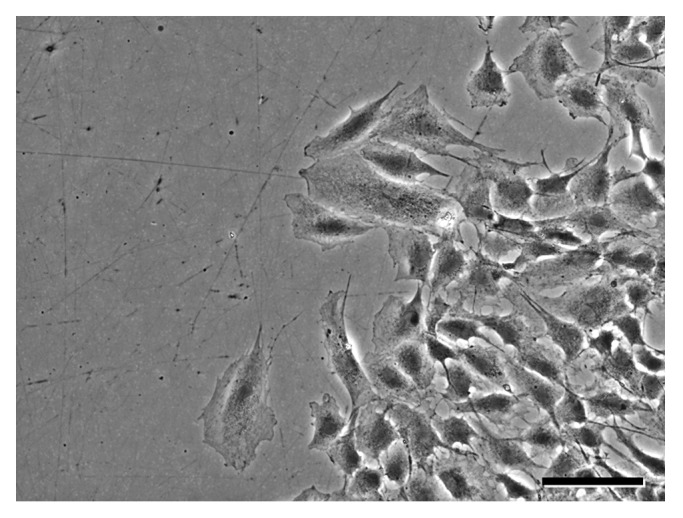
Figure 4. Imaging can be performed at higher magnifications in conjunction with contrasting optical techniques to visualize cellular detail. (A) Phase contrast image captured using a 10X objective lens shows organization of an ensemble of migrating VSMCs treated with PDGF-BB. Scale bar = 50 µm.
One difficulty that can compromise the assay is the presence of a shadow across a portion of the image of the cell-free area, precluding quantitative analysis (Fig. 5). This difficulty arises from poor positioning of masks within the culture well, and is easily avoided by careful placement of the mask near the center of the culture well, where the meniscus of the medium causes minimal refraction.
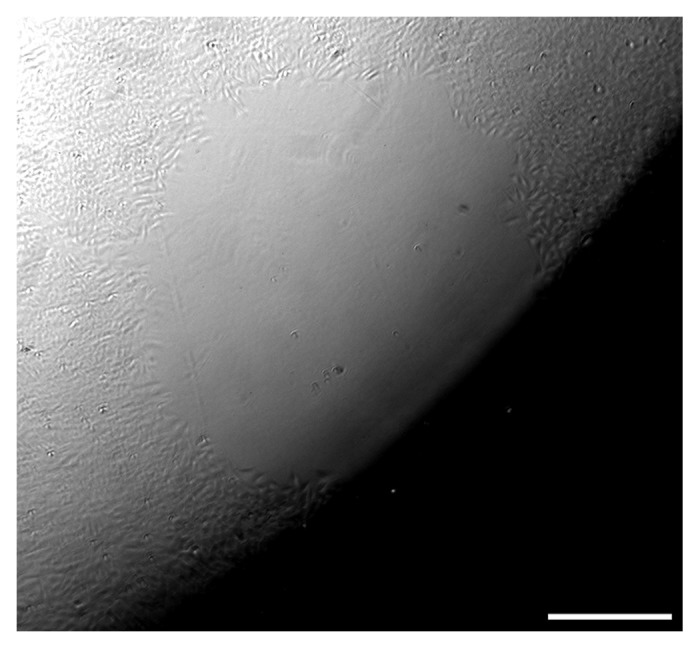
Figure 5. Placement of the mask too far from the center of the culture well can interfere with imaging and obscure some or all of the cell-free area. Scale bar = 250 µm.
Discussion
The assay method described here is a simple in vitro approach for quantitative measurement of cell migration into a defined area. Key strengths of this assay are that it does not damage cells or perturb the substrate, and that it can be performed using widely available hardware and software.
Application and imaging considerations
The assay requires repeated sampling of each cell-free area across time points. Cell-free areas can be re-located visually, but this can be challenging and time consuming. Relocating cell-free areas can be accomplished using the X-Y stage coordinates of each cell-free area on microscopes equipped with mechanical or programmable stages. Repeated sampling also can be performed by placing matching fiducial marks on the culture ware and the microscope stage, then aligning these marks to return to the cell-free area.
Imaging in this migration assay is typically performed at low magnification. The largest cell-free area that could be captured within a single image frame using our microscope configuration (see Materials and Methods) was a 2 mm circle. If a larger mask is used, a series of overlapping images can be stitched together to create a montage of the entire field using the “photomerge” function in Photoshop (Adobe Systems) or an ImageJ program plug-in for merging images (available at: http://rsbweb.nih.gov/ij/plugins/patchwork.html). To image specific cells of interest at higher magnifications, the assay can be performed with cells grown on glass coverslips for compatibility with high magnification objective lenses with short working distances. Specialized specimen chambers may be required for these applications.
A limitation of brightfield imaging in this assay preparation is intrinsically low contrast. Several imaging modalities are available to improve the contrast of brightfield images, including phase contrast (see Fig. 4), differential interference contrast (DIC or Nomarski), and Hoffmann modulation contrast microscopy. All require specialized optical components. Differential Interference Contrast microscopy can be performed using cells grown on glass coverslips but is incompatible with plastic culture ware.
Troubleshooting masks
Elastomer masks, when made properly, are consistent in size and shape but are not perfectly identical. Therefore, the size of the cell-free area produced by each mask must be measured at T0 for accurate analyses. In addition, cylindrical masks do not necessarily produce a perfectly circular cell-free region, so this shape cannot be assumed. If a precise mask size and shape is essential, then mask fabrication by other methods may be necessary (for examples, see refs. 3 and 4). If very large cell-free areas are needed, a thin coating of the elastomer can be applied directly to the bottom of the culture dish or glass coverslip.10,11 Large deformations in the mask can compromise adhesion and allow cells to colonize the area under the mask. Large inconsistencies or distortions in the shape or tears in the elastomer masks may indicate that the elastomer is incompletely polymerized or is too soft. Increasing the curing time or slightly increasing the amount of catalyst can be used to address these issues.
Proper adhesion of the mask to the substrate is critical. If cells are present in the cell-free area when the mask is removed at T0, the mask did not properly adhere. Both the mask and the culture ware must be dry when the mask is applied. Trapping air bubbles underneath the mask during placement can compromise adhesion and permit cells to invade under the mask. Elastomer masks can also be used with glass coverslips or culture ware, but may adhere better to plastic. Substrates or coatings that might be used in experiments could potentially affect mask adhesion. In the examples shown, culture ware was not pre-coated with any specific protein or substrate. Over time, the elastomer may become more rigid or lose some of its adhesive characteristics. In this case, make fresh elastomer.
Additional considerations for analysis of results
The characteristics of cell migration can vary widely across different cell types and contexts.12 Therefore, the media, experimental treatments, image sampling rate, size of cell-free area needed, and other conditions used are experiment-specific. For example, S31 VSMCs stimulated with PDGF migrate relatively slowly, so a 24 h imaging interval is appropriate. In contrast, epithelial cells might be expected to invade the cell-free space almost immediately after mask removal,3 requiring a shorter imaging interval. Another important consideration is that cells invading the cell-free area from different points around the perimeter could intermingle in the center. If this point is reached, the migratory margin can no longer be distinguished and it is not possible to accurately measure the percentage of the cell-free area invaded as described here. However, other potentially relevant measures of migration, such as time for cells to reach the center, % of cell-free area occupied, or time required to fill the cell-free area can be assessed.
Further applications
Elastomer masks could be used to test the effects of different substrates on cell migration by coating the bottom of the culture well with a substrate of interest prior to mask placement. The silicone elastomer should not interfere with use of molecular reagents such as anti-sense oligodeoxynucleotides, siRNAs, or plasmid or viral vectors used to overexpress or knockdown expression of specific genes. The ability to record the spatial information regarding specific cells in the culture as X-Y coordinates permits the investigator to fix the culture, immunolabel for antigens of interest, and then examine their expression and distribution in specific migratory cells. If analysis of cell structure at the ultrastructural level is needed, cultured cells can be fixed and embedded in resin for subsequent sectioning and analysis at the electron microscopic level. Cells can also be collected at the end of the migration assay for analysis of protein or gene expression by western blotting or polymerase chain reaction analysis.
An important limitation of the assay, as described, is that it is not intrinsically designed for analysis of migration in a chemotactic gradient. Several well-established two-chamber methods are available specifically for analysis of chemotaxis, such as Boyden, Dunn, or Zigmond chambers.13-15 The assay also could be adapted to assess chemotaxis in the presence of local microgradients established using pulsatile ejection of the chemotactic agent from a microelectrode16 or by placement of beads loaded with the agent of interest.17
Materials and Methods
Information regarding materials and vendors is provided in Table 1.
Table 1. Reagents and materials.
| Name of reagent/material | Company | Catalog number | Comments |
|---|---|---|---|
| Sylgard 184 | Ellsworth Adhesives | 184 SIL ELAST KIT 0.5KG | |
| Recombinant rat PDGF-BB | R&D Systems | 520-BB-050 | |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Invitrogen | 12100-061 | |
| Lactalbumin hydrolysate | Invitrogen | 895086IP | |
| 1X antibiotic-antimycotic | Invitrogen | 15240096 | |
| Heat-inactivated fetal bovine serum. | Invitrogen | 11150 | |
| BD Falcon 24-well culture plate | VWR | 21100-004 | 6- or 12-well culture plates or individual petri dishes of any size also will work. |
| Miltex biopsy punches (2mm) with ejector | VWR | 95039-098 | Also available in other diameters up to 6mm |
| Forceps, Dumont Style 2 | Electron Microscopy Sciences | 0103-2-PO | Straight or curved forceps will work according to user preference |
| Electron microscopy embedding molds (polyethylene) | Electron Microscopy Sciences | 70904-01 | Other sizes and shapes of molds also available. Silicone rubber molds not recommended. |
| Tissue-Tek Cryomold, biopsy molds (vinyl) | Electron Microscopy Sciences | 62534-10 | Other sizes and shapes of molds also available. Silicone rubber molds not recommended. |
Fabrication and placement of elastomer masks
Elastomer masks were prepared by adding the elastomer and catalyst components of Sylgard 184 (Ellsworth Adhesives) in an 8:1 ratio and mixing thoroughly. For masks used to create circular cell-free assay areas, elastomer was poured into a clean 60 mm polystyrene petri dish to a depth of 3–5 mm and allowed to polymerize completely at room temperature (1–2 d). Cylindrical masks were cut from the elastomer sheet in the petri dish using a fresh 2 mm diameter biopsy punch (Miltex, VWR) and gently removed from the surrounding elastomer using forceps. For masks used to create cell-free areas with linear edges, a thin layer of elastomer was poured into rectangular polyethylene electron microscopy embedding molds or square vinyl histology molds (Electron Microscopy Sciences) and was allowed to polymerize completely at room temperature. Polymerized masks were gently removed from the mold using forceps. Silicone embedding molds may interfere with elastomer polymerization and are not recommended. Masks were sterilized by immersion in 100% ethanol for 10–15 min at room temperature, rinsed in sterile water, and then allowed to air dry completely prior to use.
To create cell-free areas for analysis, elastomer masks were placed into sterile polystyrene culture dishes or multi-well plates, or onto glass coverslips prior to the addition of any media or cells. In the experiments described here, circular cell-free areas were created by placing cylindrical masks vertically at the center of the wells of disposable polystyrene 12- or 24-multi-well culture plates (BD Falcon, VWR) using sterile forceps and gentle pressure to ensure adherence. To create rectangular cell-free areas, rectangular masks were used.
Cell culture
Cells were plated into wells or onto coverslips with a pre-placed elastomer mask. Culture ware and coverslips were not pre-coated with any specific protein or other substrate. For the examples shown, S31 VSMCs were plated into mask-containing wells of 12- or 24-well culture dishes in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat inactivated fetal bovine serum and then were allowed to grow to post-confluence in a 5% CO2 atmosphere at 37 °C.7,8 Cells were synchronized by rinsing in PBS, then culturing in serum-free DMEM containing 0.02% lactalbumin hydrolysate and 1X antibiotic-antimycotic.8,9
Initiation of migration assay
To initiate the migration assay, the elastomer masks were removed from the multi-well plate using sterile forceps in a culture hood to create a cell-free zone, taking care to avoid touching the bottom of the well to avoid creating a wound or scratching the surface. Used masks were discarded. Immediately after removing the masks, the lid was replaced, and the multi-well plate was moved to an inverted microscope to capture the initial T0 images of the cell-free area for analysis (see details below under “imaging and analysis”). For our migration assays using VSMCs, which do not migrate in the absence of migratory stimulators, cultures were allowed to remain in control serum-containing medium for up to 30 min after mask removal to permit serum components such as vitronectin to adhere to the unmasked area and promote subsequent cell attachment during migration. For cell types that initiate migration immediately after creation of open space (for example, see ref. 3) it may be necessary to omit this treatment.
After capturing T0 images for each cell-free area in each culture well, medium was replaced with serum-free medium containing PDGF (25 ng/ml) to stimulate migration or with serum-free control medium without PDGF. The culture plate was returned to the incubator until the next imaging time point at 24 h. PDGF was replenished every 24 h by adding an aliquot of PDGF to the existing medium.9
Imaging
To image the cell-free area at T0, the lid was replaced on the multi-well plate to maintain sterility, and the plate was moved to an Olympus IX70 inverted microscope (Olympus America) fitted with a mechanical X-Y stage, long working distance condenser with five positions for inserts, and a 2X brightfield objective lens. An infrared cutoff filter was used in the illumination path to protect the cells from heating. A QiCAM cooled CCD camera controlled via QCapture Pro software (QImaging) was used to capture 8-bit greyscale TIFF images. The 2 mm circular cell-free area in the first well of the plate was located using the 2X objective lens, and the insert ring in the condenser was manually rotated slightly out of position to produce a shadowing effect to increase image contrast (this mis-alignment should not be performed with automated condensers as damage potentially could result). A brightfield image was captured, and the X-Y stage coordinates were recorded to facilitate repeated imaging at later time points. This image represents Time 0 (T0), the starting point for the assay, prior to the onset of migration. The process was repeated to capture the T0 image of the cell-free area in each well of the culture plate, and the cells were returned to the culture incubator. Subsequent images of each cell-free area were captured using the 2X objective lens at T24 and T48. Shorter sampling intervals are used for cell types that show a more rapid migratory response. After collecting images at the final time point in the assay, cultures can be fixed for immunolabeling or collected for western blotting or molecular biological analysis, if desired.
Analysis of migration
The migratory response was determined by measuring the proportion of the cell-free area invaded by VSMCs using the image sequence collected for each mask at T0, T24, and T48. Analysis was performed using ImageJ software (freeware available at www.download-imagej.com). To prevent accidental modification of the original image data or data loss, all analyses were performed only on duplicate copies of the original images.
To determine the extent of migration, the T0 image for the mask in the first culture well was imported into ImageJ, and the image scale (pixels/µm) was set. Brightness and contrast were adjusted to highlight the edge of the cell-free area, the polygon selection tool was used to trace the margins of contiguous cells bordering the entire cell-free area, and the area of the cell-free zone at T0 was measured. These steps were repeated for the matching T24 and T48 images to determine the area of the cell-free area remaining at these time points. The percentage of the cell-free area invaded at each time point was calculated using the following formula:
% area invaded = 100% - [(area at Tx/area at T0) x 100]; where Tx = T24 or T48.
Alternatively, the data can be expressed in units of area. This process was repeated for each mask and treatment condition in the culture plate to assess the migratory behavior of the cells under different treatment conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by awards from the College of Medicine Alumni Association at the University of Oklahoma Health Sciences Center and OCAST (HR08-149S, HR10-012).
Glossary
Abbreviations:
- GFP
green fluorescent protein
- PDGF
platelet-derived growth factor-BB
- T0
time 0, initial assay time point
- T24
time 24 hours after start of assay
- T48
time 48 hours after start of assay
- VSMC
vascular smooth muscle cell
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/27294
References
- 1.Todaro GJ, Lazar GK, Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965;66:325–33. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- 2.Liang CC, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 3.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci U S A. 2007;104:15988–93. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroening S, Goppelt-Struebe M. Analysis of matrix-dependent cell migration with a barrier migration assay. Sci Signal. 2010;3:pl1. doi: 10.1126/scisignal.3126pl1. [DOI] [PubMed] [Google Scholar]
- 5.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 6.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 7.Phelps ED, Updike DL, Bullen EC, Grammas P, Howard EW. Transcriptional and posttranscriptional regulation of angiopoietin-2 expression mediated by IGF and PDGF in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C352–61. doi: 10.1152/ajpcell.00050.2005. [DOI] [PubMed] [Google Scholar]
- 8.Risinger GM, Jr., Hunt TS, Updike DL, Bullen EC, Howard EW. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Biol Chem. 2006;281:25915–25. doi: 10.1074/jbc.M513513200. [DOI] [PubMed] [Google Scholar]
- 9.Risinger GM, Jr., Updike DL, Bullen EC, Tomasek JJ, Howard EW. TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol. 2010;298:C191–201. doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townes-Anderson E, St Jules RS, Sherry DM, Lichtenberger J, Hassanain M. Micromanipulation of retinal neurons by optical tweezers. Mol Vis. 1998;4:12. [PubMed] [Google Scholar]
- 11.Clarke R, Wang J, Townes-Anderson E. Using laser tweezers for manipulating isolated neurons in vitro. J Vis Exp. 2008;19:911. doi: 10.3791/911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rørth P. Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev Cell. 2011;20:9–18. doi: 10.1016/j.devcel.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–66. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zigmond SH. Orientation chamber in chemotaxis. Methods Enzymol. 1988;162:65–72. doi: 10.1016/0076-6879(88)62064-7. [DOI] [PubMed] [Google Scholar]
- 15.Zicha D, Dunn GA, Brown AF. A new direct-viewing chemotaxis chamber. J Cell Sci. 1991;99:769–75. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- 16.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–4. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 17.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]