Abstract
Phosphorylations control all aspects of vasodilator-stimulated phospho-protein (VASP) function. Mapped phosphorylation sites include Y39, S157, S239, T278, and S322, and multiple kinases have been shown to mediate their phosphorylation. Recently, Protein Kinase D1 (PKD1) as a direct kinase for S157 and S322 joined this group. While S157 phosphorylation generally seems to serve as a signal for membrane localization, phosphorylations at S322 or at S239 and T278 have opposite effects on F-actin accumulation. In migrating cells, S322 phosphorylation increases filopodia numbers and length, while S239/T278 phosphorylations decrease these and also disrupt formation of focal adhesions. Therefore, the kinases mediating these phosphorylations can be seen as switches needed to facilitate cell motility.
Keywords: VASP, migration, cytoskeleton, phosphorylation, leading edge, filopodium
VASP Family Members—Functions, Structural Composition, and Phosphorylation
VASP and other members of the Ena/VASP protein family, such as mammalian-enabled (Mena) and Ena-VASP-like (EVL) have been linked to many human diseases including cancer, thrombosis, cardiomyopathy, arteriosclerosis, and nephritis.1 Ena/VASP proteins link cellular signaling pathways downstream of RhoGTPases to actin dynamics.2,3 Such Ena/VASP-regulated actin-related processes include epithelial cell adhesion, cell polarity, cell motility, axon outgrowth and guidance, and pathogen F-actin tail formation.4-6
In epithelial cells, VASP contributes to cell–cell adhesions through regulation of actin polymerization and bundling. Similarly, in motile cells, VASP localizes to regions of dynamic actin reorganization including focal adhesions and filopodia at the leading edge, where it contributes to F-actin filament elongation and bundling.7-9 VASP promotes F-actin stress fiber assembly and elongation by antagonizing the capping protein CapZ, reducing Arp2/3-mediated branching activities, promoting the transfer of G-actin bound to profilin at the barbed end of growing filaments, and increasing the bundling of filaments.2,10-12 Overall, this leads to longer, less-branched filaments. Consequently, fibroblasts from VASP-knockout mice have thicker, more stable actin stress fibers and enlarged focal adhesions.13
VASP proteins consist of three conserved domains, a N-terminal Ena/VASP homology-1 (EVH-1) domain, a poly-proline region (PPR) and a C-terminal EVH-2 domain (Fig. 1A). While the EVH-1 domain mediates interactions with WASP, zyxin, and vinculin, the PPR mediates association with profilin, Src, and Abl. The EVH2 domain facilitates oligomerization and binding to G- and F-actin and is of imminent importance for VASP functions at the leading edge of cells.11,14-16 It maintains clustering of the barbed ends of actin filaments and organizes filopodia formation at the leading edge.17 The EVH2 domain also enhances actin polymerization in the presence of capping proteins at the barbed end.10
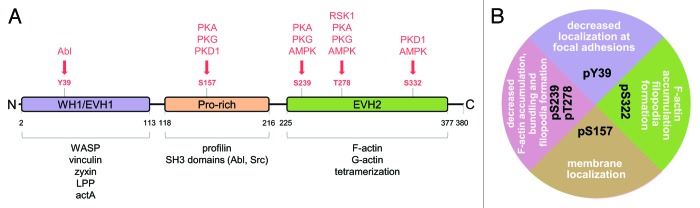
Figure 1. VASP domains, interacting proteins, phosphorylation sites, and upstream kinases. (A) VASP consists of a WH1/EVH1 domain (aa2-aa113), a prolin-rich domain (aa118-aa216), and an EVH2 domain (aa225-aa377). The EVH1 domain interacts with WASP, vinculin, zyxin, LPP, and actA; the prolin-rich (PR) domain with profilin and SH3 domains of Abl and Src; the EVH2 domain binds to F- and G-actin, but also is required for tetramerization of VASP. Multiple phosphorylation sites have been mapped including Y39 (phosphorylated by Abl) in the EVH1 domain, S157 in the PR domain (mediated by PKA, PKG, and PKD1), as well as S239 and T278 (mediated by RSK1, PKA, PKG, and AMPK, as indicated) and S322 (mediated by PKD1 and AMPK) in the EVH2 domain. (B) The VASP “phosphorylation code” with respect to its functions in regulating cell motility. Phosphorylation at Y39 decreases localization at the focal adhesions. Phosphorylation at S157 mediates membrane localization. Additional phosphorylation at S322 mediates F-actin accumulation and increases numbers of filopodia, while additional phosphorylation at S239 (and phosphorylation at T278) prevents F-actin bundling, decreases F-actin accumulation and decreases filopodia formation.
Phosphorylation events regulate most aspects of VASP localization and function. For example, VASP phosphorylation can reduce its association with actin, has negative effects on actin polymerization, and modulates interaction with other proteins such as Abl and Src.18 However, binding of focal adhesion proteins to the EVH-1 domain and of profilin to the PPR domain is independent of phosphorylation of VASP.18,19 Several tyrosine, serine, and threonine residues have been identified by mass spectroscopy (summarized in www.phosphosite.org). Five of these sites have been experimentally confirmed using site-specific methods and/or phospho-specific antibodies. These phosphorylation sites include Y39, S157, S239, T278, and S322 (Fig. 1). Altered functional consequences for cell motility have been attributed to their phosphorylation (a “phosphorylation” code for cell motility is shown in Fig. 1B), and this will be discussed in the following.
VASP Phosphorylation at S157—Signal for Membrane Localization
Phosphorylation at S157 abrogates interaction of VASP with the SH3 domains of Abl, Src, and αII spectrin and controls cellular distribution of VASP.16,20 In migrating cells, VASP phosphorylation at S157 provides a signal for membrane or leading edge localization,21,22 but has a minor impact on actin filament formation or the G-actin/F-actin ratio.21 S157 phosphorylation can be mediated through cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG).23 However, in response to serum stimulation leading to actin filament elongation, VASP phosphorylation at S157 is independent of PKA and PKG, but dependent on PKC.24 Recently, it was shown that PKD1 is also a direct kinase for S157.22 Moreover, other stimuli such as PMA and thrombin that regulate VASP via PKC are upstream activators for PKD1. Additionally, thrombin-mediated phosphorylation of VASP also is dependent on ROCK1. Since PKC and ROCK1 have been shown to be upstream of PKD1 in RhoA signaling pathways, it is very likely that PKC-dependent phosphorylation of VASP is mediated through PKD1.25,26
Following S157 phosphorylation, additional phosphorylation events can diverge the functions of VASP at the leading edge of migrating cells.
Kinases that Additionally Phosphorylate VASP at S239 and T278
Multiple kinases, including PKA, PKG, and AMP-activated protein kinase (AMPK) phosphorylate VASP at S157, S239, and T278.27-29 Often phosphorylation of T278 requires prior phosphorylation of VASP at S157 and S239. However, the ribosomal S6 kinase 1 (RSK1) interacts with VASP and phosphorylates it specifically at T278 without affecting S157 and S239 phosphorylation.30 Phosphorylation of both S239 and T278 has been linked to altered cell growth, cell adhesion, and cell motility. For example, phosphorylation of VASP by PKA in vitro abolished VASP anticapping activity,10 regulated filopodia formation,6 but also correlated with membrane ruffle formation and chemotaxis.31 PKG phosphorylation of VASP at S157, S239, and T278 disrupted Rac-1-dependent focal adhesion assembly.32 It further reduced the number of membrane protrusions and caused rounding of cells. A VASP.S239D mutant also mainly was enriched in the cytosol indicating that S239 phosphorylation may prevent binding to actin fibers.33 In summary, phosphorylations at S239 and T278 impair F-actin accumulation21 and suppress number and length of filopodia.34
Kinases that Additionally Phosphorylate VASP at S322
In contrast to phosphorylations at S239 and T278 that impair F-actin accumulation,21 S322 phosphorylation has been shown to increase F-actin accumulation.22 S322 is a newly identified phosphorylation site in the EVH-2 domain that is phosphorylated by PKD1 and AMPK, which share the same substrate motif.22,35
Activities of PKD family members are regulated by RhoGTPases.36,37 For example, PKD1 is negatively regulated by Rac1, a driver of actin reorganization and leading edge progression, and activated by RhoA, a driver of filament formation and bundling.26,38,39 When activated by RhoA, PKD1 interacts with F-actin,40 negatively regulates barbed-end formation and F-actin re-organization at the leading edge,41,42 and stabilizes F-actin filaments.22 In response to active RhoA, PKD1 phosphorylates VASP at two serine residues, S157 and S322, but not at S239. Phosphorylation at S322 alters VASP binding to F-actin filaments.35 Combined phosphorylation of VASP at S157 and S322 leads to a translocation of VASP from focal adhesions to the leading edge region.22 Consequently, PKD1-mediated phosphorylations at both S157 and S322 increased stress fiber formation and resulted in filopodia formation. In presence of active PKD1, the number and length of filopodia was dramatically increased,22 suggesting that PKD1 is part of the process leading to both filopodia formation and extension. However, when PKD1 activity was persistent or driven by a constitutive signal this led to membrane ruffling and decreased cell migration.22 Phosphorylation at S322 also affects the epithelial phenotype, since inhibition of phosphorylation at this site results in accumulation of VASP at cell–cell contacts and increased cell–cell adhesion.35
No homolog sites to S322 have been found in Mena or EVL. However, PKD1 interacts with and phosphorylates EVL-1, a splice variant of EVL (Ena/VASP-like protein), at S345 in the EVH2 domain. This site is conserved in human and mouse, but not rat EVL-1.43 Moreover, the PKD1 phosphorylation sites in EVL-1 (S345) and VASP (S322) have similar outcome. Like VASP, when phosphorylated by PKD, EVL-1 supports filopodia formation. Consequently, phosphorylated EVL-1 is located at the filopodial tips, ruffling lamellipodia, and at cell–cell contacts.43
Phosphorylation at Y39 and Potential Function in Cell Migration
Another phosphorylation of VASP is mediated by the tyrosine kinase Abl. Abl interactor- 1 (Abi-1) promotes the interaction between Abl and Ena/VASP proteins and mediates phosphorylation of VASP by Abl at Y39.44,45 Abl also phosphorylates other members of this protein family, including Mena at Y296.44 Another substrate of Abl is lamellipodin (Lpd) and its phosphorylation recruits Ena/VASP proteins (Mena, VASP, EVL) to the leading edge of cells. Signaling through c-Abl, VASP and lamellipodin mediates dorsal ruffling of fibroblasts and regulates cell motility.46 The regulation of VASP by Abl is interesting in the context that Abl is also a kinase that can phosphorylate PKD1 and lead to its activation in response to oxidative stress.47,48 Therefore, it will be interesting to determine the contribution of Y39 phosphorylation of VASP to PKD1-mediated regulation of VASP.
Expression and Phosphorylation Status of Ena/VASP Proteins as Marker for Tumor Progression
The Ena/VASP family members are differentially expressed in cancer. Mena is upregulated in invasive mammary tumor cells,49 and overexpressed in benign lesions of the breast with a high risk of transformation,50 and in over 70% of primary breast cancers.51 Overexpression of Mena in Her2-positive cancers decreases overall patient survival.52 Increased expression of Mena was also shown for colorectal carcinomas and correlated with advanced TNM stages.53 In these cancers, upregulation of Mena mainly occurred in the invasive front, where a transition from a glandular structure to single invasive cells occurred. More recent data suggest that Mena is alternately spliced to produce an invasion-promoting isoform.54,55
In breast cancer cells, expression of EVL-1 leads to increased actin bundling and suppression of protrusive activity and cell motility.56 Consequently, in samples of human breast cancer, decreased expression of EVL-1 correlates with high invasiveness and poor patient outcome.56
In contrast to Mena and EVL-1, VASP expression is generally high in both cancer and normal tissue. Only in lung adenocarcinomas increased VASP expression was described when compared with normal epithelium.57 However, a random homozygous knockout approach in NIH-3T3 cells suggested an rather inhibitory role for VASP on soft agar colony and tumor formation.58 Thus, VASP expression levels alone may not serve as good predictive tumor markers. S157 phosphorylation of VASP has been suggested as a marker for prostate cancer cell motility and potential of metastatic progression.59 However, using phosphorylation of S157 solely as a marker needs to be pursued with caution, since additional phosphorylations may direct to opposing effects for the cell’s potential to migrate, invade, and metastasize. While so far no data are available correlating the S322 or the S239/T278 phosphorylation status with tumor progression, it was shown that the phosphorylation status of VASP at S239 can be used as endogenous cellular biomarker for anticancer agents that cause cGMP-mediated apoptosis in colon cancer.60
Conclusions
Although a multitude of phosphorylation sites and upstream kinases of VASP have been described, the code by which they regulate VASP cellular localization and function is not fully understood. In migrating cells, phosphorylation of VASP at S157 drives it to the leading edge. Further function is dependent on additional phosphorylations. While a RhoA-PKC-PKD1 pathway leads to additional phosphorylation at S322 and mediates F-actin filament elongation and accumulation, activation of PKG impairs serum- and RhoA-mediated actin filament accumulation through phosphorylation at S239 and T278.21,28 Consequently, phosphorylation of VASP by PKG suppresses filopodia formation,34 while phosphorylation by PKD1 leads to increased number and length of filopodia.22 In the future, to fully understand the phosphorylation code that determines VASP function, the phosphorylation status at all of the mapped sites including Y39 need to be determined for each known stimulus and upstream kinase and needs to be correlated with localization and outcome. Once these phosphorylation patterns are fully mapped, definitive conclusions may be drawn of how phosphorylations of VASP affect cell motility and how this knowledge may be used to predict progression of cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the NIH grants GM086435 and CA140182 (to PS). The content is solely the responsibility of the authors. The funders had no role in decision to publish, or preparation of the manuscript.
Glossary
Abbreviations:
- AMPK
AMP-activated protein kinase
- EVL
Ena-VASP-like
- F-actin
filamentous actin
- Mena
Mammalian Enabled
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKD
protein kinase D
- PKG
cGMP-dependent protein kinase
- RSK1
ribosomal S6 kinases 1
- VASP
vasodilator-stimulated phosphoprotein
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/27351
References
- 1.Pula G, Krause M. Role of Ena/VASP proteins in homeostasis and disease. Handb Exp Pharmacol 2008:39-65. [DOI] [PubMed] [Google Scholar]
- 2.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003;13:386–92. doi: 10.1016/S0962-8924(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 4.Krause M, Bear JE, Loureiro JJ, Gertler FB. The Ena/VASP enigma. J Cell Sci. 2002;115:4721–6. doi: 10.1242/jcs.00218. [DOI] [PubMed] [Google Scholar]
- 5.Reinhard M, Jarchau T, Walter U. Actin-based motility: stop and go with Ena/VASP proteins. Trends Biochem Sci. 2001;26:243–9. doi: 10.1016/S0968-0004(00)01785-0. [DOI] [PubMed] [Google Scholar]
- 6.Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/S0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Jouvenal K, Tripier D, Walter U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein) Proc Natl Acad Sci U S A. 1995;92:7956–60. doi: 10.1073/pnas.92.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–53. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trichet L, Sykes C, Plastino J. Relaxing the actin cytoskeleton for adhesion and movement with Ena/VASP. J Cell Biol. 2008;181:19–25. doi: 10.1083/jcb.200710168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280:28653–62. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–57. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- 12.Skoble J, Auerbuch V, Goley ED, Welch MD, Portnoy DA. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J Cell Biol. 2001;155:89–100. doi: 10.1083/jcb.200106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galler AB, García Arguinzonis MI, Baumgartner W, Kuhn M, Smolenski A, Simm A, Reinhard M. VASP-dependent regulation of actin cytoskeleton rigidity, cell adhesion, and detachment. Histochem Cell Biol. 2006;125:457–74. doi: 10.1007/s00418-005-0091-z. [DOI] [PubMed] [Google Scholar]
- 14.Kühnel K, Jarchau T, Wolf E, Schlichting I, Walter U, Wittinghofer A, Strelkov SV. The VASP tetramerization domain is a right-handed coiled coil based on a 15-residue repeat. Proc Natl Acad Sci U S A. 2004;101:17027–32. doi: 10.1073/pnas.0403069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball LJ, Kühne R, Hoffmann B, Häfner A, Schmieder P, Volkmer-Engert R, Hof M, Wahl M, Schneider-Mergener J, Walter U, et al. Dual epitope recognition by the VASP EVH1 domain modulates polyproline ligand specificity and binding affinity. EMBO J. 2000;19:4903–14. doi: 10.1093/emboj/19.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275:36143–51. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 17.Applewhite DA, Barzik M, Kojima S, Svitkina TM, Gertler FB, Borisy GG. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18:2579–91. doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbeck B, Hüttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem. 2000;275:30817–25. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- 19.Ferron F, Rebowski G, Lee SH, Dominguez R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007;26:4597–606. doi: 10.1038/sj.emboj.7601874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renné T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180:205–19. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, Renné T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–65. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Döppler HR, Bastea LI, Lewis-Tuffin LJ, Anastasiadis PZ, Storz P. Protein kinase D1-mediated phosphorylations regulate vasodilator-stimulated phosphoprotein (VASP) localization and cell migration. J Biol Chem. 2013;288:24382–93. doi: 10.1074/jbc.M113.474676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006;393:555–64. doi: 10.1042/BJ20050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitaley K, Chen L, Galler A, Walter U, Daum G, Clowes AW. Vasodilator-stimulated phosphoprotein is a substrate for protein kinase C. FEBS Lett. 2004;556:211–5. doi: 10.1016/S0014-5793(03)01435-2. [DOI] [PubMed] [Google Scholar]
- 25.Storz P, Döppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–26. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowell CF, Yan IK, Eiseler T, Leightner AC, Döppler H, Storz P. Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J Cell Biochem. 2009;106:714–28. doi: 10.1002/jcb.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–17. [PubMed] [Google Scholar]
- 28.Zhuang S, Nguyen GT, Chen Y, Gudi T, Eigenthaler M, Jarchau T, Walter U, Boss GR, Pilz RB. Vasodilator-stimulated phosphoprotein activation of serum-response element-dependent transcription occurs downstream of RhoA and is inhibited by cGMP-dependent protein kinase phosphorylation. J Biol Chem. 2004;279:10397–407. doi: 10.1074/jbc.M313048200. [DOI] [PubMed] [Google Scholar]
- 29.Blume C, Benz PM, Walter U, Ha J, Kemp BE, Renné T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007;282:4601–12. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- 30.Lara R, Mauri FA, Taylor H, Derua R, Shia A, Gray C, Nicols A, Shiner RJ, Schofield E, Bates PA, et al. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene. 2011;30:3513–21. doi: 10.1038/onc.2011.61. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Chung CY. Role of VASP phosphorylation for the regulation of microglia chemotaxis via the regulation of focal adhesion formation/maturation. Mol Cell Neurosci. 2009;42:382–90. doi: 10.1016/j.mcn.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Routray C, Liu C, Yaqoob U, Billadeau DD, Bloch KD, Kaibuchi K, Shah VH, Kang N. Protein kinase G signaling disrupts Rac1-dependent focal adhesion assembly in liver specific pericytes. Am J Physiol Cell Physiol. 2011;301:C66–74. doi: 10.1152/ajpcell.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defawe OD, Kim S, Chen L, Huang D, Kenagy RD, Renné T, Walter U, Daum G, Clowes AW. VASP phosphorylation at serine239 regulates the effects of NO on smooth muscle cell invasion and contraction of collagen. J Cell Physiol. 2010;222:230–7. doi: 10.1002/jcp.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuzga DS, Pelta-Heller J, Li P, Bombonati A, Waldman SA, Pitari GM. Phosphorylation of vasodilator-stimulated phosphoprotein Ser239 suppresses filopodia and invadopodia in colon cancer. Int J Cancer. 2012;130:2539–48. doi: 10.1002/ijc.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson DM, Ascione MP, Grange J, Nelson C, Hansen MD. Phosphorylation of VASP by AMPK alters actin binding and occurs at a novel site. Biochem Biophys Res Commun. 2011;414:215–9. doi: 10.1016/j.bbrc.2011.09.059. [DOI] [PubMed] [Google Scholar]
- 36.Machesky LM. Putting on the brakes: a negative regulatory function for Ena/VASP proteins in cell migration. Cell. 2000;101:685–8. doi: 10.1016/S0092-8674(00)80879-X. [DOI] [PubMed] [Google Scholar]
- 37.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–23. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Yuan J, Rey O, Rozengurt E. Activation of protein kinase D3 by signaling through Rac and the alpha subunits of the heterotrimeric G proteins G12 and G13. Cell Signal. 2006;18:1051–62. doi: 10.1016/j.cellsig.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Döppler H, Bastea LI, Eiseler T, Storz P. Neuregulin mediates F-actin-driven cell migration through inhibition of protein kinase D1 via Rac1 protein. J Biol Chem. 2013;288:455–65. doi: 10.1074/jbc.M112.397448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eiseler T, Schmid MA, Topbas F, Pfizenmaier K, Hausser A. PKD is recruited to sites of actin remodelling at the leading edge and negatively regulates cell migration. FEBS Lett. 2007;581:4279–87. doi: 10.1016/j.febslet.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 41.Eiseler T, Döppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–56. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spratley SJ, Bastea LI, Döppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem. 2011;286:34254–61. doi: 10.1074/jbc.M111.259424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssens K, De Kimpe L, Balsamo M, Vandoninck S, Vandenheede JR, Gertler F, Van Lint J. Characterization of EVL-I as a protein kinase D substrate. Cell Signal. 2009;21:282–92. doi: 10.1016/j.cellsig.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tani K, Sato S, Sukezane T, Kojima H, Hirose H, Hanafusa H, Shishido T. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J Biol Chem. 2003;278:21685–92. doi: 10.1074/jbc.M301447200. [DOI] [PubMed] [Google Scholar]
- 45.Maruoka M, Sato M, Yuan Y, Ichiba M, Fujii R, Ogawa T, Ishida-Kitagawa N, Takeya T, Watanabe N. Abl-1-bridged tyrosine phosphorylation of VASP by Abelson kinase impairs association of VASP to focal adhesions and regulates leukaemic cell adhesion. Biochem J. 2012;441:889–99. doi: 10.1042/BJ20110951. [DOI] [PubMed] [Google Scholar]
- 46.Michael M, Vehlow A, Navarro C, Krause M. c-Abl, Lamellipodin, and Ena/VASP proteins cooperate in dorsal ruffling of fibroblasts and axonal morphogenesis. Curr Biol. 2010;20:783–91. doi: 10.1016/j.cub.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storz P, Döppler H, Johannes FJ, Toker A. Tyrosine phosphorylation of protein kinase D in the pleckstrin homology domain leads to activation. J Biol Chem. 2003;278:17969–76. doi: 10.1074/jbc.M213224200. [DOI] [PubMed] [Google Scholar]
- 48.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–20. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 50.Di Modugno F, Mottolese M, Di Benedetto A, Conidi A, Novelli F, Perracchio L, Venturo I, Botti C, Jager E, Santoni A, et al. The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res. 2006;12:1470–8. doi: 10.1158/1078-0432.CCR-05-2027. [DOI] [PubMed] [Google Scholar]
- 51.Di Modugno F, Bronzi G, Scanlan MJ, Del Bello D, Cascioli S, Venturo I, Botti C, Nicotra MR, Mottolese M, Natali PG, et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer. 2004;109:909–18. doi: 10.1002/ijc.20094. [DOI] [PubMed] [Google Scholar]
- 52.Di Modugno F, Mottolese M, DeMonte L, Trono P, Balsamo M, Conidi A, Melucci E, Terrenato I, Belleudi F, Torrisi MR, et al. The cooperation between hMena overexpression and HER2 signalling in breast cancer. PLoS One. 2010;5:e15852. doi: 10.1371/journal.pone.0015852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyoda A, Kawana H, Azuhata K, Yu J, Omata A, Kishi H, Higashi M, Harigaya K. Aberrant expression of human ortholog of mammalian enabled (hMena) in human colorectal carcinomas: implications for its role in tumor progression. Int J Oncol. 2009;34:53–60. [PubMed] [Google Scholar]
- 54.Di Modugno F, Iapicca P, Boudreau A, Mottolese M, Terrenato I, Perracchio L, Carstens RP, Santoni A, Bissell MJ, Nisticò P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc Natl Acad Sci U S A. 2012;109:19280–5. doi: 10.1073/pnas.1214394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouneimne G, Hansen SD, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22:615–30. doi: 10.1016/j.ccr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dertsiz L, Ozbilim G, Kayisli Y, Gokhan GA, Demircan A, Kayisli UA. Differential expression of VASP in normal lung tissue and lung adenocarcinomas. Thorax. 2005;60:576–81. doi: 10.1136/thx.2004.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu K, Li L, Nisson PE, Gruber C, Jessee J, Cohen SN. Reversible tumorigenesis induced by deficiency of vasodilator-stimulated phosphoprotein. Mol Cell Biol. 1999;19:3696–703. doi: 10.1128/mcb.19.5.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasegawa Y, Murph M, Yu S, Tigyi G, Mills GB. Lysophosphatidic acid (LPA)-induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells. Mol Oncol. 2008;2:54–69. doi: 10.1016/j.molonc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deguchi A, Soh JW, Li H, Pamukcu R, Thompson WJ, Weinstein IB. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol Cancer Ther. 2002;1:803–9. [PubMed] [Google Scholar]


