Abstract
Although human papillomavirus (HPV)+ oropharyngeal cancers often present with metastasis, most patients have excellent long-term survival. The reason underlying such an apparent contradiction remains unclear, but we have recently demonstrated that the improved survival of HPV+ oropharyngeal cancer patients has an immunological component, as the levels of tumor-infiltrating lymphocytes (TILs) can be used to stratify HPV+ patients into high-risk and low-risk groups.
Keywords: human papillomavirus, oropharyngeal cancer, prognosis, survival, tumor-infiltrating lymphocytes
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) in the Western world has significantly increased, and only in recent times has human papillomavirus type 16 (HPV-16) been identified as the principal causative agent of this neoplasm, presently accounting for around 40–80% of cases.1
Although HPV+ OPSCCs often present at advanced stage with regional lymph node metastasis, studies report significantly better long-term survival for most HPV+ OPSCC patients compared with those affected by HPV− (tobacco-related) disease.1 The reason for this survival advantage is unclear, and may be multifactorial, perhaps linked to the retained expression of wild-type p53 (TP53) and retinoblastoma 1 (Rb1) tumor suppressor genes, or the absence of field cancerization.1 Of note, the improved survival of HPV+ OPSCC patients appears to be independent of treatment modality (be it surgery, radiotherapy, chemotherapy, or combinations thereof),1,2 raising the possibility that some of these patients receive unnecessary treatment. Based on these observations, various clinical trials are currently testing treatment de-escalation, in order to reduce therapy-related morbidity. However, a minority of HPV+ OPSCC patients respond poorly to treatment and have a poor prognosis. It has been suggested that future clinical investigations focus on this patient group, in order to improve outcome (and to avoid treatment de-intensification in this setting). Although smoking has been shown to reduce the survival benefit of individuals with HPV+ OPSCC patients,3 there is as yet, no single clinical or morphological feature for identifying those HPV+ OPSCC patients who will have poor disease outcome.
We hypothesized that the difference in survival between HPV+ and HPV− patients is due to an adaptive immune response directed against the viral antigens expressed by tumor cells. The abundance of tumor-infiltrating lymphocytes (TIL) has been shown to be a predictor of positive disease outcome in several tumor types, particularly colorectal cancer, suggesting that the adaptive immune system plays a role in suppressing tumor progression. Importantly, immune responses against foreign viral antigens are less likely to be suppressed since there is no central immunological tolerance to confound the immune system’s attempts to control cancer. We therefore conducted a study to examine the effects of HPV status and TIL levels on the survival of OPSCC patients using cohorts from independent clinical centers.
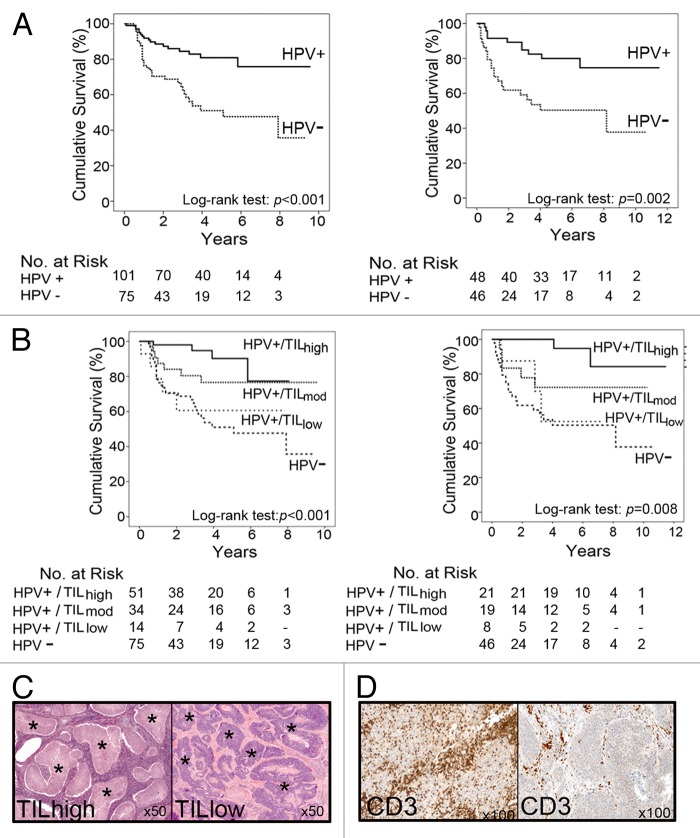
We recently reported that HPV+ OPSCCs are associated with significantly improved survival (3-y survival rate of combined cohorts: 79% vs. 51% for HPV+ and HPV− OPSCC patients, respectively; P < 0.001).4 We found that most (85%) of HPV+ tumors contain high or moderate levels of TILs, and that TIL levels stratify HPV+ OPSCC patients into those with good and poor prognosis (Fig. 1). The 3-y survival rate (of combined cohorts) for patients with HPV+/TILhigh tumors was 94%, compared with 72% for HPV+/TILmod and 56% for HPV+ /TILlow tumors. Of note, the survival of individuals with HPV+/TILlow tumors was similar to that of patients with HPV− OPSCCs (3-y survival rate 51%). We also quantified the densities of CD3+, CD4+, CD8+ and Foxp3+ T cells and performed receiver operating characteristic (ROC) analyses to determine the predictive value of the absolute or relative levels of these TIL subsets. These more complex analyses did not outperform the simple quantification of TIL performed on hematoxylin and eosin (H+E)-stained sections. Several groups have reported that heavy smoking reduces survival benefit in HPV+ OPSCC patients. This was reflected in our prognostic model, developed using a “training” cohort from one center, where the combination of TIL levels, heavy smoking, and T-stage resulted in a statistically significant prediction (area under the ROC curve, AUROC = 0.87). This model was validated on patients from the other centers (detection rate = 67%; false-positive rate = 5.6%; AUROC = 0.82).
Figure 1. Disease-specific survival of oropharyngeal squamous cell carcinoma (OPSCC) patients from independent cohorts. (A) Kaplan–Meier curves depicting the disease-specific survival of OPSCC patients stratified according to human papillomavirus (HPV) status (P < 0.001, P = 0.002, respectively, as per log rank test). (B) Kaplan–Meier curves depicting the disease-specific survival of OPSCC patients stratified according to HPV status and tumor-infiltrating lymphocyte (TIL) levels. The survival of patients with HPV+/TILlow tumors is similar to that of HPV− patients. (C) Hematoxylin and eosin (H+E)-stained sections showing examples of OPSCC with high and low TIL levels. Tumor islands are marked with an asterix. In TILhigh OPSCCs, lymphocytes fill the stromal compartment and infiltrate into tumor islands. (D) Immunochemistry showing typical examples of HPV+ OPSCCs with high and low infiltrates of CD3+ T cells.
Our findings suggest that the improved survival of most HPV+ OPSCC patients results from an antitumor immune response, indicated by the presence of TIL. HPV preferentially targets the reticulated epithelium that lines tonsillar crypts, and it could be argued that the abundant lymphocytic infiltrate seen in most HPV+ OPSCCs simply reflects the anatomical location of the tumor. However, our analysis of a publically available head and neck squamous cell carcinoma (HNSCC) microarray data set revealed enrichment of genes associated with CD8+ T-cell effector functions in patients with improved survival, arguing that the T cells are not simply “innocent bystanders’’ of the biological events in the tumor, but play an active role in tumor recognition. HPV-16-specific T cells have been isolated from HPV+ OPSCCs,5 and E6/E7 seropositivity has been reported to be a favorable prognostic factor in HPV+ OPSCC patients.6 The impact of the immune system may also explain why improved patient survival is seen independent of treatment modality in several studies, including our own.
If most HPV+ OPSCC are recognized by the immune system, the question is raised as to how tumors develop in the first place. The HPV proteins E5 and E7 have been reported to downregulate expression of MHC class I on the cell surface,7,8 thereby limiting antigen recognition by CD8+ T cells. In addition, several studies have shown that a degree of immunological tolerance may exist within the tonsils, which contain Foxp3+CD8+ T lymphocytes with a regulatory T cell (Treg) phenotype, as well as lymphocytes expressing CD28 family members, cytotoxic T lymphocyte-associated protein 4 (CTLA4), inducible T-cell co-stimulator (ICOS) and programmed cell death protein 1 (PDCD1, best known as PD-1).9,10 We can speculate that surgery- or chemoradiotherapy-induced inflammation promotes the interferon γ (IFNγ)-dependent upregulation of MHC class I molecules or disrupts the immunosuppressive environment of OPSCC lesions in favor of an immunostimulatory one.
There is much interest in de-intensifying the treatment of HPV+ OPSCC patients, in particular for avoiding the morbidity associated with standard chemotherapeutic regimens. Our data suggest that most of these patients have a pre-existing (albeit ineffective) antitumor immune response. Thus, the administration of antibodies targeting molecules involved in the co-stimulation or co-inhibition of T cells including CTLA4, PD-1, PD-L1 and CD40, perhaps combined with a therapeutic anti-HPV-16 vaccine, may provide a useful therapeutic strategy for patients with HPV+ OPSCCs. It will be interesting to determine whether an effective immune response can be generated by this approach in TILlow OPSCC patients. However, evaluation of TIL levels is an effective tool for identifying the HPV+ OPSCC patients who are likely to respond poorly to conventional treatments, making it highly relevant for clinical decision making.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Cancer Research UK, The Health Foundation and the NIHR Cancer Research Network.
Citation: King EV, Ottensmeier CH, Thomas GJ. The immune response in HPV+ oropharyngeal cancer. OncoImmunology 2013; 2:e27254; 10.4161/onci.27254
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27254
References
- 1.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D’Souza G, Gravitt PE, Westra W, Psyrri A, Kast WM, Koutsky LA, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009;31:1393–422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 2.Hong AM, Dobbins TA, Lee CS, Jones D, Harnett GB, Armstrong BK, Clark JR, Milross CG, Kim J, O’Brien CJ, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2010;103:1510–7. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, Webb A, Hampton C, Patel NN, Randall CJ, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2013 doi: 10.1038/bjc.2013.639. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, Smit VT, Langeveld AP, Jansen JC, van der Burg SH. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131:E74–85. doi: 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 6.Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, Clark JR, Wein RO, Grillone GA, Houseman EA, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72:5004–13. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai Y, Okazaki T, Nishimura H, Kawasaki A, Yagita H, Honjo T. Microanatomical localization of PD-1 in human tonsils. Immunol Lett. 2002;83:215–20. doi: 10.1016/S0165-2478(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 8.Siegmund K, Rückert B, Ouaked N, Bürgler S, Speiser A, Akdis CA, Schmidt-Weber CB. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J Immunol. 2009;182:2124–30. doi: 10.4049/jimmunol.0802271. [DOI] [PubMed] [Google Scholar]
- 9.Bottley G, Watherston OG, Hiew YL, Norrild B, Cook GP, Blair GE. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene. 2008;27:1794–9. doi: 10.1038/sj.onc.1210798. [DOI] [PubMed] [Google Scholar]
- 10.Ashrafi GH, Haghshenas MR, Marchetti B, O’Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113:276–83. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]