Abstract
Glioblastoma is a deadly brain cancer with limited treatment options. Targeting chondroitin sulfate proteoglycan 4 (CSPG4, best known as NG2) with the monoclonal antibody mAb9.2.27 and activated natural killer (NK) cells abrogated the tumor growth and prolonged the survival of glioblastoma-bearing animals by favoring the establishment of a pro-inflammatory microenvironment. The combination of NK cells and mAb9.2.27 recruited ED1+CCR2low macrophages that stimulated ED1+ED2lowMHCIIhigh microglial cells to exert robust cytotoxicity. Our findings demonstrate the therapeutic potential of targeting salient tumor associated-antigens.
Keywords: CNS immunosurveillance, CSPG4, glioblastoma, NK cells, passive immunotherapy
Glioblastoma (GBM) is the most frequent primary tumor of the brain in adults. The median survival of GBM patients is 14.6 mo, 1 despite aggressive multimodal therapy. Thus, there is an urgent need for novel therapies for the treatment of GBM. One promising approach in this sense is represented by approaches that target functionally validated tumor-associated antigens (TAAs). Chondroitin sulfate proteoglycan 4 (CSPG4, best known as NG2) is involved in several processes that favor GBM progression and high NG2 expression levels have been shown to negatively impact disease outcome. 2 - 5 As a proteoglycan on the cells surface, NG2 is amenable to antibody-based immunotherapy.
Although the immunoprivileged status of the nascent brain has been contested, there is a general consensus that GBM establishes multiple, coordinated mechanisms of escape from immunosurveillance, 6 both de novo and as result of treatment. GBM cells express high levels of MHC class I molecules, that are ligands for inhibitory killer immunoglobulin-like receptors (KIRs), thus inhibiting the antineoplastic activity of natural killer (NK) cells. In the GBM microenvironment, the cytokine balance is skewed toward the elicitation of anti-inflammatory TH2 responses and cancer cells mainly recruit immune cells with immunosuppressive properties. Although NK cells are not abundant within GBM lesions, representing 2.11 ± 0.54% of all lymphocytes, they exhibit the unusual CD56dimCD16- phenotype 6 . Moreover, GBM-infiltrating NK cells generally expressed activating killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) receptors, which recognize stress ligands expressed by malignant cells. 6 Pellegata and colleagues observed an increase in circulating, interferon γ (IFNγ)-producing NK cells that was associated with improved survival in GBM patients subjected to dendritic cell-based vaccination. 7 Thus, anticancer treatments may activate endogenous NK cells against GBM. Taken together, these findings suggest that adoptively transferred, allogeneic NK cells may constitute a therapeutic option for GBM patients. Nevertheless, the therapeutic potential of purified NK cells against solid tumors has not been fully explored yet.
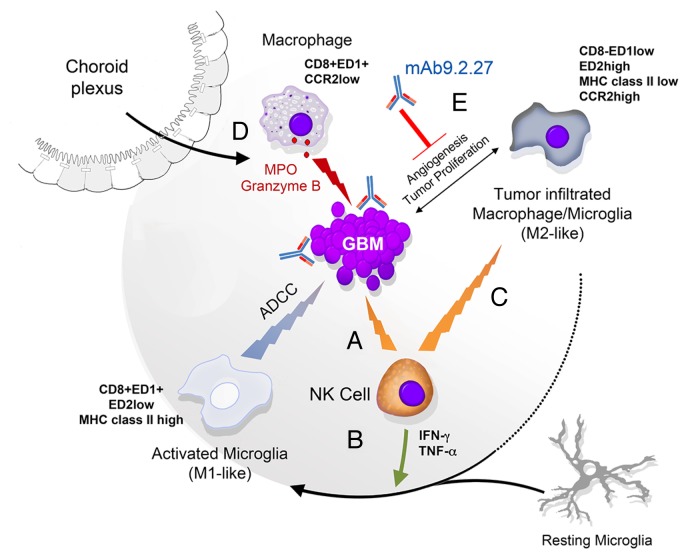
We have recently demonstrated that combining activated NK cells with a monoclonal antibody targeting NG2 (mAb9.2.27) inhibits tumor growth and prolongs the survival of GBM-bearing rats. 8 The administration of NK cells plus mAb9.2.27 resulted in the recruitment to neoplastic lesions of pro-inflammatory ED1+CCR2low macrophages via the choroid plexus and blood vessels (Fig. 1). These macrophages differentiated into and/or drove the differentiation of ED1+ED2lowMHCIIhigh microglial cells that exerted robust cytotoxic effects against GBM cells. The depletion of ED1+CCR2low macrophages abrogated the therapeutic effect of NK cells plus mAb9.2.27 while promoting the accumulation of tumor-associated macrophages (TAMs) exhibiting an ED2+CCR2high anti-inflammatory phenotype as well as the differentiation of ED2high microglial cells that promoted GBM survival ex vivo. 8 Remarkably, mAb9.2.27 reversed the tumor-promoting effects of TAMs derived from a GBM biopsy or tumor xenografts. However, the ability of NK cells to kill GBM cells was not augmented by mAb9.2.27 in vitro, indicating that the major role of NK cells in this setting is to secrete cytokines. Indeed, NK cells produced pro-inflammatory TH1 cytokines including IFNγ and tumor necrosis factor α (TNFα) upon exposure to GBM cells in vitro. Of note, the amounts of IFNγ and TNFα were increased in the cerebral spinal fluid of rats receiving NK cells plus mAb9.2.27, whereas the levels of other (immunosuppressive) cytokines such as interleukin (IL)-10, IL-6, and IL-1β were diminished. IFNγ increased the cytotoxic activity of the microglia against GBM cells in vitro. The synergistic interaction between NK cells and mAb9.2.27 might therefore originate from the combined effects of NK cell-derived IFNγ and mAb9.2.27 on the cytotoxic activity of microglial cells.
Figure 1. Adoptively transferred natural killer cells as initiators of glioblastoma regression. The intracranial administration of activated natural killer (NK) cells promotes cellular-dependent cytotoxicity against glioblastoma (GBM) (A) The NK cell-GBM cell interaction leads to the secretion of interferon γ (IFNγ) and tumor necrosis factor α (TNFα) by NK cells, in vitro and in vivo (B) IFNγ and TNFα can activate the resting microglia and/or anti-inflammatory tumor-associated macrophages (TAMs) to promote the establishment of pro-inflammatory conditions, ex vivo. Such a pro-inflammatory environment is maintained by NK cells, which kill anti-inflammatory microglial cells (C) Activated microglial cells and/or macrophages mediate natural as well as antibody-dependent cytotoxic functions, for instance upon the binding of mAb9.2.27 to NG2+ GBM cells (D) Moreover, mAb9.2.27 inhibits tumor growth and angiogenesis, mainly in this rat tumor model as it blocks the tumor-supporting functions of TAMs and microglial cells (E).
Our study did not elucidate the molecular mechanisms mediating the conversion of TAMs from tumor-supporting ED2highCCR2high cells to ED1+ED2lowCCR2low cells exhibiting robust pro-inflammatory activity. This process may be regulated by miR-124, which has previously been shown to regulate the activation of microglial cells and macrophages in the central nervous system. 9 It is therefore conceivable that the pro-inflammatory environment established by NK cells plus mAb9.2.27 modulates miR-124, promoting the activation of macrophages and microglial cells as well as their maintenance in a classically activated phenotype.
Our findings support the notion that the immunomodulation properties of NK cells, and notably (1) their ability to secrete pro-inflammatory cytokines, and (2) their capacity to influence the activity of the microglia and macrophages, may be exploited to boost the efficacy of passive immunotherapies targeting validated TAAs. One novelty of our approach related to the use of purified NK cells for the treatment of GBM, as previous attempts near-to-invariably employed autologous lymphokine activated killer (LAK) cells, which are a mixture of NK and T cells. The T-cell component of LAKs may actually generate immunosuppressive regulatory T cells (Tregs), which would limit the therapeutic efficacy of such an approach.
The use of mAb9.2.27 as a standalone therapeutic intervention induced temporary tumor regressions, presumably due to the immunoediting of NG2-expressing cells. Thus, simultaneously using monoclonal antibodies that are specific for several TAAs might reduce the selection of antigen loss tumor variants. Nevertheless, combining mAb9.2.27 with NK cells converted the tumor-promoting, anti-inflammatory microenvironment into a setting that allowed for therapeutically relevant tumor-specific immune responses. The selection of donors with NK cells expressing particular activating KIRs cognate to the MHCligands expressed by the patient GBM cells might further enhance the therapeutic potency of NK cells against GBM. However since NK cells may induce thrombocytopenia, and activated microglia are implicated in several neurological diseases, the safety, tolerable doses and duration of such a therapeutic approach requires stringent evaluations. Although it is desirable to study heterogeneous, patient-derived GBMs that pose significant therapeutic challenges, the role of Tregs might have been largely underestimated in studies relying on immunocompromised models. As NG2 is expressed on both malignant and angiogenic pericytes, humanized and/or bispecific monoclonal antibodies that recognize NG2 epitopes on both these cell compartments may induce antibody-dependent cell-mediated cytotoxicity (and hence mediate tumor destruction) more efficiently than molecules targeting either compartment alone. Furthermore, mAb9.2.27 may render cancer cells more susceptible to adjuvant chemo- and radiotherapy, as we have previously demonstrated that NG2 signaling augments chemo and radioresistance in GBM cells. 4 , 5 Moreover, the therapeutic potential of NK cells might be further enhanced by the co-administration of multiple agents, such as the proteasome inhibitor bortezomib, which not only sensitized GBM cells to death receptor-dependent apoptosis, but also stimulates the expression of stress-induced ligands. Additional NK cell-stimulatory agents include ligands for activatory receptors, anti-KIR antibodies, Toll-like receptor ligands and CpG oligodeoxynucleotides. 10 In summary, NK cells plus mAb9.2.27 converted the anti-inflammatory GBM microenvironment into a pro-inflammatory one dominated by M1-like macrophages and microglial cells that mediated tumor rejection. Our findings demonstrate the potential of targeting the tumor microenvironment as a means to stimulate endogenous immune responses and exert therapeutic effects against GBM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from The Bergen Medical Research Foundation, Meltzer Fund, The Norwegian Research Council (FRIFORSK) and The Norwegian Cancer Society.
Citation: Kmiecik J, Gras Navarro A, Poli A, Zimmer J, Chekenya M. Combining NK cells and mAb9.2.27 to combat NG2-dependent and anti-inflammatory signals in glioblastoma. OncoImmunology 2013; 2:e27185; 10.4161/onci.27185
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27185
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mayhani MT, Grenfell R, Narita M, Piccirillo S, Kenney-Herbert E, Fawcett JW, Collins VP, Ichimura K, Watts C. NG2 expression in glioblastoma identifies an actively proliferating population with an aggressive molecular signature. Neuro Oncol. 2011;13:830–45. doi: 10.1093/neuonc/nor088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chekenya M, Hjelstuen M, Enger PO, Thorsen F, Jacob AL, Probst B, Haraldseth O, Pilkington G, Butt A, Levine JM, et al. NG2 proteoglycan promotes angiogenesis-dependent tumor growth in CNS by sequestering angiostatin. FASEB J. 2002;16:586–8. doi: 10.1096/fj.01-0632fje. [DOI] [PubMed] [Google Scholar]
- 4.Chekenya M, Krakstad C, Svendsen A, Netland IA, Staalesen V, Tysnes BB, Selheim F, Wang J, Sakariassen PØ, Sandal T, et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–94. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svendsen A, Verhoeff JJ, Immervoll H, Brøgger JC, Kmiecik J, Poli A, Netland IA, Prestegarden L, Planagumà J, Torsvik A, et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011;122:495–510. doi: 10.1007/s00401-011-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, Zimmer J, Chekenya M. Elevated CD3(+) and CD8(+) tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E, Cuppini L, Cuccarini V, et al. The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology. 2013;2:e23401. doi: 10.4161/onci.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poli A, Wang J, Domingues O, Planagumà J, Yan T, Rygh CB, Skaftnesmo KO, Thorsen F, McCormack E, Hentges F, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–46. doi: 10.18632/oncotarget.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kmiecik J, Zimmer J, Chekenya M. Natural killer cells in intracranial neoplasms: presence and therapeutic efficacy against brain tumours. J Neurooncol. 2013;116:1–9. doi: 10.1007/s11060-013-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]