Abstract
The Caenorhabditis elegans heterochronic gene pathway, which consists of a set of regulatory genes, plays an important regulatory role in the timing of stage-specific cell lineage development in nematodes. Research into the heterochronic gene pathway gave rise to landmark microRNA (miRNA) studies and showed that these genes are important in stem cell and cancer biology; however, their functions in vertebrate development are largely unknown. To elucidate the function of the heterochronic gene pathway during vertebrate development, we cloned the zebrafish homologs of the C. elegans let-7 miRNA-binding protein, Lin-28, and analyzed their function in zebrafish development. The zebrafish genome contains two Lin28-related genes, lin-28a and lin-28b. Similar to mammalian Lin28 proteins, both zebrafish Lin-28a and Lin-28b have a conserved cold-shock domain and a pair of CCHC zinc finger domains, and are ubiquitously expressed during early embryonic development. In a reciprocal fashion, the expression of downstream heterochronic genes, let-7 and lin-4/miR-125 miRNA, occurred subsequent to lin-28 expression. The knockdown of Lin-28a or Lin-28b function by morpholino microinjection into embryos resulted in severe cell proliferation defects during early morphogenesis. We found that the expression of let-7 miRNA was upregulated and its downstream target gene, lin-41, was downregulated in these embryos. Interestingly, the expression of miR-430, a key regulator of maternal mRNA decay, was downregulated in lin-28a and lin-28b morphant embryos, suggesting a role for Lin-28 in the maternal-to-zygotic transition in zebrafish. Taken together, our results suggest an evolutionarily conserved and pivotal role of the heterochronic gene pathway in early vertebrate embryogenesis.
Introduction
The spatial and temporal coordination of gene expression during development is essential for the correct morphogenesis of animals. Genetic studies of Caenorhabditis elegans (C. elegans) have provided significant insight into the evolutionarily conserved networks of gene regulation that underlie development [1]. The C. elegans heterochronic gene pathway, the study of which led to the landmark discovery of the first microRNA (miRNA)-mediated gene regulatory network during development, was also discovered through conventional forward genetic screens in C. elegans for heterochronic (developmental timing) mutants [2]–[6].
miRNAs are a class of post-transcriptional regulatory noncoding small RNAs (18–25 nucleotides in length) that bind specific target sequences, usually located in the 3′-untranslated region (UTR) of regulated mRNAs, and block their translation and/or initiate their degradation [7], [8]. In vertebrate miRNA biogenesis, the primary transcripts of miRNA genes (pri-miRNAs) are cleaved into short hairpin intermediates (pre-miRNAs) by the nuclear RNase III Drosha and the Dgcr8 complex, and are further processed to mature miRNAs by the cytosolic RNase III-related enzyme Dicer. Then, the mature miRNA molecule binds to an RNA-induced silencing complex, which regulates protein expression [9].
The functional significance of miRNAs in vertebrate development has been clearly demonstrated using Dicer mutants [10], [11]. In Danio rerio (zebrafish), embryos deficient in maternal and zygotic Dicer activity (MZ dicer mutants) cannot generate mature miRNAs and display severe morphogenetic defects [12]. For example, MZ dicer mutants develop more slowly than wild-type embryos and have reduced extension of the body axis, resulting in shortening of the embryo and morphological malformation of the heart and brain [12]. Interestingly, the injection of miR-430, which has a crucial function in deadenylation and the clearance of maternal mRNAs, rescues these brain defects, suggesting a critical role for miR-430 in early zebrafish development [12].
In the C. elegans heterochronic pathway, let-7 and lin-4/miR-125 miRNA play an essential regulatory role in the timing of stage-specific cell lineage development in nematodes, in part by directly regulating their target genes [5], [6], [13], [14]. For example, lin-4/miR-125 miRNA promotes the transition between the first and second larval stages (L1 and L2, respectively) by binding to complementary sites in the 3′UTR of the upstream heterochronic genes lin-14 and lin-28 [3], [6], [15]–[17]. Since LIN-28 prevents the premature accumulation of let-7 miRNA in L2, let-7 miRNA expression occurs during the third larval stage (L3) and controls the fourth larval stage (L4)-to-adult transition by repressing multiple target genes, including the TRIM protein Lin-41 [6], [18], [19]. Interestingly, recent works have shown that several members of the heterochronic pathway are highly conserved by sequence and play a critical role in humans and mice [20]–[22]. However, it is unknown whether these heterochronic homologs function to regulate the timing of specific developmental events in vertebrates.
In this study, to elucidate the function of the heterochronic pathway during vertebrate development, we used zebrafish as an animal model to investigate the role of Lin-28 during development. We cloned two zebrafish homologs of lin-28, lin-28a and lin-28b, and analyzed their functions in development. Zebrafish lin-28a and lin-28b were ubiquitously expressed in early embryonic development, and their expression decreased at 12 h post-fertilization (hpf) and 24 hpf, respectively. On the other hand, the expression of let-7 and lin-4/miR-125b miRNA, a downstream heterochronic gene of lin-28, was expressed subsequent to lin-28b expression. The knockdown of Lin-28a or Lin-28b function by morpholino (MO) microinjection resulted in severe cell proliferation defects during early morphogenesis, and the expression of both let-7 and lin-41 was modulated. Interestingly, a microarray-based analysis of miRNAs showed that miR-430 expression was inhibited in lin-28a and lin-28b morphant embryos, suggesting that the clearance of maternal mRNAs was affected in these morphants. These results suggest an evolutionarily pivotal role of Lin-28 for the heterochronic pathway in early vertebrate embryogenesis, and provide novel insight into Lin28 function.
Materials and Methods
Fish Handling and Care
Wild type zebrafish strains AB were maintained according to The zebrafish book: A Guide for the Laboratory Use of Zebrafish. All animals were handled in strict accordance with good animal practice as defined by national and local animal welfare bodies, and all efforts were made to minimize suffering. All animal work was approved by the the Committee on the Ethics of Animal Experiments and the Institutional Animal Care and Use of Chubu University; serial permit numbers were not necessarily assigned to fish experiments in Chubu University.
Genomic Analysis
We used the VISTA Web server to align genomic sequences (http://www.gsd.lbl.gov/VISTA/). All the genomic sequences were found by Ensembl and ZFIN database search and the coordinates are as follows: Homo sapiens (human) LIN28A, chromosome 1, 26,737,269–26,756,213 bp, human LIN28B, chromosome 6, 105404923–105531207 bp, Gallus gallus (Chicken) LIN28A, chromosome 23, 148,871–160,164 bp, Gallus gallus LIN28B, chromosome 3, 71550790–71626325 bp, Mus muculus (Mouse) Lin28A, chromosome 4, 134,003,330–134,018,841 bp, Mouse Lin28B, chromosome 10, 45,376,620–45,470,201 bp, Danio rerio (zebrafish) lin-28a, chromosome 19, 14873056–14901221 bp, Danio rerio lin-28b, chromosome 20, 47,677,565–47,707,773 bp.
Transposable elements were masked prior to submission using the RepeatMasker program, and the annotated mouse sequence was used as the base reference track. Pair-wise sequence comparisons were calculated with a threshold of 70% identity in a 10-bp window, with a 0% minimum identity shown.
Cloning and RT-PCR
RT-PCR was performed as previously described [27]. Briefly, total RNA was purified from zebrafish embryos (TRIzol reagent; Invitrogen-Gibco, Carlsbad, CA), and cDNA was synthesized (Superscript II; Invitrogen-Gibco). The primer sets were tested over a range of thermal cycles using ExTaq (Takara, Shiga, Japan), and the semiquantitative cycle number was determined for each primer set. Bands were visualized with ethidium bromide. The primer sequences used for the PCR were shown 5′ to 3′:
Lin41Fw: 5′-ATGGCTTCGTTTCCAGACTC-3′, Lin41Rv: 5′-TCATGTCCCTCACATTCTAC-3′, Lin28AFw: 5′-TAAAGATGCCCCCGGCAAAT-3′, Lin28ARv: 5′-CTCTGCTAATCAGTGCTCTC-3′, Lin28ARv2: 5′-GAGCCGTGAAAAGAGCCTGA-3′(For knockdown analysis), Lin28BFw: 5′-TTCGCTGGAACTTTGGAACG-3′, Lin28BRv: 5′-TTCAGTCCCGGCTCTTTCTC-3′, Lin28BRv2: 5′-TGCGGACATTGAACCACTTG-3′ (For knockdown analysis), Sox2Fw: 5′-AAGGAACACCCGGATTACAA-3′, Sox2Rv: 5′-TCATGTCAGCCTTTGCAGAA-3′, Otx2Fw: 5′-ATGATGTCGTATCTCAAGCA-3′, Otx2Rv: 5′-CTTGGTCCTTATAATCCAAG-3′, gapdhFw: 5′-CTGCCAAGGCTGTGGGCAAG-3′, gapdhRv: 5′-TTACTCCTTGGAGGCCATGT -3′,
The amplified PCR products were subcloned into pCS2+ or pGEM-T easy (Promega) vector and confirmed by sequencing analysis. Amino acid sequence comparisons and phylogenic tree analysis were carried out with APE and Jalview software. The sequence was submitted to GenBank (Accession number; AB828400).
Whole Mount in situ Hybridization
Whole-mount in situ hybridization was done as previously described [28]. Digoxigenin (DIG)-labeled sense and antisense RNA probes were made by in vitro transcription with T7 or SP6 RNA polymerase (Roche Diagnostics GmbH, Mannheim, Germany), using templates generated by PCR. Embryos were fixed in 4% paraformaldehyde (PFA) and stored in methanol. Rehydrated embryos were sequentially treated with Proteinase K and then acetylated in acetic anhydrate solution. The pretreated samples were next hybridized at 60°C with DIG-labeled RNA probe. The probe was detected with an anti-DIG antibody-conjugated to alkaline phosphatase (Roche Diagnostics GmbH, Mannheim, Germany) and visualized by using a BCIP/NBT solution kit (Nacalai Tesque Inc.).
Reverse Transcription and Real-time PCR Quantification of miRNA
For miRNA quantification, cDNA was synthesized from total RNA using gene-specific primers according to the TaqMan MicroRNA Assay protocol as per the manufacturer’s protocol (Applied Biosystems). Quantitative PCR of miRNA was performed using an Applied Biosystems 7300 Sequence Detection system. The 10 µl PCR reaction contained 0.67 µl RT product, 1 × TaqMan Universal PCR master mix, and 1 µl of the primer and probe mix, according to the TaqMan MicroRNA Assay protocol (Applied Biosystems). The threshold cycle data were determined using default threshold settings. The threshold cycle was defined as the fractional cycle number at which the fluorescence exceeded the fixed threshold. Gapdh or miR-26a were used as internal control.
Microinjection of Morpholinos or rescueRNA into Zebrafish Embryos
Antisense oligonucleotide Morpholinos (MO) were designed and obtained from Gene Tools, LLC. The lin-28a and lin-28b splicing MOs were designed splicing donor region of intron between exon 1 and exon 2. The sequences of the MO were shown 5′ to 3′: lin-28a MO: 5′-GAAGTTCTCACCTGTGTGATTGAGA-3′, lin-28b MO: 5′-AAAGCATCGTAACCTTCGGCCATGT-3′, Control MO: 5′-CCTCTTACCTCAGTTACAATTTATA-3′.
For RNA synthesis, capped sense RNAs were synthesized by using an mMessage mMachine in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer's instructions. The synthesized RNAs were diluted to an appropriate concentration with RNase-free water.
In all experiments, MOs and mRNAs were resuspended in H2O with Phenol red (0.05%). Fertilized eggs from wild-type zebrafish at the one- to two-cell stage were injected with approximately 1–3 nl of the solution (10 µM) by using a microinjector (IM-300; Narishige) as described elsewhere [29].
BrdU Labeling and Immunodetection
For BrdU incorporation, 4 hours postfertilization (hpf) embryos were treated with 10 mM BrdU in egg water for 60 minutes and washed two times with egg water, followed by 4% paraformaldehyde fixation overnight at 4°C. After gradual rehydration, embryos were incubated in 2N HCl for 1 hour at 37°C. After rinsing with PBS, they were permeabilized with 0.1% Triton X/PBS, washed with PBS, blocked with 2% BSA/PBS for at least 1 hour at room temperature, and incubated with rat anti-BrdU antibody (Roche) in 2% BSA/PBS overnight at 4°C. After washing three times with PBS for 5 minutes each, anti-BrdU antibody was visualized using secondary antibodies conjugated with Alexa Fluor 594 (Molecular Probes). Nuclei were counterstained with DAPI. Embryos were mounted in 1% agarose and confocal fluorescence images were obtained using a confocal microscope (FV-1000; Olympus).
Microarray Analysis
RNA was isolated using Trizol from control MO, lin-28a MO and lin-28b MO injected embryos at 5hpf. For miRNA expression analysis, 250 ng of total RNA was subjected to microarray analysis using a miRCURY LNA microRNA Array (Exiqon). Labeling, hybridization, washing, and scanning of the microarray were performed by Cosmo Bio Co., Ltd. (Tokyo, Japan), following the manufacturer’s instructions. Data analyses were performed using the Mev software (MultiExperiment Viewer). The experimental data used in this manuscript are freely available in the NCBI gene expression omnibus (GEO) repository with the accession number GSE52492.
Statistical Analysis
Data were analyzed by the two-tailed Student’s t-test. Values were expressed as mean ± SEM. Changes were deemed significant if the p-value was <0.05. Statistical significance is indicated as follows: * p<0.05, ** p<0.01, *** p<0.001, N.S., not significant.
Results
Cloning of Lin28 Homologs in Zebrafish
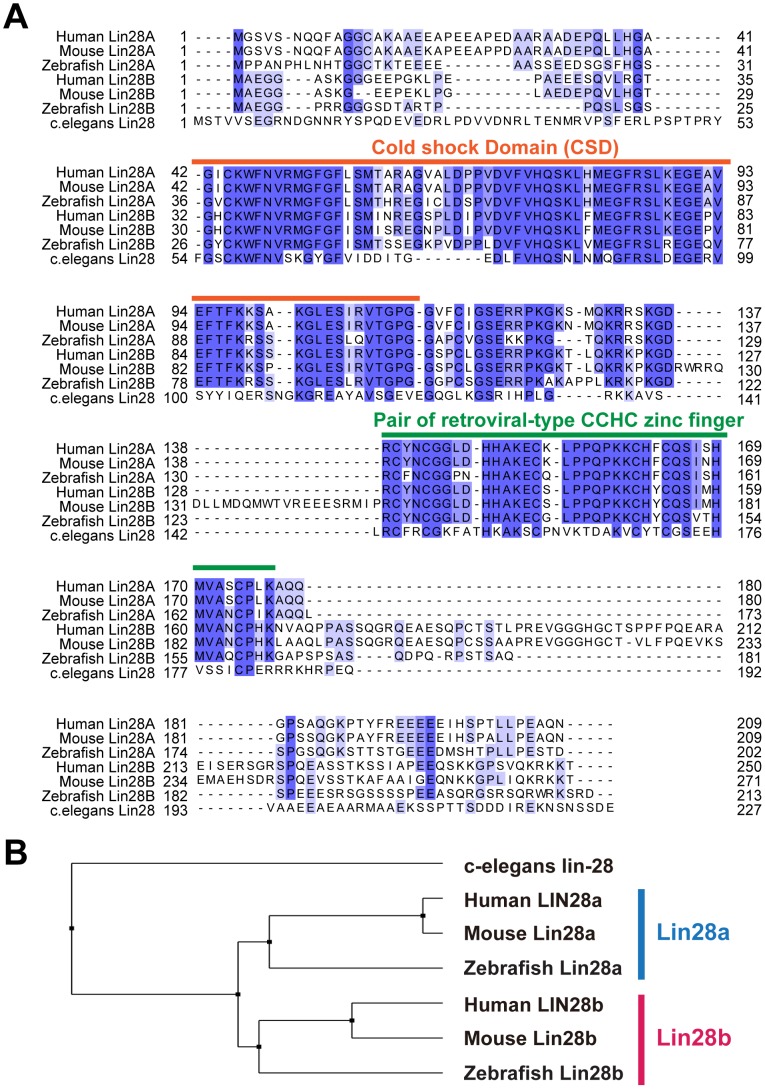
To analyze the developmental function of Lin28 using zebrafish as a model system, we first searched the zebrafish Ensembl Genome Server (www.ensembl.org/Danio_rerio/) for sequences homologous to human Lin28a and Lin28b, which were identified in mammalian genomes as two homologs of the C. elegans heterochronic gene lin-28, Lin28a and Lin28b [30], [31]. However, since the zebrafish Lin28 homolog was not fully assigned in the database, we only found one Lin28 homolog at Chromosome (Chr.)19 and a partially expressed sequence tag at Chr. 20. To clone and examine these genes, we first used VISTA genome alignment tools to align and compare the sequences of their corresponding genomic loci with those from other species, including human, rat, mouse, and chicken. As a result, we identified a highly conserved candidate genomic region of zebrafish lin-28a and lin-28b at Chr. 19 and Chr. 20, respectively (Figure S1A and B). Polymerase chain reaction (PCR) primers were designed using sequence information from the surrounding conserved exons and amplified PCR products were sequenced to obtain full-length zebrafish lin-28a and lin-28b. The deduced amino acid sequences of lin-28a and lin-28b encoded 202 and 213 amino acids, respectively. Two domains containing RNA-binding motifs, an N-terminal cold-shock domain and a pair of retroviral-type CCHC zinc fingers near the C-terminus, presumably play an important role in let-7 miRNA binding [32]. Since the cold-shock domain and retroviral-type CCHC finger regions of zebrafish Lin28A and Lin28B had 79–90% homology at the amino acid level with corresponding regions in mouse and human LIN28A and LIN28B (Figure 1A), the function of Lin-28a and Lin-28b may be conserved across animal phylogeny. Furthermore, a phylogenetic analysis suggested that these two Lin-28 proteins belong to the LIN28A and LIN28B groups (Figure 1B), as expected.
Figure 1. Cloning and characterization of zebrafish Lin-28a and Lin-28b.
A. Deduced amino acid sequences of zebrafish Lin-28a and Lin-28b aligned against human, mouse and c.elegans Lin28 homologs. Blue boxes represent identical and similar amino acids. The characteristic Lin28 structure including cold-shock domain and two CCHC-Zn finger domains was highly conserved in zebrafish Lin-28a and Lin-28b. B. The phylogeny of the Lin28 family based on the alignment of full length of amino acid sequence. The branch length (X axis) in the rectangular cladogram represent the distances among those sequences calculated using BLOSUM62 substitution matrix.
Expression Pattern of Heterochronic Genes during Zebrafish Development
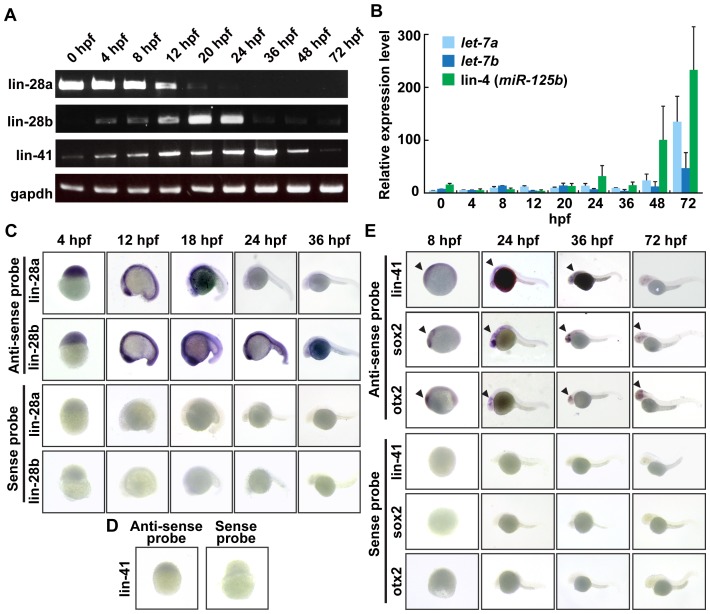
To characterize the expression pattern of zebrafish homologs of heterochronic genes (including lin-28, let-7, lin-41/TRIM71, and lin-4/miR-125b) in zebrafish development, reverse transcription-PCR (RT-PCR), TaqMan quantitative RT-PCR (qRT-PCR), and whole-mount in situ hybridization were performed on embryos at various developmental stages. First, RT-PCR analyses of lin-28a, lin-28b, and lin-41 mRNA expression during development showed strong lin-28a expression from the one-cell stage until 30% epiboly and then gradually decreased at 12 hpf, indicating that lin-28a is maternally expressed (Figure 2A). In contrast, lin-28b was expressed subsequent to lin-28a expression, and gradually increased after fertilization with a peak at 20 hpf and then decreased (Figure 2A). Faint lin-41 expression was first detected at 0 hpf; expression gradually increased to its peak at 36 hpf (Figure 2A). After 48 hpf, lin-41 expression began to decrease and was undetectable at 72 hpf (Figure 2A). Interestingly, consistent with decreased lin-28a and lin-28b expression, let-7a, let-7b, and lin-4/miR125b miRNA expression was dramatically increased at 72 hpf (Figure 2B).
Figure 2. Expression patterns of zebrafish heterochronic genes during embryonic development.
A. Semiquantitative RT-PCR analysis of lin-28a, lin-28b and lin-41 gene mRNA expression. Total RNA was isolated from wild-type embryos at the indicated developmental stages and analyzed by using specific primers for lin-28a, lin-28b and lin-41. gapdh was used as a control. B. Real-time PCR analysis of let-7a, let-7b and lin-4/miR-125b miRNA expression. Total RNA was isolated from wild-type embryos at the indicated developmental stages. Expression levels of let-7a, let-7b and lin-4/miR-125b was analyzed using TaqMan miRNA assay. C. Developmental expression analysis of zebrafish lin-28a and lin-28b mRNAs by whole mount in situ hybridization. Zebrafish embryos at various stages were hybridized with antisense probes. D, E. Developmental expression analysis of zebrafish lin-41 mRNAs by whole mount in situ hybridization. sox2 and otx2 probes were used as a CNS maker. Arrowhead indicated expression in the CNS.
We next examined the spatial distribution of lin-28a, lin-28b, and lin-41 mRNA in developing embryos by whole-mount in situ hybridization using DIG-labeled RNA as a probe. A hybridization signal for both lin-28a and lin-28b was observed in the entire embryonic region of eggs at 4 hpf, whereas a very faint or no lin-41 signal was detected (Figure 2C and Figure 2D). Later, while a lin-28b mRNA signal was observed throughout the entire body until 24 hpf, lin-28a expression disappeared from the caudal half of the embryos and was undetectable after 24 hpf (Figure 2C). At 36 hpf, we did not observe significant expression of lin-28a or lin-28b mRNA, suggesting that lin-28a and lin-28b play a role in early development. On the other hand, a relatively strong lin-41 mRNA signal began to appear in the anterior neural plate at 8 hpf, where the earliest neural molecular markers, sox2 and otx2, were expressed (Figure 2E). At 24–36 hpf, lin-41 was restricted to the entire anterior half of the embryo, where the sox2 and otx2 were also expressed, and become undetectable by 72 hpf (Figure 2E). These expression patterns suggest that an evolutionarily conserved heterochronic gene regulatory hierarchy is used even in vertebrate development.
Lin-28a and Lin-28b are Required for Proper Gastrulation in Zebrafish Embryos
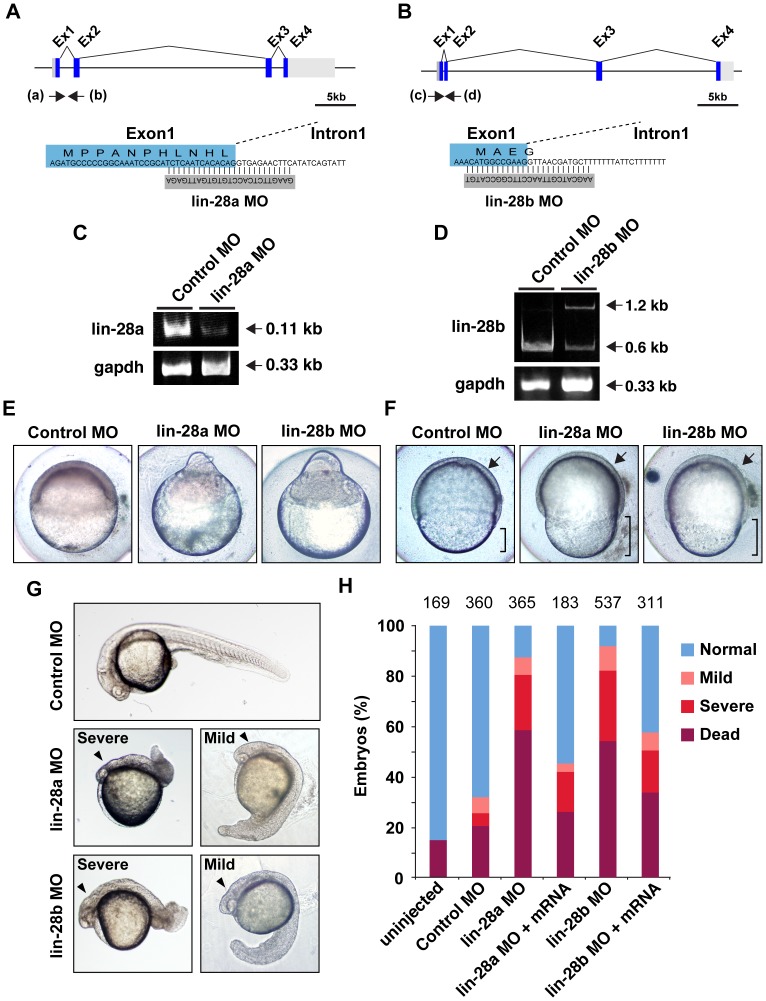
To examine the role of Lin-28a and Lin-28b in the development of zebrafish embryos, we designed and injected MO oligonucleotides that blocked the normal splicing of lin-28a and lin-28b in fertilized eggs (Figure 3A and Figure 3B). To confirm the knockdown, we performed RT-PCR using lin-28a- and lin-28b-specific primer sets that amplified normal or abnormal transcripts with exon skipping (Figure 3A and Figure 3B). In 3 ng of lin-28a MO-injected embryos, although a band corresponding to aberrant splicing was not observed, the morphants displayed a significant decrease in lin-28A transcripts (Figure 3C). Similarly, in lin-28b MO-injected embryos, a band corresponding to normal splicing (0.6 kb) was reduced by lin-28b MO (Figure 3D). In addition, a band corresponding to aberrant splicing (1.2 kb) was observed in the lin-28b MO-injected sample (Figure 3D). These results suggest that the lin-28a and lin-28b MOs effectively knocked down lin-28a and lin-28b in zebrafish. As we expected, the lin-28a and lin-28b morphants displayed severe growth retardation and reduced epiboly as compared to the controls (Figure 3E and Figure 3F). Morphants of the most severe phenotype, failed to initiate blastoderm epiboly (Figure 3E). As a result, approximately 40% of the injected embryos died around 12 hpf. Later, both the remaining lin-28a and lin-28b morphants displayed reduced extension of the body axis, resulting in shortened embryos and a reduced head size. At 30 hpf, both the remaining lin-28a and lin-28b morphant embryos exhibited a severe phenotype in which the embryo had a very small head and tail (Figure 3G). The survival rates and phenotype percentages at 30 hpf are shown in Figure 3H. Both MO groups had a significant increase in death and abnormal morphology relative to control MOs (Figure 3H; p<0.0001, control MO vs. lin-28a MO, control MO vs. lin-28b MO, Fisher’s exact test); 58.4% of lin-28a morphants and 54.6% of lin-28b morphants exhibited the dead phenotype, while the percentage of dead in the control was 20.3%.
Figure 3. Morpholino-mediated knockdown of lin-28a and lin-28b affects early development.
A. B. Schematic representation shows the genomic organization of the lin-28a and lin-28b genes in zebrafish. Regions targeted by splice-blocking morpholinos are shown. Arrows depict the location of the two primer sets to amplify exon and intron sequences, which were utilized in the RT-PCR analysis. C. D. The efficacy of MO was validated by RT-PCR using primers as indicated in A, B. Total RNA was isolated from lin-28a, lin-28b or control MO-injected embryos at 5 hpf and analyzed by using specific primers for lin-28a and lin-28b. Upper panel shows a reduced expression of lin-28a and lin-28b mRNA, and an increase of an aberrantly spliced lin-28b transcripts corresponding to the presence of intron 1. Lower panel shows RT-PCR products with gapdh primers used as internal control. E. Representative images of severe phenotype of lin-28a and lin-28b morphants at 5 hpf. F. Vegetal view of control, lin-28a and lin-28b morphants at 8 hpf. Arrow shows the similar extent of prechordal plate extension in control, lin-28a and lin-28b morphant embryos; bracket shows a reduced extent in epiboly in lin-28a and lin-28b morphant embryos compared with controls. G. Representatives of mild and severe phenotypes in lin-28a and lin-28b morphants at 30 hpf. Both lin-28a and lin-28b morphants develop morphological phenotypes displaying shorten body axis, small anterior structures and aberrant tail morphology ranging from mild (Right) to severe (Left) compare to control MO injected embryos (Top). Arrowheads indicate the smaller head. H. Quantification of the efficiency of rescue from gastrulation defects following co-injection of lin-28a MO, lin-28b MO and mRNAs. More than half of the lin-28a MO or lin-28b MO injected embryos had died by 30 hpf. The frequencies of the phenotypes were similar between lin-28a and lin-28b morphants. The frequency of the dead, mild and severe phenotypes decreased with a rescue experiment in which 300 pg of lin-28a and lin-28b mRNAs were co-injected with the MO. (p<0.0001, lin-28a MO vs. lin-28a MO+lin-28a mRNA, lin-28b MO vs. lin-28b MO+lin-28b mRNA, Fisher’s exact test). The total number of embryos is noted above each bar.
To ensure the specificity of the lin-28a and lin-28b MOs, we performed a rescue experiment. Co-injection of zebrafish lin-28a or lin-28b mRNA (300 pg) with the splice-blocking MO resulted in significant rescue of the gastrulation defect; the percentage of the “dead” phenotype was reduced from 58.4% in lin-28a MO-injected embryos to 25.7% and from 54.6% in lin-28b MO-injected embryos to 33.4% (Figure 3H). Moreover, the “severe” phenotype was also reduced in both mRNA co-injected embryos. This rescue experiment confirmed that both lin-28a and lin-28b are required for early morphogenesis in zebrafish.
Both Lin-28a and Lin-28b Regulate Cell Proliferation and Neural Expansion during Early Development
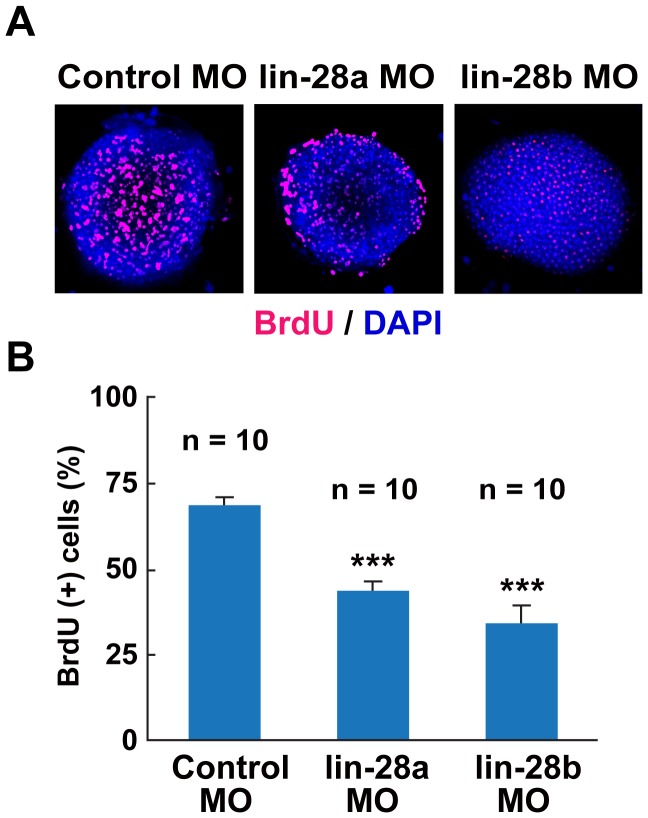
Lin28a and Lin28b play a critical role in the proliferation of various mammalian cells, including ES cells, and in the formation of iPS cells [23]–[25]. Thus, to characterize the severe growth retardation phenotype observed in the early stage of lin-28a and lin-28b morphants, we investigated cellular proliferation in these morphants using BrdU. Lin-28a or lin-28b morphant embryos were treated with BrdU egg water at 4 hpf for 1 h and the percentage of BrdU-positive cells was examined by immunohistochemistry (Figure 4A). We observed a significant reduction in BrdU incorporation in both lin-28a and lin-28b morphants as compared with the controls (Figure 4A and B). These findings indicate that the severe morphological defects observed in the lin-28a and lin-28b morphants may have resulted from impaired cellular proliferation.
Figure 4. Effect of lin-28a and lin-28b knockdown on cell proliferation.
A. Confocal images of BrdU (Red) incorporation in lin-28a and lin-28b morphant embryos at 5hpf. Both lin-28a and lin-28b MO-injected embryos were exposed to BrdU at 4 hpf for 1 hour. The nuclei were stained with DAPI (blue). B. Percentage of BrdU incorporated cells in lin-28a and lin-28b morphants embryos. The percentage of BrdU-incorporated cells was calculated by dividing the number of BrdU-positive cells by the total number of cells as determined by DAPI-stained cells. The percentage of BrdU-positive cells was significantly lower in both lin-28a MO- and lin-28b MO-injected embryos compared with control MO-injected embryos (***p<0.001, control MO vs. lin-28a MO, control MO vs. lin-28b MO, two-tailed test, n = 10, mean ± SEM).
Lin-28a and Lin-28b Regulate the Expression of Downstream Heterochronic Genes, as well as the miR-430 miRNA family
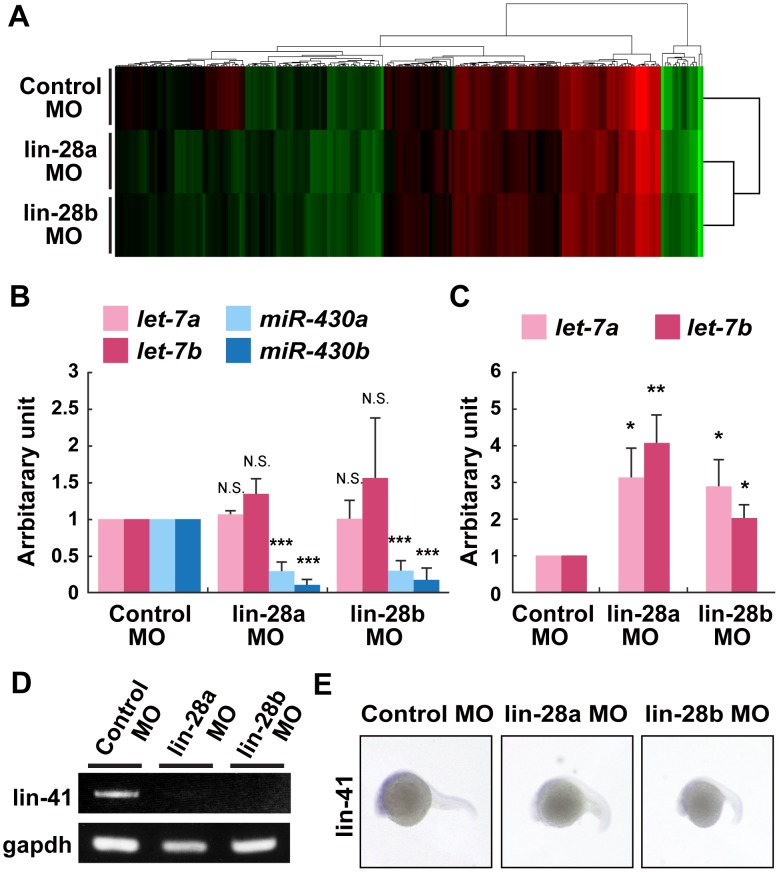
In the C. elegans heterochronic pathway, miRNAs, including let-7 and lin-4/miR-125, play a critical role as downstream genes of lin28. To determine the downstream events following lin-28 expression, we conducted an miRNA microarray analysis and examined the global changes in miRNA expression in lin-28a and lin-28b morphant embryos. Total RNA from control, lin-28a, and lin-28b MO-injected zebrafish embryos was isolated at 5 hpf and subjected to microarray analysis using an miRCURY LNA microRNA array for differential miRNA expression (Figure 5A). The heat map from the two-way hierarchical clustering analysis showed that most of the miRNAs on the microarray had a similar expression pattern among the samples, but that some of the miRNAs had substantial differences in their expression patterns to be clustered (Figure 5A). Additionally, the miRNA expression pattern in lin-28a MO-injected embryos clustered more closely with those in lin-28b MO-injected embryos than with those in control MO-injected embryos, suggesting that these two genes have comparable functions (Figure 5A).
Figure 5. Expression of miRNAs and downstream heterochronic genes in lin-28a and lin-28b morphant embryos.
A. miRNA expression profiling in lin-28a and lin-28b morphants embryos at 5 hpf. Hierarchically clustered heat-map representing differences in miRNA expression between control MO-injected embryos and lin-28a MO- or lin-28b MO-injected embryos. Relative levels of expression are colored from green (low) to red (high). B. Real-time PCR analysis of let-7a, let-7b, miR-430a and miR-430b expression in control MO-, lin-28a MO- and lin-28b MO-injected embryos. Expression levels of let-7a, let-7b, miR-430a and miR-430b were analyzed at 5 hpf using TaqMan miRNA assay. The expression level of miR-430a and miR-430b was downregulated in both lin-28a MO- and lin-28b MO-injected embryos compared with control MO-injected embryos (***p<0.001, control MO vs. lin-28a MO, control MO vs. lin-28b MO, two-tailed test, n = 3, mean ± SEM). C. Real-time PCR analysis of let-7a and let-7b expression in control MO-, lin-28a MO- and lin-28b MO-injected embryos. Expression levels of let-7a and let-7b were analyzed using TaqMan miRNA assay at 28 hpf. The expression level of let-7a and let-7b was increased in both lin-28a MO- and lin-28b MO-injected embryos embryos compared with control MO-injected embryos (*p<0.05, **p<0.01, control MO vs. lin-28a MO, control MO vs. lin-28b MO, two-tailed test, n = 4, mean ± SEM). D. Semiquantitative RT-PCR analysis of lin-41 gene expression in control MO-, lin-28a MO- and lin-28b MO-injected embryos. Total RNA was isolated from lin-28a MO-, lin-28b MO- or control MO-injected embryos at 24 hpf and analyzed by using specific primers for lin-41. The expression of lin-41 was decreased in both lin-28a MO- and lin-28b MO-injected embryos compared with control MO-injected embryos. E. Control, lin-28a and lin-28b MO-injected embryos labeled by in situ hybridization with lin-41 probe. Expression of lin-41 was repressed in CNS of both lin-28a and lin-28b morphant embryos at 24 hpf.
To detect significant changes in miRNA expression, we chose miRNAs that had at least a 1.5-fold change with high Hy3 signal (sample) in expression in the lin-28a or lin-28b morphants as compared with control MO-injected embryos (Table 1). Using this criterion, several miRNAs, including miR-203a, were downregulated in both lin-28a and lin-28b morphant embryos. Interestingly, we found a marked decrease in the expression of members of the miR-430 family, which was recently demonstrated to play a crucial role in deadenylation and clearance of maternal mRNAs during early zebrafish development (Table 1). To confirm this, we examined the expression level of these miRNAs by qRT-PCR (Figure 5B). Consistent with our microarray data, we observed a significant decrease in the expression of miR-430a and miR-430b (Figure 5B). Surprisingly, the expression level of let-7 miRNA in lin-28a and lin-28b morphant embryos was very low, and we did not detect significant upregulation in these morphants, suggesting that the downregulation of Lin-28a or Lin-28b is itself insufficient to induce the expression of the let-7 family, at least at 5 hpf (Figure 5B).
Table 1. List of differentially expressed probe sets (Fold change >1.5, %CV <50, High Hy3 signal >100).
| Probe Name | Name | Hy3/Hy5 | Fold change | |||
| Control MO | Lin28a MO | Lin28b MO | Lin28a MO/Control MO | Lin28b MO/Control MO | ||
| 49301 | dre-miR-203a | 1.029 | 0.939 | 0.651 | 0.913 | 0.633 |
| 48937 | dre-miR-430a | 1.322 | 0.81 | 0.783 | 0.613 | 0.592 |
| 48938 | dre-miR-430b | 1.288 | 0.662 | 0.808 | 0.514 | 0.627 |
| 50268 | dre-miR-430c | 1.18 | 0.695 | 0.606 | 0.589 | 0.513 |
| 48939 | dre-miR-430j | 1.35 | 0.64 | 0.766 | 0.475 | 0.568 |
To further examine the expression level of factors downstream of lin-28a and lin-28b, we isolated total RNA from surviving control, lin-28a, and lin-28b MO-injected zebrafish embryos at 28 hpf, which is the stage just before the expression of let-7a and let-7b was observed, and performed qRT-PCR to determine the relative expression levels of these miRNAs. As expected, the miRNA expression of both let-7a and let-7b was increased two- to four-fold in lin-28a and lin-28b morphant embryos (Figure 5C).
Furthermore, since lin-41 is a direct target of let-7 in C. elegans and mice, we performed RT-PCR and in situ hybridization to investigate whether the expression of lin-41 was downregulated in lin-28a or lin-28b morphant embryos. Consistent with a prominent increase in let-7 miRNA, RT-PCR analysis of lin-41 expression showed that lin-41 mRNA was downregulated in both lin-28a and lin-28b morphants as compared to the control sample at 24 hpf (Figure 5D). In situ hybridization of lin-41 mRNA confirmed that the hybridization signal in the anterior half of the embryos was barely detectable in the surviving lin-28a or lin-28b morphants at 24 hpf (Figure 5E). These results suggest the existence of an evolutionarily conserved heterochronic gene regulatory network in vertebrate development, and a possible correlation between the expression of lin-28 genes and the miR-430 family, which is a key regulator of maternal mRNA clearance.
Discussion
In C. elegans, heterochronic genes play a critical role in the timing of organ formation during development [2]–[5]. In this report, we identified zebrafish homologs of C. elegans lin-28 heterochronic genes and showed that the C. elegans heterochronic gene pathway is conserved and could be involved in regulating key temporally controlled events during early zebrafish development. Similar to mammals, zebrafish has two Lin28 homologs, lin-28a and lin-28b, in distinct genomic regions. An alignment of the sequences of the zebrafish lin-28a and lin-28b gene products showed that they are highly homologous with mammalian Lin28a and Lin28b. In particular, two functional domains for pri- and pre-let-7 miRNA binding, a cold-shock domain and a pair of CCHC zinc finger domains, were highly conserved. This high degree of sequence conservation suggests that the function of zebrafish Lin-28a and Lin-28b is conserved across animal phylogeny. Indeed, we observed significant upregulation of let-7 family miRNA expression in both lin-28a and lin-28b morphants, implying their conserved function as a let-7 regulator.
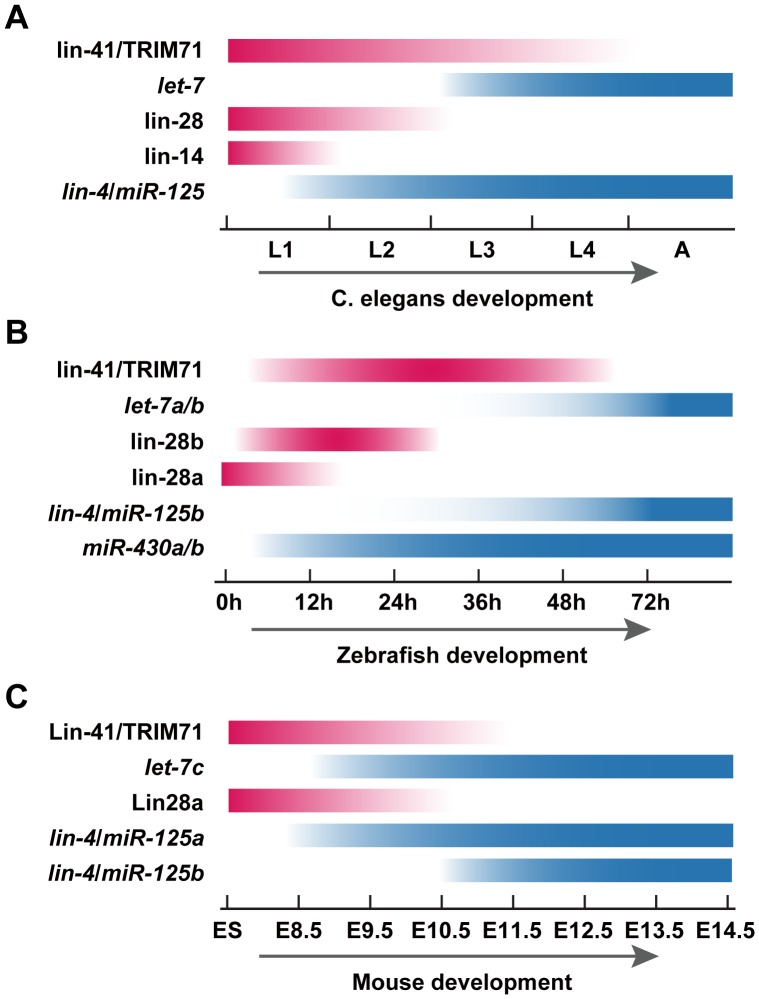
During C. elegans development, the expression pattern of heterochronic genes is temporally controlled to specify the timing of developmental events [2]–[6]. First, lin-28 and lin-41 are expressed at L1. Next, lin-4 and let-7 miRNA expression begins at L2 and L3, respectively [5], [6], [13], [14]. Since these miRNAs target the 3′UTR of lin-28 and lin-41, respectively, expression of these genes begins to decrease at L2 and L3, respectively [6], [17], [19] (Figure 6A). Consistent with the sequential and reciprocal expression of heterochronic genes during C. elegans larval development, we showed through whole-mount in situ hybridization, qRT-PCR and RT-PCR, that the heterochronic gene pathway is highly conserved in zebrafish.
Figure 6. Expression pattern of heterochronic genes in developmental timing.
A. Schematic diagram for heterochronic gene expression during C. elegans development. Consistent with critical role in the early development, expressions of LIN-14, LIN-28 and LIN-41 were observed at L1 stage. In a reciprocal fashion, Lin-4 and let-7 expressions were first detected at L1 and L3 stage, respectively and became stronger as development proceeded, thereby down-regulated the expression of their target gene. B. A schematic diagram summarizing heterochronic gene and miR-430 expression during zebrafish development. As with the expression pattern of C. elegans heterechoronic gene, expressions of lin-28a, lin-28b and lin-41 were first observed at early development. Reciprocal expression of lin-4 and let-7 expression were detected at 24 hpf and 48 hpf, respectively, and the expression level increased as development proceeds. Moreover, consistent with the timing of zygotic transcription, miR-430 expression was activated, and its levels rose. C. Model for heterochronic gene expression during mouse development. The expression of LIN28 was highly restricted in ES cells, which negatively regulated let-7 expression, and the let-7 target gene, mouse lin-41, was reported to play an important role as a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. As development proceeded, let-7 and miR-125a/b expression levels were increased, and expression levels of Lin28 and Lin41 were prominently downregulated in a reciprocal pattern. Red boxes represent the approximate timing of gene expressions and the blue boxes depicts the timing of the miRNA expressions. Developmental stages of each species are listed at bottom.
During zebrafish development, although the expression onset was somewhat different, lin-28a, lin-28b, and lin-41 were ubiquitously expressed at the early gastrulation stage. Concomitant with the increased expression of let-7 and lin-4/miR-125b, the expression of lin-28b and lin-41 began to decrease from 24 and 48 hpf, respectively (Figure 6B). Since zebrafish lin-28b and lin-41 have a lin-4/miR-125b and let-7 target sites in their 3′UTRs (using Targetscan and PITA) [33], the downregulation of lin-28b and lin-41 might be directly regulated by these miRNAs.
Recent studies have shown that several members of the heterochronic pathway are highly conserved in mammals, not only in sequence but also through regulation by an evolutionarily conserved genetic pathway. For example, let-7 and lin-4/miR-125 are highly conserved and expressed in various species, including Drosophila and humans [20], [34], [35]. Moreover, lin-41 and lin-28 are highly conserved in mammals and are regulated by let-7 and miR-125 like C. elegans [22], [36].
In mice, Lin28a is a marker of undifferentiated ES cells [24], and is abundant in a variety of developing tissues, including the neuroepithelium at E8.5, and is then expressed throughout the neural tube, co-localized with Sox2 at E9.5 [37]. By E10.5, this expression is markedly decreased in differentiated neural lineages and becomes undetectable [37] (Figure 6C). The mouse Lin41 expression pattern is somewhat similar to that of Lin28a. Lin41/TRIM71 is highly expressed in ES cells [38], and robust and ubiquitous Lin41 expression at E8.5 has been reported [36], [39]. The expression then gradually decreases until E11.0, becoming undetectable at later developmental stages [36], [39] (Figure 6C). On the other hand, let-7 and miR-125 are reportedly expressed in a reciprocal fashion to Lin28a and Lin41 during mouse development [36], [39] (Figure 6C).
Considering our and other observations, it seems that an evolutionarily conserved heterochronic gene cascade exists in early vertebrate embryogenesis.
In addition, mammalian homologs of heterochronic genes play a critical roles in the regulation of various undifferentiated cells. For example, in has been suggested that Lin28, let-7 and miR125 play important roles in cell fate determination in ES cells [24]. Among others, mammalian homologs of Lin-28 significantly contribute to cancer progression, the pluripotency of embryonic stem (ES) cells, early zygote development and the reprogramming of human and mouse fibroblasts to induced pluripotent stem (iPS) cells [23]–[26], mostly by preventing the anti-proliferative function of let-7; thus, the role of Lin-28 in vertebrate development has attracted considerable interest.
Although it has recently been suggested that the Lin28-mediated repression of let-7-induced differentiation may play a major role in the maintenance of most undifferentiated cells, increasing evidence strongly suggests the existence of a let-7-independent function for Lin28. For example, in neural stem cells, Lin28 regulates the timing of cell fate competency in neural stem cells during neurogliogenesis by a let-7-independent mechanism [37].
Consistent with the essential role of Lin28a in stem cell maintenance in various types of mammalian cells, we observed a significant decrease in cell proliferation at 5 hpf in both lin-28a and lin-28b morphant embryos, although a stage-specific cell lineage function of Lin28 remainss to be determined. During the late stage, these morphants displayed a severe phenotype characterized by a shortened body axis, small anterior structures, and aberrant tail morphology. However, while the expression of let-7a and let-7b was significantly upregulated in these morphants at 24 hpf, we did not find any significant upregulation of these let-7 miRNAs in these morphants at the early blastula stage (Figure 5B). Thus, it is possible that the let-7 independent function of Lin-28 might play a critical role in these lin-28a and lin-28b morphant phenotypes. These findings are consistent with a recent publication in which gross gastrulation defects were observed in lin-28a and lin-28b morphant Xenopus embryos [40]. They did not find any significant changes in the overall level of let-7 in lin-28 morphant embryos at the early gastrula stage (10.5), proposing a let-7-independent function for Lin-28 (e.g., translational regulation of maternally-deposited mRNAs). Unlike in zebrafish, lin-28b is maternally expressed in Xenopus and then expression of both lin-28a and lin-28b is upregulated shortly after mid-blastula transition. Given the fact that the lin-28a and lin-28b morphants displays similar severe developmental defects in both Xenopus and zebrafish, both of the genes seem to be necessary for the early development and not redundant.
Although we were not able to discriminate between maternal lin-28a and lin-28b function, recent study suggests an intriguing role of Lin28a in mouse early zygote development. Consistent with our results, Vogt et al. reported that Lin28a mRNA is maternally expressed, and the Lin28a protein accumulates at the nucleolar precursor body (NPB) of mouse ES cells, where it co-localize with the nucleophosmin1 (NPM1) [26]. MO-mediated knockdown of maternal Lin28a inhibit accumulation of NPM1 at presumptive NPB, resulting in a developmental arrest at the transition of the 2-cell to the 4-cell stage and never develop to morula or blastocyst, suggesting that Lin28a is a novel essential factor of nucleologenesis during early zygote development [26]. Further studies are needed to elucidate the functional difference of lin-28a and lin-28b.
Surprisingly, our microarray-based analysis of miRNA expression in lin-28a and lin-28b morphants revealed that the expression of miR-430 family miRNAs was significantly downregulated in these morphants. In the early development of zebrafish, miR-430 accumulates during the maternal-to-zygotic transition and promotes deadenylation and the clearance of hundreds of maternal mRNAs [12]. Since miR-430 can rescue the severe developmental defects in MZ dicer mutant embryos, miR-430 is suggested to play a central role in early development [12]. Intriguingly, our lin-28a and lin-28b morphants displayed a phenotype similar to that of MZ dicer mutants, which is characterized by reduced extension of the body axis, resulting in shortening of the embryo and morphological malformation of the heart and brain. Thus, we speculate that the function of Lin-28 during early zebrafish development involves the miR-430-mediated clearance of maternal mRNAs. Currently, we do not have an explanation for the genetic interaction between lin-28 and miR-430. It would be important to consider this interaction in future experiments.
We also observed the expression of lin-41, a down-stream target of let-7 in the presumptive anterior neural plate. Since it was downregulated in lin-28a and lin-28b morphant embryos, there may exist a conserved functional role of heterochronic gene cascade in zebrafish. In mouse ES cells, Lin41 regulates let-7 activity in cooperation with Lin28 and acts as a stem cell-specific E3 ubiquitin ligase [38]. Moreover, Lin28 is expressed in developing mouse neural tube, co-localizes with SOX2, and has been demonstrated to play a role in neurogenesis [37]. Lin41-knockout mice display a striking neural tube closure defect during development and embryonic lethality [39]. Thus, these results suggest that a significant gene regulatory network exists between Lin28, Sox2, and Lin41 during early vertebrate embryogenesis.
Based on homology and expression pattern, zebrafish lin-28a, lin-28b, and the miRNAs studied here may be involved in regulating specific genes directing key developmental events. These findings will aid in the understanding of the evolutionarily conserved roles of the heterochronic gene cascade underlying early embryonic development in vertebrates.
Supporting Information
Genomic organization of LIN28A/B and its homologous locus in various species. VISTA plot of the human LIN28A (A) and LIN28B (B) genomic sequence (x-axis) vs. mouse, rat, chicken and zebrafish genomic sequence (y-axis). Regions of high conservation are colored according to the annotation as exons (dark blue), untranslated regions (light blue) or non-coding (pink).
(EPS)
Acknowledgments
We thank Dr. Yukihiro Baba and Dr. Hideto Koso for their helpful discussion of this work, Takae Hiraide, Taeko Nakashima, Yuya Banno, Hidenori Tsuruda and Yuhei Hakumoto for technical assistance.
Funding Statement
This study was supported in part by Center of Excellence Project for Private Universities (no. S0801055) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 3. Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855–862. [DOI] [PubMed] [Google Scholar]
- 4. Ambros V, Horvitz HR (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226: 409–416. [DOI] [PubMed] [Google Scholar]
- 5. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, et al. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403: 901–906. [DOI] [PubMed] [Google Scholar]
- 6. Rougvie AE (2001) Control of developmental timing in animals. Nat Rev Genet 2: 690–701. [DOI] [PubMed] [Google Scholar]
- 7. Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23: 175–205. [DOI] [PubMed] [Google Scholar]
- 8. Rana TM (2007) Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 8: 23–36. [DOI] [PubMed] [Google Scholar]
- 9. Kim VN (2005) MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385. [DOI] [PubMed] [Google Scholar]
- 10. Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, et al. (2005) Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, et al. (2003) Dicer is essential for mouse development. Nat Genet 35: 215–217. [DOI] [PubMed] [Google Scholar]
- 12. Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, et al. (2005) MicroRNAs regulate brain morphogenesis in zebrafish. Science 308: 833–838. [DOI] [PubMed] [Google Scholar]
- 13. Feinbaum R, Ambros V (1999) The timing of lin-4 RNA accumulation controls the timing of postembryonic developmental events in Caenorhabditis elegans. Dev Biol 210: 87–95. [DOI] [PubMed] [Google Scholar]
- 14. Johnson SM, Lin SY, Slack FJ (2003) The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev Biol 259: 364–379. [DOI] [PubMed] [Google Scholar]
- 15. Ha I, Wightman B, Ruvkun G (1996) A bulged lin-4/lin-14 RNA duplex is sufficient for Caenorhabditis elegans lin-14 temporal gradient formation. Genes Dev 10: 3041–3050. [DOI] [PubMed] [Google Scholar]
- 16. Olsen PH, Ambros V (1999) The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol 216: 671–680. [DOI] [PubMed] [Google Scholar]
- 17. Moss EG, Lee RC, Ambros V (1997) The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88: 637–646. [DOI] [PubMed] [Google Scholar]
- 18. Vella MC, Reinert K, Slack FJ (2004) Architecture of a validated microRNA::target interaction. Chem Biol 11: 1619–1623. [DOI] [PubMed] [Google Scholar]
- 19. Vella MC, Choi EY, Lin SY, Reinert K, Slack FJ (2004) The C. elegans microRNA let-7 binds to imperfect let-7 complementary sites from the lin-41 3′UTR. Genes Dev 18: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, et al. (2000) Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408: 86–89. [DOI] [PubMed] [Google Scholar]
- 21. Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, et al. (2000) The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell 5: 659–669. [DOI] [PubMed] [Google Scholar]
- 22. Moss EG, Tang L (2003) Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol 258: 432–442. [DOI] [PubMed] [Google Scholar]
- 23. Martinez NJ, Gregory RI (2010) MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell 7: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melton C, Judson RL, Blelloch R (2010) Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920. [DOI] [PubMed] [Google Scholar]
- 26. Vogt EJ, Meglicki M, Hartung KI, Borsuk E, Behr R (2012) Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development 139: 4514–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouchi Y, Tabata Y, Arai K, Watanabe S (2005) Negative regulation of retinal-neurite extension by beta-catenin signaling pathway. J Cell Sci 118: 4473–4483. [DOI] [PubMed] [Google Scholar]
- 28. Muto A, Arai K, Watanabe S (2006) Rab11-FIP4 is predominantly expressed in neural tissues and involved in proliferation as well as in differentiation during zebrafish retinal development. Dev Biol 292: 90–102. [DOI] [PubMed] [Google Scholar]
- 29. Kurita R, Sagara H, Aoki Y, Link BA, Arai K, et al. (2003) Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev Biol 255: 113–127. [DOI] [PubMed] [Google Scholar]
- 30. Guo Y, Chen Y, Ito H, Watanabe A, Ge X, et al. (2006) Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384: 51–61. [DOI] [PubMed] [Google Scholar]
- 31. Viswanathan SR, Daley GQ, Gregory RI (2008) Selective blockade of microRNA processing by Lin28. Science 320: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam Y, Chen C, Gregory RI, Chou JJ, Sliz P (2011) Molecular basis for interaction of let-7 microRNAs with Lin28. Cell 147: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin YC, Hsieh LC, Kuo MW, Yu J, Kuo HH, et al. (2007) Human TRIM71 and its nematode homologue are targets of let-7 microRNA and its zebrafish orthologue is essential for development. Mol Biol Evol 24: 2525–2534. [DOI] [PubMed] [Google Scholar]
- 34. Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, et al. (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735–739. [DOI] [PubMed] [Google Scholar]
- 35. Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, et al. (2003) The microRNAs of Caenorhabditis elegans. Genes Dev 17: 991–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schulman BR, Esquela-Kerscher A, Slack FJ (2005) Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Dev Dyn 234: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balzer E, Heine C, Jiang Q, Lee VM, Moss EG (2010) LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development 137: 891–900. [DOI] [PubMed] [Google Scholar]
- 38. Rybak A, Fuchs H, Hadian K, Smirnova L, Wulczyn EA, et al. (2009) The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nat Cell Biol 11: 1411–1420. [DOI] [PubMed] [Google Scholar]
- 39. Maller Schulman BR, Liang X, Stahlhut C, DelConte C, Stefani G, et al. (2008) The let-7 microRNA target gene, Mlin41/Trim71 is required for mouse embryonic survival and neural tube closure. Cell Cycle 7: 3935–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faas L, Warrander FC, Maguire R, Ramsbottom SA, Quinn D, et al. (2013) Lin28 proteins are required for germ layer specification in Xenopus. Development 140: 976–986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic organization of LIN28A/B and its homologous locus in various species. VISTA plot of the human LIN28A (A) and LIN28B (B) genomic sequence (x-axis) vs. mouse, rat, chicken and zebrafish genomic sequence (y-axis). Regions of high conservation are colored according to the annotation as exons (dark blue), untranslated regions (light blue) or non-coding (pink).
(EPS)