Abstract
During sepsis, a complex network of cytokine, immune, and endothelial cell interactions occur and disturbances in the microcirculation cause organ dysfunction or even failure leading to high mortality in those patients. In this respect, numerous experimental and clinical studies indicate sex-specific differences in infectious diseases and sepsis.
Female gender has been demonstrated to be protective under such conditions, whereas male gender may be deleterious due to a diminished cell-mediated immune response and cardiovascular functions. Male sex hormones, i.e., androgens, have been shown to be suppressive on cell-mediated immune responses. In contrast, female sex hormones exhibit protective effects which may contribute to the natural advantages of females under septic conditions. Thus, the hormonal status has to be considered when treating septic patients.
Therefore, potential therapies could be derived from this knowledge. In this respect, administration of female sex hormones (estrogens and their precursors) may exert beneficial effects. Alternatively, blockade of male sex hormone receptors could result in maintained immune responses under adverse circulatory conditions. Finally, administration of agents that influence enzymes synthesizing female sex hormones which attenuate the levels of pro-inflammatory agents might exert salutary effects in septic patients. Prospective patient studies are required for transferring those important experimental findings into the clinical arena.
Keywords: sex steroids, estrogen, immune depression, immune modulation
Introduction
Recently there has been increasing interest in the immuno-neuroendocrine system, including the relationship between gender, sex hormones, and their effects on pathophysiological parameters and the immune response following adverse circulatory conditions, i.e., hemorrhage1 and sepsis.2-4 These research efforts may disclose important links between steroid hormones and the immune system, thereby offering chances for novel therapeutic options. Several clinical and experimental studies demonstrated significant effects of sex hormones on cell mediated immune responses.5,6 With respect to humoral immune responses, a remarkable preponderance of females are susceptible to autoimmune diseases, i.e., systemic lupus erythematosus (SLE), Hashimoto thyroiditis, rheumatoid arthritis, and primary biliary cirrhosis.7-9 These have been documented in both human and experimental studies.10,11 Male sex steroids appear to be immunodepressive whereas female sex steroids increase the activity of humoral immune responses.12-14 This concept was also evident in a mouse model of autoimmune lupus. Female mice of this strain normally develop lupus erythematosus. Administration of male sex steroids prior to maturation prevented the disease development. Interestingly, castration of male mice resulted in the development of autoimmune diseases that are not evident in normal male mice of this strain.15 Also, in humans, lower antigen expression and increased levels of active estrogen metabolites were evident in women suffering from SLE when compared with age-matched healthy female controls.16 Similar effects of sex steroids have been demonstrated on cell-mediated immune responses.17
The above mentioned studies collectively suggest that male and female sex steroids have immune-modulating properties in humoral as well as cell-mediated immune responses under normal conditions and in various disease processes.
Effect of Gender on Sepsis and Septic Shock Associated Morbidity and Mortality
Epidemiological studies demonstrate gender differences with respect to the development of septic complications and multiple organ failure in trauma victims. In this respect, Offner et al. identified male gender as an independent risk factor for the development of severe infection in surgical patients.18 McGowan et al. also reported a significantly higher incidence of bacteremic infections in males compared with females.19
A retrospective analysis incorporating 30 286 severely injured trauma victims (injury severity score, ISS, >15) displayed a higher incidence of pneumonia in males.20 However, no differences in the mortality of pneumonia were evident in this study.20 In addition to the incidence of sepsis, the clinical course also appears to be affected by gender. A retrospective analysis of 261 255 consecutive patients, reviewing the data available in the APACHE database, revealed an increased mortality in males younger than 50 y compared with women of the same age group.21 This difference was not evident in patients older than 50 y.21 A prospective observational cohort study of 2183 patients with community-acquired pneumonia, however, also revealed a lower survival in older men.22 This was associated with an increased expression of TNF, IL-6, IL-10, d-dimer, antithrombin-III, and factor IX in men with pulmonary infection.22 Conversely, anti-inflammatory mediators were increased in females which may contribute to the better prognosis.22 Support for the advantage of female gender in sepsis comes from a prospective trial demonstrating an increased survival in females with sepsis compared with male patients (survival rate: 74% in women vs. 31% in men).23 An analysis of 373 370 patients from the US National Trauma Data Bank similarly found male gender to be an independent prognostic variable for patients’ outcome in sepsis using multivariate analysis.24 In pediatric patients, gender-specific responses to sepsis have also been documented. Nonetheless, this was beyond the scope of the present manuscript.
Experimental studies in mice revealed a significantly increased survival rate of proestrus female mice following polymicrobial sepsis induced by cecal ligation and puncture (CLP) compared with male animals.25 Moreover, the susceptibility to sepsis was also lower in proestrus female mice compared with males following trauma and severe blood loss.26
In summary, the above studies collectively suggest that male gender is a risk factor for the development of infectious complications after trauma. In patients with manifested sepsis, females are better positioned to survive the insult. This gender difference may only be evident in patients younger than 50 y, suggesting that postmenopausal women are no longer in an advantaged condition.
Despite these cited studies regarding gender-dimorphic responses following sepsis, conflicting studies with varied conclusions have also been published. In this regard, a huge cohort of trauma victims involving 22 332 patients did not detect gender differences in patient outcome.27
In the text that follows, we will offer potential explanations for this conflicting data. In particular, sex hormones and not gender itself have been shown to be responsible for gender-specific findings following sepsis. In this respect, differences in plasma sex hormone levels due to the stage of the menstrual cycle or age might explain why some clinical studies have failed to demonstrate gender differences. Stratification for hormone levels due to menstrual cycle or menopause has not been included in those clinical analyses investigating gender-specific outcomes in these patients.
Gender-Specific Immune Response after Sepsis
As outlined above, several clinical studies indicate a differential outcome in patients after septic conditions with respect to patients’ gender. As a potential mechanism for this observation, a sex-specific expression of pro- and anti-inflammatory cytokines has been found in surgical patients at the molecular level.
Significantly elevated levels of pro-inflammatory cytokines (i.e., IL-6, procalcitonin) have been described in sepsis in male compared with female patients.28-30 The importance of IL-6 has recently been emphasized in a clinical study by Wang et al. which demonstrated the predictive role of this cytokine for the severity of septic episodes.31
Similarly, experimental studies described an increased early cytokine response in males after the induction of endotoxemia. Four hours after LPS injection, significantly increased IL-1 levels were found in male as compared with female mice.32
Conversely, Ongaro et al. showed an elevated LPS-induced TNF-α secretion in plasma of adult male rats after androgen receptor antagonism or testosterone depletion with neonatal flutamide treatment or prepubertal orchidectomy, respectively.33 In this regard, our studies have shown that flutamide administration following trauma increases aromatase activity which promotes the synthesis of 17β-estradiol from testosterone.34-36 Thus, the salutary effects of flutamide in males may not be mediated by androgen receptor antagonism but due to increased estrogen levels. Similarly, stimulatory effects of estradiol on the responsiveness of human peripheral blood mononuclear cells to LPS in vitro have been demonstrated.37 Thus, this early immune response may contribute to the enhanced immune resistance of females in subsequent phases of sepsis.
Despite these findings, gender-specific differences in the activation of lymphocytes remain controversial.38-40 While Wichmann et al. reported a gender dimorphism in those cells following abdominal trauma,38 Majetschak et al. failed to demonstrate differences in the cytokine response after activation of the immune system.39 In a model of experimental sepsis in mice, protective effects of a precursor of testosterone, androstenedione, as indicated by decreased levels of pro-inflammatory cytokines in animals treated with this drug, have been found.41 It should be noted, however, that testosterone is an intermediate and is converted to 17β-estradiol by aromatase and to 5α-dihydrotestosterone (DHT; active compound) by 5α-reductase. Thus, depending upon the prevailing enzymatic milieu, testosterone may be converted to 17β-estradiol or DHT.
Sepsis is often the consequence of operative interventions. In their clinical study, Wichmann et al. reported a significant depression of immune competent cells following surgery in men.38 As a potential explanation, this study describes a sex-specific expression of different types of lymphocytes as well as natural killer cells in the postoperative course.38
In addition to a gender-specific immune response following sepsis, differences in survival have been demonstrated in various sepsis models. In this respect, in an experimental “two-hit” model of trauma-hemorrhage and subsequent sepsis, male gender and age have recently been shown to increase mortality rates.42 Moreover, maintained immune responses in proestrus females were associated with significantly improved survival following cecal ligation and puncture.25
One explanation might be the fact that the number of resting resident leukocytes occupying the peritoneal and pleural cavities is higher in female than male rats, comprising more T and B lymphocytes, as recently shown by Scotland et al.43 This altered immune cell composition of the female peritoneum is controlled by elevated tissue chemokine expression. Female resident macrophages also exhibit greater Toll-like receptor (TLR)-4 expression, enhanced phagocytosis, and NADPH oxidase-mediated bacterial killing.40 On the other hand, addition of testosterone to isolated mouse macrophages decreased TLR-4 expression and sensitivity to a TLR-4-specific ligand as demonstrated by Rettew et al.44 This enhanced immunological competence of local immune cells in females led to improved antimicrobial host defense after polymicrobial sepsis as indicated by Newsome et al.45 They demonstrated that intraperitoneal application of the biological modifier poly-(1,6)-b-d-glucopyranosyl-(1,3)-b-d-glucopyranose glucan (PGG glucan) at the onset of sepsis enhanced survival in female mice over a 10-d period, but survival in males was improved for only 24 h. This effect was associated with decreased IL-6 and IL-10 levels and reduced bacterial burden in the liver in female compared with male mice.
These gender-specific effects on different immune cell functions and compartments are potentially influenced by an X-chromosome mosaicism that exists naturally in females. Therefore, heterozygous cellular mosaicism presents a unique biological circumstance in females due to the fact that either the maternal or the paternal X-chromosomes are inactivated in each individual cell whereas males carry exclusively the maternal X-chromosome. Experimental studies revealed that this female X-chromosome mosaicism diversifies leukocyte responses during endotoxemia and may contribute to the dimorphic character of the inflammatory response.46 Moreover, male and female sex hormone receptors have been identified on immune cells suggesting direct effects of androgen and estrogen. In particular, the estrogen receptor β expressed on immune cells plays a pivotal role in mediating immunoprotective effects of female sex hormones. In this respect, experimental studies indicate that the estrogen receptor β agonist WAY-202196 preserved gastrointestinal barrier function and improved outcome in models of systemic infection and inflammation.47
Sex Steroids Differently Affect the Cardiovascular System
Recent studies indicate that gender also affects cardiovascular responses.1 Sex hormones have been demonstrated to affect outcomes after trauma-hemorrhage, ischemia/reperfusion (I/R) injury, and sepsis.48,49 Castration of male rats two weeks prior to the onset of trauma-hemorrhage prevented the depression of myocardial function as evidenced by significantly higher values of heart performance in vivo.50 Similarly, treatment of male rats with the androgen receptor antagonist flutamide has also been found to improve and restore cardiovascular responses following trauma and severe blood loss.51 Recently, differences in the restitution of plasma and tissue volumes between males and proestrus females during and after trauma-hemorrhage have been found.52 In this respect, proestrus females showed faster restitution of blood volume during and after trauma-hemorrhage, which might contribute to the improved immune and organ functions in females under adverse circulatory conditions.40
There is increasing evidence that administration of female sex steroids, i.e., estrogen, result in maintained cardiovascular functions through paracrine actions on immune cells, host tissue, and endothelial cell function.53-55 In this respect, several studies involving males and ovariectomized females given exogenous estrogen indicated that females might be better positioned in mitigating against pathology from microcirculatory disorder and sepsis.48,49 In particular, Sharawy et al. showed that independent of whether septic males or ovariectomized females were treated with agonists for the estrogen receptor (ER)-α or -β, a significantly reduced sepsis-induced leukocyte–endothelial interaction (rolling, adherent leukocytes and neutrophil extravasations) and improved intestinal muscular functional capillary density was observed.56 Those results suggest that the observed effects of estradiol receptors on different phases of leukocyte recruitment with the improvement of the functional capillary density could partially explain salutary effects of estradiol on the intestinal microcirculation during sepsis.
Moreover, direct effects of sex hormones on myocardial function appear to be relevant for gender-specific cardiovascular functions in sepsis. In this respect, hearts from proestrus females displayed a significantly better post-ischemic functional recovery than males. Administration of estradiol in males and ovariectomized females improved post-ischemic myocardial functional recovery, reduced the production of TNF-α, IL-1β, and IL-6, and decreased the activation of p38 MAPK and caspase-3 when compared with their untreated counterparts.49 These beneficial effects of estradiol were also evident in endotoxemia as shown by Zhu et al.57 They demonstrated that male mice had greater myocardial dysfunction compared with female animals in a model of endotoxemia. Administration of estrogen reversed those alterations in the studies of Zhu et al.57 Particular signaling pathways have been identified to be involved in this estrogen-mediated effect on myocardial function during endotoxemia. Generally, endotoxin induces Rac1 activation, which contributes to NADPH oxidase activity and phosphorylation of ERK1/2/p38 MAPK, leading to TNF-α expression in the heart. Estrogen attenuated this endotoxin-induced RAC 1 activation thereby reducing potentially detrimental myocardial TNF-α expression.57
Potential Therapeutic Strategies
Androgen receptor antagonists
Several animal studies have indicated that testosterone depletion exerts beneficial effects on immune responses after infectious diseases and sepsis.58,59 In order to transfer those effects into the clinical arena, concepts mimicking castration by the use of the androgen receptor antagonist flutamide have been conducted, revealing modulation of a variety of pathways.34-36 Administration of flutamide at a dosage of 25 mg/kg BW following trauma-hemorrhage and resuscitation normalized the depressed splenic and peritoneal macrophage cytokine release.60 Additionally, LPS-induced TNF-α secretion in plasma in adult male rats after neonatal flutamide treatment was increased, indicates the depressive effects of testosterone.33 In addition, flutamide administration on three consecutive days following trauma-hemorrhage not only restored the depressed splenocyte and splenic macrophage cytokine release even after the induction of subsequent sepsis, but also significantly decreased the mortality of post-hemorrhaged animals subjected to a subsequent septic challenge.59 This maintenance of the immune system after flutamide treatment in male mice was also seen after induction of heatstroke in another animal model associated with massive inflammation. In this model flutamide administration resulted in improved survival.61 In particular, treatment with flutamide in male mice subjected to heatstroke significantly attenuated hypothermia, reduced the number of apoptotic cells in the hypothalamus, the spleen, the liver, the kidney, and attenuated the plasma index of toxic oxidizing radicals (e.g., nitric oxide metabolites and hydroxyl radicals), diminished the plasma index of the organ injury index (e.g., lactate dehydrogenase), attenuated plasma systemic inflammation response molecules (e.g., TNF-α and IL-6), and reduced the index of infiltration of polymorphonuclear neutrophils in the lung (e.g., myeloperoxidase activity). Since therapeutic use of flutamide in patients with testicular cancer for longer periods of time did not exhibit major adverse effects, the short-term use of this androgen receptor antagonist in male patients with a septic constellation may be a safe and useful therapeutic option for the treatment of immune and cardiovascular depression.
The steroid hormone DHEA
Dehydroepiandrosterone (DHEA) is the major circulating steroid hormone in humans. As an intermediate in the sex steroid synthesis it can be metabolized to both testosterone and estrogen. DHEA administered at a dose of 100 μg per animal in a mouse model prevented the depression of cell-mediated immune responses following trauma-hemorrhage and resuscitation, as evidenced by maintained splenic and peritoneal macrophage cytokine release and normalized splenocyte lymphokine release under those conditions.62,63 In addition, administration of DHEA following trauma-hemorrhage significantly improved the survival rate of animals subjected to subsequent sepsis compared with vehicle-treated animals.62 DHEA has been demonstrated to maintain TNF-α mRNA expression in the lung of a murine model of polymicrobial sepsis and trauma.64 In particular, natural killer (NK) cells may be one of the effector cells of the protective mechanisms of DHEA in polymicrobial sepsis.65 Furthermore, DHEA treatment in an experimental sepsis model restored splenocyte proliferation and delayed type hypersensitivity reaction, decreased cellular apoptosis rate of splenocytes, and attenuated cytokine releases.66 DHEA has been shown to increase expression of Toll-like receptors in polymicrobial sepsis, which in turn preserves innate immunity and confers a longer survival in mice.67
These studies are in accordance with the observation that DHEA normalized the depressed pro-inflammatory cytokine release capacities of human PBMC in vitro following major abdominal surgery.68 The effect of DHEA on PBMC function followed a dose-dependent manner. Studies demonstrated that DHEA can be administered intravenously or subcutaneously in septic male mice to achieve improvement of immune functions.63-65 In the United States, DHEA is widely used as an over-the-counter drug without any serious side effects. Therefore, DHEA might be a promising treatment option in male patients with sepsis by preventing the depression of cell-mediated immune responses. Finally, it is noteworthy that a DHEA metabolite, androstenediol, when administered in experimental sepsis and trauma-hemorrhage, reduces organ damage, improves cardiac function, attenuates inflammation, reduces apoptosis, and extends survival.66-71
Treatment with female mesenchymal stem cells
Recent studies have demonstrated the capability of mesenchymal stem cells (MSCs) to augment the immune system when faced with various challenges. MSCs attenuate the excessive inflammation occurring in sepsis by specific signal pathways thereby antagonizing disorders of the organ systems.72,73 As a potential mechanism, these cells may increase the antibacterial defense as well as the expression of anti-inflammatory substances. Furthermore, MSCs have been shown to reduce an overwhelming expression of inflammatory cytokines, as well as decreasing apoptosis under septic conditions.74-76 In mice, MSCs have further been shown to improve survival in an experimental model of pulmonary sepsis.76 In other models (i.e., endotoxemia or cecal ligation and puncture-induced sepsis) MSCs have been shown to increase survival which was mediated by reducing organ dysfunction as well as influencing the immune response.73,77 Interestingly, MSCs possess gender-specific characteristics. Following stimulation, an enhanced expression of vascular endothelial growth factor, lower TNF-α production, as well as a reduced inflammatory response has been observed in female-derived murine MSCs compared with male-derived MSCs.78 Moreover, female MSCs may be more effective than male MSCs in the treatment of myocardial recovery following cardiac I/R.79 Gender-specific expression of receptors and intracellular signaling enzymes have been discussed as potential mechanisms for these differences. In a knockout model of TNF receptor 1 (TNFR1) female MSCs stimulated with LPS show greater resistance to the increased pro-inflammatory cytokine and a decreased growth factor production compared with male MSCs.80 However, gender discrepancies exist in MSC function after injury, and injury-induced TNF signaling via TNFR1 may have disparate effects in males and females.
Summary
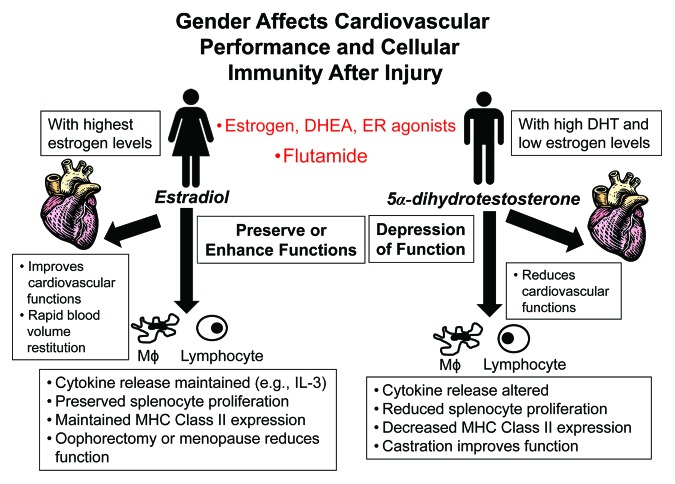
Despite the fact that gender differences in the morbidity and mortality from trauma, shock, and sepsis have been observed in several clinical studies, alterations in the immune functions following shock have been investigated primarily using young male laboratory animals. Numerous studies have recently been initiated investigating the effect of gender, age, and sex hormones on immune responses following sepsis. The findings of those studies suggest that low DHT and/or high estradiol appear to be protective for the host following adverse circulatory conditions, i.e., septic shock (Fig. 1). Although the exact underlying mechanism(s) for the immunomodulatory properties of sex hormones on cell-mediated immune responses and cardiovascular functions following sepsis remain unknown, there is evidence that both direct and indirect effects of sex steroids synergistically modulate the immune and cardiovascular responses (Table 1). In this respect, sex hormone receptors have been identified on various immune and non-immune cells suggesting receptor-mediated processes. Other studies suggest the release of secondary mediators which alter immune responses following adverse circulatory conditions. In view of these findings, clinically relevant therapeutic strategies should use the androgen receptor antagonist flutamide and/or estrogen or agents with estrogenic effects, i.e., DHEA, which might yield safe and useful therapeutic adjunct approaches for the treatment of immune and cardiovascular depression in sepsis. Moreover, gender should be taken into account when studying the immune or cardiovascular responses following sepsis.
Figure 1. Schematic illustration of the effect of gender on cardiovascular performance and cellular immunity following trauma and severe blood loss.
Table 1. Effect of sex steroids/agents on immune/cardiovascular responses following injury/sepsis in murine models and humans.
| Author | Reference | Species | Model | Agent | Effect |
|---|---|---|---|---|---|
| Angele et al.,1997 | 59 | Murine male C3H/HeN mice | Hemorrhage, sepsis | Testosterone receptor blockade (flutamide) | Splenocyte proliferation ↑, IL-2 ↑, splenic macrophage IL-1,6 ↑, survival ↑ |
| Yu et al., 2005 | 34 | Murine male Sprague–Dawley rats | Trauma, hemorrhage, cardiac function | Testosterone receptor blockade (flutamide) | Cardiac output ↑, estrogen receptors in cardiomyocytes ↑ |
| Lin et al., 2012 | 61 | Murine inbred male mice | Heatstroke | Testosterone receptor blockade (flutamide) | Systemic inflammation ↓, survival ↑, apoptosis ↓ |
| Barkhausen et al., 2009 | 64 | Murine male NMRI mice | Hemorrhage, sepsis | DHEA | Survival ↑, restoration of TNF-α mRNA expression in lung and liver |
| Frantz et al., 2005 | 68 | Human | PBMCs from patients undergoing abdominal surgery | DHEA | Proinflammatory cytokine release ↑ (TNF-α, IL-6, IL-1β) |
| Shimizu et al., 2004 | 70 | Murine Sprague–Dawley rats | Trauma, hemorrhage | Androstenediol/DHEA | Cardiac function ↑, splanchnic perfusion ↑, inflammatory response (IL-6) ↓ |
| Kiang et al., 2007 | 71 | Murine Sprague–Dawley rats | Trauma, hemorrhage | Androstenediol/DHEA | Apoptosis ↓ via caspase 3 ↓, iNOS ↓ |
| Gonzalez-Rey et al., 2009 | 74 | Human/murine | Experimental colitis | Female human MSCs | Restoration of immune functions, overwhelming inflammatory response ↓ (TNF-α, IFN-γ, IL-6, IL-1β, IL-12) |
| Nemeth et al., 2009 | 77 | Human/murine | Experimental sepsis | Female human MSCs | Survival and organ function ↑, inflammation ↓ via macrophage derived IL-10 |
Clinical studies, however, have been unable to consistently reproduce the experimental findings following low flow conditions. There continues to be a gap between the “bench and bedside” in regard to our understanding of gender-based differences following sepsis.81 Relative to controlled animal experiments, predisposing comorbidities, injury characteristics, additional medication (analgesics, pain killers, inotropic agents, antibiotics, allogeneic blood transfusions, etc.), and a lack of information about the hormone milieu of the septic patient disallow reproducible results from clinical analyses. Continued clinical research into potential sex hormone-based differences, genetic differences, and the cellular and molecular mechanisms responsible for these gender-based differential responses is required to close this gap. There is hope that this research may ultimately promote sex-based therapeutic interventions, which will allow for improved outcomes for male and female trauma victims in the near future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grants RO1 GM37127 and RO1 GM39519 (IH Chaudry).
References
- 1.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–21. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 2.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. ACCESS Study Group Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–62. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunnington A, Nadel S. New therapies for sepsis. Curr Top Med Chem. 2008;8:603–14. doi: 10.2174/156802608783955601. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol. 2013;13:576–81. doi: 10.1016/j.coph.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Bello R, Nava-Castro K, Muñiz-Hernández S, Nava-Luna P, Trejo-Sánchez I, Tiempos-Guzmán N, Mendoza-Rodríguez Y, Morales-Montor J. Beyond the reproductive effect of sex steroids: their role during immunity to helminth parasite infections. Mini Rev Med Chem. 2012;12:1071–80. doi: 10.2174/138955712802762149. [DOI] [PubMed] [Google Scholar]
- 7.Pan Z, Chang C. Gender and the regulation of longevity: implications for autoimmunity. Autoimmun Rev. 2012;11:A393–403. doi: 10.1016/j.autrev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Nussinovitch U, Shoenfeld Y. The role of gender and organ specific autoimmunity. Autoimmun Rev. 2012;11:A377–85. doi: 10.1016/j.autrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Smyk DS, Rigopoulou EI, Pares A, Billinis C, Burroughs AK, Muratori L, Invernizzi P, Bogdanos DP. Sex differences associated with primary biliary cirrhosis. Clin Dev Immunol. 2012;2012:610504. doi: 10.1155/2012/610504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luckey D, Medina K, Taneja V. B cells as effectors and regulators of sex-biased arthritis. Autoimmunity. 2012;45:364–76. doi: 10.3109/08916934.2012.665528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha S, Tieng A, Pepeljugoski KP, Zandamn-Goddard G, Peeva E. Prolactin, systemic lupus erythematosus, and autoreactive B cells: lessons learnt from murine models. Clin Rev Allergy Immunol. 2011;40:8–15. doi: 10.1007/s12016-009-8182-6. [DOI] [PubMed] [Google Scholar]
- 12.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–72. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard RC, Spinedi E. Sex- and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest Anim Endocrinol. 1998;15:345–52. doi: 10.1016/S0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- 14.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 15.Panchanathan R, Shen H, Bupp MG, Gould KA, Choubey D. Female and male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J Immunol. 2009;183:7031–8. doi: 10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidler C, Härle P, Schedel J, Schmidt M, Schölmerich J, Straub RH. Patients with rheumatoid arthritis and systemic lupus erythematosus have increased renal excretion of mitogenic estrogens in relation to endogenous antiestrogens. J Rheumatol. 2004;31:489–94. [PubMed] [Google Scholar]
- 17.Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3:pe36. doi: 10.1126/scisignal.3143pe36. [DOI] [PubMed] [Google Scholar]
- 18.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–8, discussion 938-40. doi: 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 19.McGowan JE, Jr., Barnes MW, Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975;132:316–35. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- 20.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–4. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood K, Eldeirawi K, Wahidi MM. Association of gender with outcomes in critically ill patients. Crit Care. 2012;16:R92. doi: 10.1186/CC11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reade MC, Yende S, D’Angelo G, Kong L, Kellum JA, Barnato AE, Milbrandt EB, Dooley C, Mayr FB, Weissfeld L, et al. Genetic and Inflammatory Markers of Sepsis Investigators Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med. 2009;37:1655–62. doi: 10.1097/CCM.0b013e31819da853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–5. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 24.Kisat M, Villegas CV, Onguti S, Zafar SN, Latif A, Efron DT, Haut ER, Schneider EB, Lipsett PA, Zafar H, et al. Predictors of sepsis in moderately severely injured patients: an analysis of the National Trauma Data Bank. Surg Infect (Larchmt) 2013;14:62–8. doi: 10.1089/sur.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–10. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–9. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- 27.Gannon CJ, Napolitano LM, Pasquale M, Tracy JK, McCarter RJ. A statewide population-based study of gender differences in trauma: validation of a prior single-institution study. J Am Coll Surg. 2002;195:11–8. doi: 10.1016/S1072-7515(02)01187-0. [DOI] [PubMed] [Google Scholar]
- 28.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–7. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27:151–6. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 30.Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, Hermann C. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res. 2006;26:887–92. doi: 10.1089/jir.2006.26.887. [DOI] [PubMed] [Google Scholar]
- 31.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. J Crit Care. 2013;28:549–55. doi: 10.1016/j.jcrc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 33.Ongaro L, Castrogiovanni D, Giovambattista A, Gaillard RC, Spinedi E. Enhanced proinflammatory cytokine response to bacterial lipopolysaccharide in the adult male rat after either neonatal or prepubertal ablation of biological testosterone activity. Neuroimmunomodulation. 2011;18:254–60. doi: 10.1159/000324125. [DOI] [PubMed] [Google Scholar]
- 34.Yu HP, Yang S, Choudhry MA, Hsieh YC, Bland KI, Chaudry IH. Mechanism responsible for the salutary effects of flutamide on cardiac performance after trauma-hemorrhagic shock: Upregulation of cardiomyocyte estrogen receptors. Surgery. 2005;138:85–92. doi: 10.1016/j.surg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Suzuki T, Bland KI, Chaudry IH. Flutamide attenuates pro-inflammatory cytokine production and hepatic injury following trauma-hemorrhage via estrogen receptor-related pathway. Ann Surg. 2007;245:297–304. doi: 10.1097/01.sla.0000232523.88621.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh YC, Yang S, Choudhry MA, Yu HP, Bland KI, Schwacha MG, Chaudry IH. Flutamide restores cardiac function after trauma-hemorrhage via an estrogen-dependent pathway through upregulation of PGC-1. Am J Physiol Heart Circ Physiol. 2006;290:H416–23. doi: 10.1152/ajpheart.00865.2005. [DOI] [PubMed] [Google Scholar]
- 37.Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340–3. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Wichmann MW, Müller C, Meyer G, Adam M, Angele MK, Eisenmenger SJ, Schildberg FW. Different immune responses to abdominal surgery in men and women. Langenbecks Arch Surg. 2003;387:397–401. doi: 10.1007/s00423-002-0346-2. [DOI] [PubMed] [Google Scholar]
- 39.Majetschak M, Christensen B, Obertacke U, Waydhas C, Schindler AE, Nast-Kolb D, Schade FU. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma. 2000;48:832–9, discussion 839-40. doi: 10.1097/00005373-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 41.Brunnemer U, Zeckey C, Hildebrand F, Frink M, Mommsen P, van Griensven M, Andruszkow H, Krettek C, Barkhausen T. Androstenediol exerts salutary effects on chemokine response after trauma-hemorrhage and sepsis in mice. J Orthop Trauma. 2011;25:511–5. doi: 10.1097/BOT.0b013e3182251044. [DOI] [PubMed] [Google Scholar]
- 42.Drechsler S, Weixelbaumer K, Raeven P, Jafarmadar M, Khadem A, van Griensven M, Bahrami S, Osuchowski MF. Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post-traumatic sepsis. PLoS One. 2012;7:e51457. doi: 10.1371/journal.pone.0051457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–27. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–7. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 45.Newsome CT, Flores E, Ayala A, Gregory S, Reichner JS. Improved antimicrobial host defense in mice following poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose glucan treatment by a gender-dependent immune mechanism. Clin Vaccine Immunol. 2011;18:2043–9. doi: 10.1128/CVI.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandra R, Federici S, Haskó G, Deitch EA, Spolarics Z. Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit Care Med. 2010;38:2003–10. doi: 10.1097/CCM.0b013e3181eb9ed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristofaro PA, Opal SM, Palardy JE, Parejo NA, Jhung J, Keith JC, Jr., Harris HA. WAY-202196, a selective estrogen receptor-beta agonist, protects against death in experimental septic shock. Crit Care Med. 2006;34:2188–93. doi: 10.1097/01.CCM.0000227173.13497.56. [DOI] [PubMed] [Google Scholar]
- 48.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(Suppl 1):101–6. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40:205–12. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Remmers DE, Cioffi WG, Bland KI, Wang P, Angele MK, Chaudry IH. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg. 1998;227:790–9. doi: 10.1097/00000658-199806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–25. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 52.Kuebler JF, Toth B, Rue LW, 3rd, Wang P, Bland KI, Chaudry IH. Differential fluid regulation during and after soft tissue trauma and hemorrhagic shock in males and proestrus females. Shock. 2003;20:144–8. doi: 10.1097/01.shk.0000072127.33223.f1. [DOI] [PubMed] [Google Scholar]
- 53.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008;83:499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 54.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock. 2009;31:227–37. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007;245:971–7. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharawy N, Ribback S, Al-Banna N, Lehmann C, Kern H, Wendt M, Cerny V, Dombrowski F, Pavlovic D. Estradiol receptors agonists induced effects in rat intestinal microcirculation during sepsis. Microvasc Res. 2013;85:118–27. doi: 10.1016/j.mvr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhu H, Shan L, Peng T. Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J Mol Cell Cardiol. 2009;47:264–74. doi: 10.1016/j.yjmcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Angele MK, Chaudry IH. Surgical trauma and immunosuppression: pathophysiology and potential immunomodulatory approaches. Langenbecks Arch Surg. 2005;390:333–41. doi: 10.1007/s00423-005-0557-4. [DOI] [PubMed] [Google Scholar]
- 59.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–14. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 60.Wichmann MW, Angele MK, Ayala A, Cioffi WG, Chaudry IH. Flutamide: a novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8:242–8. doi: 10.1097/00024382-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Lin CY, Hsu CC, Lin MT, Chen SH. Flutamide, an androgen receptor antagonist, improves heatstroke outcomes in mice. Eur J Pharmacol. 2012;688:62–7. doi: 10.1016/j.ejphar.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Angele MK, Catania RA, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Dehydroepiandrosterone: an inexpensive steroid hormone that decreases the mortality due to sepsis following trauma-induced hemorrhage. Arch Surg. 1998;133:1281–8. doi: 10.1001/archsurg.133.12.1281. [DOI] [PubMed] [Google Scholar]
- 63.Catania RA, Angele MK, Ayala A, Cioffi WG, Bland K, Chaudry IH. Dehydroepiandrosterone (DHEA) restores immune function following trauma-hemorrhage by a direct effect on T-lymphocytes. Cytokine. 1998;11:443–50. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- 64.Barkhausen T, Hildebrand F, Krettek C, van Griensven M. DHEA-dependent and organ-specific regulation of TNF-alpha mRNA expression in a murine polymicrobial sepsis and trauma model. Crit Care. 2009;13:R114. doi: 10.1186/cc7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeckey C, Hildebrand F, Hoevel P, Müller K, Krettek C, Barkhausen T, van Griensven M. Activity of lymphocyte subpopulations in polymicrobial sepsis and DHEA treatment in IL-6 knockout mice. J Innate Immun. 2010;2:469–77. doi: 10.1159/000284369. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz D, Lendemans S, Oberbeck R. Androstenediol Modulates Sepsis Induced Alterations of Survival and Immune Functions in a Murin Model of Sepsis. Med Chem. 2013 doi: 10.2174/157340641002140131155607. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda A, Furukawa K, Suzuki H, Matsutani T, Tajiri T, Chaudry IH. Dehydroepiandrosterone modulates toll-like receptor expression on splenic macrophages of mice after severe polymicrobial sepsis. Shock. 2005;24:364–9. doi: 10.1097/01.shk.0000180624.36811.97. [DOI] [PubMed] [Google Scholar]
- 68.Frantz MC, Prix NJ, Wichmann MW, van den Engel NK, Hernandez-Richter T, Faist E, Chaudry IH, Jauch KW, Angele MK. Dehydroepiandrosterone restores depressed peripheral blood mononuclear cell function following major abdominal surgery via the estrogen receptors. Crit Care Med. 2005;33:1779–86. doi: 10.1097/01.CCM.0000172278.91959.38. [DOI] [PubMed] [Google Scholar]
- 69.Szalay L, Shimizu T, Suzuki T, Hsieh YC, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Androstenediol administration after trauma-hemorrhage attenuates inflammatory response, reduces organ damage, and improves survival following sepsis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G260–6. doi: 10.1152/ajpgi.00390.2005. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu T, Choudhry MA, Szalay L. Rue LW3, Bland KI, Chaudry IH. Salutary effects of androstenediol on cardiac function and splanchnic perfusion after trauma-hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2004;287:386–90. doi: 10.1152/ajpregu.00214.2004. [DOI] [PubMed] [Google Scholar]
- 71.Kiang JG, Peckham RM, Duke LE, Shimizu T, Chaudry IH, Tsokos GC. Androstenediol inhibits the trauma-hemorrhage-induced increase in caspase-3 by downregulating the inducible nitric oxide synthase pathway. J Appl Physiol (1985) 2007;102:933–41. doi: 10.1152/japplphysiol.00919.2006. [DOI] [PubMed] [Google Scholar]
- 72.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Kelly ML, Meldrum DR. Stem cells in sepsis. Ann Surg. 2009;250:19–27. doi: 10.1097/SLA.0b013e3181a77b9c. [DOI] [PubMed] [Google Scholar]
- 73.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 75.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 76.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 77.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26:571–4. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]
- 79.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142:215–21. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1) J Mol Cell Cardiol. 2007;42:142–9. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37(Suppl):S30–7. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]