Abstract
Sepsis is the leading cause of death in children worldwide. Although the diagnosis and management of sepsis in infants and children is largely influenced by studies done in adults, there are important considerations relevant for pediatrics. This article highlights pediatric-specific issues related to the definition of sepsis and its epidemiology and management. We review how the capacity of the immune system to respond to infection develops over early life. We also bring attention to primary immune deficiencies that should be considered in children recurrently infected with specific types of organisms. The management of pediatric sepsis must be tailored to the child’s age and immune capacity, and to the site, severity, and source of the infection. It is important for clinicians to be aware of infection-related syndromes that primarily affect children. Although children in developed countries are more likely to survive severe infections than adults, many survivors have chronic health impairments.
Keywords: infants, children, pediatric, sepsis, septic shock, infection, innate immunity, review
Introduction
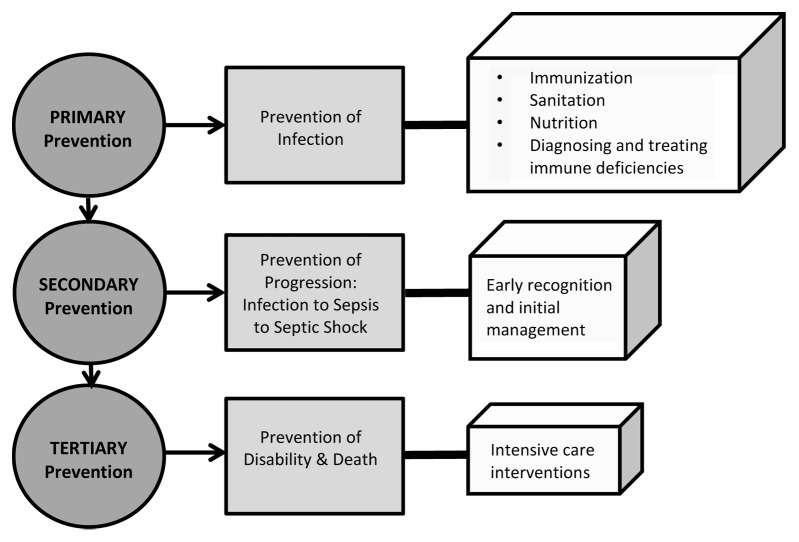
Sepsis is the most common cause of death in infants and children worldwide.1-4 Childhood pneumonia has an estimated incidence of 0.29 episodes per child-year in pre-developed and 0.05 episodes per child-year in developed countries, making it the most common cause of pediatric sepsis; it is also the leading cause of mortality in children less than 5 y of age.5 Pre-developed countries with large populations of children bear the major burden of pediatric sepsis. Of the approximately 156 million new cases of pneumonia per year worldwide, 151 million are estimated to be in the developing world where a combination of contaminated water, poor sanitation, indoor air pollution, crowding, low birth weight, and insufficient immunization and nutrition allow pathogens to invade and multiply relatively unchecked in the body.5 This is why the first tier of the three tiered approach to the prevention of pediatric sepsis and its sequelae outlined in Figure 1 requires escalation of public health initiatives to remedy these issues. The second tier includes early identification and intervention to prevent sepsis progression from infection to sepsis to septic shock with end-organ damage. The third prevention tier includes intensive care supportive interventions to prevent sepsis-related death and disability.
Figure 1. Depiction of primary, secondary, and tertiary pediatric sepsis prevention efforts (modified, with permission from Dr Bala Totapally).
In this review, we limit our discussion to sepsis in term infants that have been exposed to the home environment through adolescents under 18 y of age. We exclude much of the discussion of perinatal and neonatal sepsis which is covered elsewhere in this special issue. The purpose of this article is to highlight the major differences between adults and children in the diagnosis of sepsis, the response of the immune system to infection, and the management of infants, children, and adolescents with life-threatening infections.
Pediatric Definitions of Sepsis, Severe Sepsis, and Septic Shock
When pediatricians use the term “sepsis”, they usually are referring to an infection that overwhelms the host causing capillary leak, hypotension, and/or respiratory failure. Relatively stable children admitted to the hospital with complicated pneumonia, pyelonephritis, invasive cellulitis, or bronchiolitis are given these descriptive discharge diagnoses even though most could be given the diagnosis of “sepsis” according to the definitions published in 2005 from the International Consensus Conference on Pediatric Sepsis (see Table 1). Those sepsis definitions were created to facilitate enrollment of children into clinical trials of anti-sepsis agents.6 They were modified only slightly from the consensus sepsis definitions created for adult patients originally published in 1992.7
Table 1. Definitions of illnesses in the sepsis continuum.
| Clinical syndrome | Criteria |
|---|---|
| Systemic inflammatory response syndrome (SIRS) | Must have at least 2 of the following of which at least one must be abnormal temperature or abnormal leukocyte count: |
| 1. Abnormal heart rate (HR) defined as tachycardia (HR >2 SD above normal for age in the absence of external stimulus, drugs, or painful stimuli; or otherwise unexplained elevation over 0.5–4 h) or bradycardia (HR <10th percentile for age in absence of external vagal stimulus, drugs, congenital heart disease; or otherwise unexplained HR depression >0.5 h). | |
| 2. Tachypnea >2 SD above normal for age or mechanical ventilation for process other than anesthesia or underlying neuromuscular disease | |
| 3. Abnormal temperature defined as fever (core temperature >38.5 °C) or hypothermia (core temperature <36 °C). | |
| 4. Abnormal leukocyte profile with counts either elevated or depressed for age (not due to chemotherapy); or >10% immature neutrophils | |
| Infection | A suspected infection or one proven by positive culture, tissue stain, or molecular testing caused by any pathogen or a clinical syndrome associated with a high probability of infection. Acceptable evidence can include physical exam, laboratory, or radiologic findings |
| Sepsis | SIRS resulting from or occurring in the presence of proven infection |
| Severe sepsis | Sepsis plus the following: cardiovascular organ dysfunction, acute respiratory distress syndrome (ARDS), or two or more other organ dysfunctions |
| Septic shock | Sepsis (defined above) and the following signs of cardiovascular organ dysfunction that remain after initial fluid resuscitation (40 ml/kg intravascularly in ≤1 h): |
| • Decrease in BP (hypotension) <5th percentile for age or systolic BP >2 SD below normal for age; OR | |
| • Need for vasoactive drug to maintain BP in normal range (dopamine >5 µg/kg/min or epinephrine, or norepinephrine at any dose); OR | |
| • At least two of the following | |
| - Unexplained metabolic acidosis: base deficit >5.0 mEq/L; | |
| - Increased arterial lactate >2 times upper limit of normal | |
| - Oliguria: urine output <0.5 mL/kg/h | |
| - Prolonged capillary refill: >5 s | |
| - Core to peripheral temperature gap >3 °C |
Adapted from reference 6.
Briefly, to be septic, a child must have a confirmed or suspected infection and signs of a systemic response to that infection. Severe sepsis requires diagnosis of end organ system involvement. Septic shock requires cardiovascular dysfunction that is not resolved by initial fluid resuscitation. These definitions were aimed at identifying sepsis in an early stage to facilitate early intervention, with the goal of stopping further spread of infection and preventing a severe life-threatening inflammatory reaction to infection.6,7
One major difference in the definition of sepsis in children vs. adults are the age-specific cutoffs for physiologic and organ system-related laboratory parameters. The healthy pediatric cardiovascular system can maintain cardiac output by employing extreme tachycardia for a prolonged period without inducing myocardial ischemia. Compared with adults, hypotension presents later in children and often portends imminent and potentially non-reversible cardiovascular collapse.8 As a result, the pediatric consensus guidelines are designed to identify patients with compensated septic shock in the hope that early intervention will prevent cases of profound decompensation leading ultimately to death. Consequently, children whose physical exam reveals cold extremities with delayed capillary refill despite receipt of boluses of intravenous fluid are diagnosed with septic shock and are treated similarly to children with life-threatening vasopressor-dependent decompensated septic shock.9 Although rigorous studies on the impact of these broadly encompassing sepsis definitions on clinical outcomes are lacking, there are some data to support that early recognition and treatment of sepsis can be life-saving for children in developed10 and pre-developed11 countries.
Response to Infection by the Developing Immune System
A child’s immune system is remarkably different from adults in terms of innate and adaptive immune function; in fact, full immunologic maturity is not reached until adolescence.12 The transition from a sterile, intrauterine environment, to the ecologically complex and changing microbiologic milieu that the infant must confront for the remainder of his or her lifetime, requires extreme shifts in immune function. Neonates are the most profoundly immune compromised, and their immune system is typified by comparatively poor innate and adaptive immune responses.13-19 In part, this gives them a survival advantage because a relatively suppressed immune system allows the newborn to tolerate colonization of previously sterile skin and gastrointestinal tract with normal bacterial flora without triggering an overwhelming inflammatory response.20 In neonates, phagocytes are less responsive to pathogen-associated molecular patterns (PAMPs) than adult cells, have diminished adhesion and extravasation activity, produce fewer pro-inflammatory cytokines, and have diminished antigen presentation activity to adaptive immune cells.21 Natural killer (NK) cells are less cytotoxic as well and complement levels are also only 10–70% of adult levels.16
Adaptive immunity is also suppressed in the very young. Although T cell levels are much higher in neonates than adults, their functionality is relatively poor, due in part to low production of interleukin-2 (IL-2). Helper CD4+ T cells are skewed toward Th-2 (humoral) responses in the neonate due to low production of interferon-γ (IFNγ), and cytotoxic CD8+ T cells are less active.22 B cells, although also abundant in the neonate, are predominantly naïve, produce mostly IgM immunoglobulins (Igs), and are poorly responsive to capsular polysaccharides. By 2 y of age, adaptive and immune responses have largely approached those of healthy adult levels, but full immune competence is not truly reached until the teenage years.
The cumulative result of these deficits in immune function is that infants and some toddlers have markedly increased susceptibility to severe infection from various organisms, particularly viruses and encapsulated bacteria. Susceptibility to severe viral infection is most prominent in children less than 2 y old due in part to unchecked viral replication caused by lower production of IFNγ and diminished cytotoxic lymphocyte responses.12 The primary mitigating factor during the first 6 mo of life is transplacentally acquired maternal antibody (primarily IgG and IgA), a reason for expanding the recommendations for maternal immunization to include coverage of common pathogens associated with severe pediatric illness.23 Additionally, the use of protein-polysaccharide conjugate vaccines can stimulate infant immune systems to produce protective levels of immunoglobulin to polysaccharide-encapsulated pathogens, including Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae (S. pneumoniae).24,25 Such vaccines can even change nasopharyngeal carriage of pathogens, conferring a benefit to the community.26 Unfortunately, vaccines to common viral pathogens, particularly influenza, are not approved for children less than 6 mo old and are poorly immunogenic in children <2 y old compared with older children and adults.27
Epidemiology and Clinical Manifestations of Pediatric Sepsis
The estimated overall prevalence of severe sepsis among children living in the United States increased steadily from 0.56 cases per 1000 children in 1995 to 0.63 cases per 1000 children in 2000, to 0.89 cases per 1000 children in 2005, with most of this increase due to newborn sepsis.3,28,29 For example, estimated prevalence of sepsis in US newborns is 9.7/1000 population, non-newborn infants 2.25/1000, and the rates are 0.23 to 0.52/1000 in subgroups of children 1–19 y of age.29 Such variation at the early extremes of life mirrors the markedly high rates reported in advanced elderly patients compared with younger adults.30 In fact, the clinical presentation of sepsis in 18- to 30-y-olds is very similar to the profile encountered in 12- to 17-y-olds and very different from patients aged 65 y and over. Also similar to adults, common pathogens causing sepsis in children differ not only by local geography but by age and medical co-morbidities.
In neonates presenting with late-onset sepsis, the most common bacterial pathogens include group B streptococci (GBS) and enteric gram-negative rods, especially Escherichia coli.31 Peripartum prophylaxis protocols have reduced the incidence of GBS-related sepsis.32 Bordatella pertussis can cause a severe illness in young infants, characterized by recurrent episodes of gagging, apnea, cyanosis, and bradycardia and with high mortality in those that develop respiratory failure and pulmonary hypertension.33-36 H. influenzae type b, previously one of the most common causes of bacterial sepsis in children <5 y old, and still a major cause of preventable pediatric mortality worldwide,37 is now uncommon in the developed world due to widespread use of the conjugate vaccine in infants.38,39 Similarly, although S. pneumoniae is still the leading cause of hospitalization for pneumonia in childhood, conjugate 7-valent and 13-valent S. pneumoniae vaccine use has decreased the incidence of invasive bacterial infection by as much as 76%.40,41
Another bacteria often isolated from infants and young children with severe sepsis in developed countries is Neisseria meningitidis.3 N. meningitidis infection, causing meningococcemia, peaks in a unique bimodal age distribution, first in infants and toddlers and again in adolescents where outbreaks can occur at schools, thus prompting recommendations for administering conjugate meningococcal vaccine for teenagers and debate among experts regarding potential vaccine strategies for infants.42 Meningococcemia most commonly occurs in previously healthy children, usually presenting with the sudden onset of fever, vomiting, headache, difficulty concentrating, and severe myalgias.43 The classic triad of fever, meningismus, and altered mental status occurs in only 27% of children with meningococcemia. Up to 25% of children with meningococcemia will progress to develop purpura fulminans, which is caused by microvascular thrombosis that leads to tissue necrosis, skin infarction, and hemorrhage.44 Children developing gangrene and tissue necrosis can require extensive amputations.45 Other causes of purpura fulminans include S. pneumoniae, S. pyogenes, and varicella.
Additional bacterial pathogens of concern include S. aureus and Streptococcus pyogenes (group A strep or GAS) which can lead to severe necrotizing pneumonias accompanied by septic shock in otherwise healthy children. S. aureus is of particular concern as it increasingly accounts for pediatric hospitalization for invasive disease and because the rising incidence of methicillin-resistant (MRSA) strains in communities impacts empiric antibiotic selection and longitudinal management.46 Increasing antimicrobial resistance among gram-negative enteric bacteria and opportunistic gram-negative pathogens (e.g., Pseudomonas, Acinetobacter, Burkholderia spp.), also raises the risk of mortality among infected children by delay of effective antibiotic treatment and/or from increased virulence that is observed in some multidrug-resistant organisms.47,48 Such organisms are most commonly identified in children hospitalized for prolonged periods with persistent indwelling devices such as intravascular catheters or tracheostomies,49 and in oncology and other immune-suppressed patients who have had multiple courses of broad-spectrum antibiotics.50 Among such children with multiple exposures to hospitals and other healthcare settings, nosocomial pathogens, including coagulase-negative staphylococci (CONS) and MRSA, should also be considered.51,52 Neutropenic patients are at high risk of mortality from gram-negative rod bacteremia (including Pseudomonas aeruginosa) and α-hemolytic streptococci (particularly in cases of mucositis).51,53
Viral-induced sepsis can result from a variety of viruses influenced by age and underlying immune status. Influenza is one of the most common cause of viral sepsis in children leading to one of the highest rate of hospitalizations and the highest number of deaths.54 Although vaccination may prevent the majority of influenza-related severe respiratory infections,55 low vaccination rates, decreased vaccine response in young children, and periods of poor match between circulating viruses and influenza vaccine lead to an ongoing healthcare burden.56 Although parainfluenza virus most commonly stays in the upper airway causing croup mostly in healthy children, it can cause severe pneumonia in the very young and in children with compromised immune or respiratory symptoms57 as can adenovirus.58
Bronchiolitis, a viral lower respiratory tract infection syndrome characterized by hyperinflation, increased mucous production, air trapping, and wheezing, affects 1–2% of infants worldwide.59 Although respiratory syncytial virus (RSV) is identified in the majority of infants hospitalized for bronchiolitis in the developed world,60 very few of these infants die if given supportive care. Human metapneumovirus and rhinovirus are increasingly identified as a cause of hospitalization for bronchiolitis in infants.60 Risk factors for life-threatening bronchiolitis and viral sepsis include premature birth, chronic lung disease, congenital cardiac abnormalities, and primary immunodeficiency.61
Viral–bacterial co-infection occurs in up to 23% of cases of severe pneumonia, resulting in a higher likelihood of respiratory failure and septic shock.62 The viral infection is thought to precede and predispose children to bacterial invasion. For example, MRSA was recently reported to be associated with mortality in previously healthy children infected with influenza,63 especially in the 2009 influenza pandemic where this fatal coinfection was a strong mortality predictor causing unrelenting destruction of lung despite appropriate antibiotics.64 Although the mechanism underlying viral–bacterial coinfection is unclear, this highest risk subgroup of children with influenza–S. aureus co-infection were shown in one study to be more likely than those with influenza alone to have cytokine storm that coexisted with a decreased monocyte response to ex vivo stimulation with lipopolysaccharide (aka “immunoparalysis”).65 Neonates are susceptible to overwhelming viral sepsis from herpes simplex virus (HSV), enterovirus, and parechoviruses,66-68 and profoundly immune-compromised children from cancer or HIV can develop sepsis from HSV, acute cytomegalovirus, adenovirus, or Epstein–Barr virus infections)69-71 Aside from influenza virus, older children and adolescents with healthy immune and cardiorespiratory systems are rarely hospitalized for viral sepsis.
Diarrheal diseases are another major cause of sepsis in infants and children, especially in the pre-developed world. Public health sanitation interventions and availability of clean water are essential and highly effective in decreasing sepsis-related mortality in children worldwide. In developed countries, rotavirus can lead to a profound diarrhea and sepsis-like picture in very young children prompting development of the rotavirus vaccine.72
Several other pathogens cause sepsis primarily in pre-developed countries. Dengue virus, a mosquito-borne flavivirus endemic to many tropical countries, causes a sepsis syndrome typified by capillary leak and disseminated intravascular coagulation (DIC).73 Malaria—particularly Plasmodium falciparum—can cause sepsis in young children and HIV-infected children; sepsis is often seen in association with cerebral malaria presenting with symptoms of altered mental status, convulsions, and acidosis.74,75 Burkholderia pseudomallei, or meliodosis, seen in southeast Asia, can present with pulmonary symptoms and fever.76
Less common causes of sepsis should be considered as well, depending on risk factors. Fungal pathogens, particularly Candida species, cause up to 10% of severe septic shock in children.3 Depending on geographic exposures, tick-borne diseases that can present as encephalitis meeting criteria for sepsis include Rocky Mountain Spotted Fever and ehrlichiosis.77 Salmonella species should be considered, particularly in children with functional asplenia and those who are malnourished.78 In endemic areas, children with immune compromise and/or asplenia are at risk for babesiosis. Finally, it is important to note that, despite advances in microbiologic detection methods, the underlying cause of sepsis remains unknown in up to 75% of pediatric cases.79
Primary and Acquired Immune Deficiency in Sepsis
Although neonates, infants, and young children are at increased risk for severe infection and sepsis compared with older children and adolescents, it is critical to recognize recurring patterns of infection or severe clinical presentations suggesting that the child may have an underlying immune deficiency.80 A thorough evaluation for a primary immune deficiency should include a detailed medical history that includes gestation, birth, growth, development, and immunizations, a family history, and a history of prior infections with special attention to pathogens identified and sites of infection. Screening labs can then be considered in conjunction with specialist consultation.81 Table 2 lists pathogens and clinical presentations associated with selected primary immune deficiency categories.
Table 2. Infections and infection-related syndromes associated with underlying immune deficiencies.
| Underlying type of immune deficiency | Example/etiology | Associated infections and infection-related syndromes |
|---|---|---|
| Agammaglobulinemias | ||
| All Ig isotypes deficient or absent | X-linked agammaglobulinemia | Hib, S. pneumoniae (recurrent respiratory infections) |
| Giardia lamblia, rotavirus (chronic diarrhea) | ||
| Enterovirus (chronic meningoencephalitis) | ||
| One or more (but not all) Ig isotypes reduced/absent | Common variable immune deficiency (CVID) | Hib, S. pneumoniae (recurrent respiratory infections) |
| C. jejuni, Salmonella spp., Giardia (gastrointestinal infections) | ||
| Autoimmune disease | ||
| Immunoglobulin class switch recombination disorders | Hyper-IgM syndromes | Recurrent/severe sinopulmonary infections |
| P. jirovecii infection in first year of life | ||
| Cryptosporidium, Giardia | ||
| Autoimmune disorders | ||
| Complement deficiencies | ||
| Common pathway | C3 | Bacteremia or sepsis from H. influenzae, S. pneumoniae, meningococci, encapsulated bacteria |
| Mannose-binding lectin (MBL) pathway | Polymorphisms with low MBL levels | Meningococci, S. pneumoniae, among others |
| Late complement defects and alternative pathway defects | C5–C9, properdin | Sepsis, disseminated infection from meningococci, N. gonorrhoeae |
| Phagocyte disorders | ||
| Absent/defective oxidative burst | Chronic granulomatous disease (CGD) | Burkholderia cepacia pneumonia, Nocardia spp., Aspergillus spp., S. aureus infections, liver abscesses |
| Decreased/absent hypochlorous acid production | Myeloperoxidase deficiency | Invasive Candida spp. infections |
| Lysosomal packaging disorder | Chediak–Higashi syndrome | Recurrent respiratory, skin, and soft tissue infections with S. aureus, oculocutaneous albinism |
| Cell-mediated immunity | ||
| T- and B-cell dysfunction | Severe combined immunodeficiency syndrome (SCID) | Opportunistic infections (incl. P. jirovecii), fungal infections, invasive bacterial infections, persistent, severe viral infections (RSV, VZV, HSV, CMV), occurring in infancy |
| T-cell and NK cell disorders | Ataxia–telangiectasia | Severe sinopulmonary infections, ± opportunistic infections |
| Hyper Ig-E syndrome | Recurent pneumonias from S. aureus, H. influenzae, S. pneumoniae, severe eczema | |
| NK cell deficiency | Severe HSV, VZV, CMV infection beyond infancy | |
Summarized from reference 114. Definitions: Ig, Immunoglobulin; Hib, H. influenzae type b; RSV, respiratory syncytial virus; VZV, varicella zoster virus; HSV, herpes simplex virus; CMV, cytomegalovirus; NK, natural killer.
Human immunodeficiency virus increases the risk for sepsis in infected children substantially, although this risk is mitigated with antiretroviral therapy use.82-84 Similar to severe combined immune deficiency syndromes (SCID) and other disorders of T-cell function, sepsis can arise from infection with typical pathogens (e.g., S. pneumoniae), from invasive fungal infections or from opportunistic infection such as disseminated mycobacterial infection. In pre-developed countries, HIV infection is significantly associated with disseminated infection from Mycobacterium tuberculosis as well.84
Management of Sepsis in Infants and Children Compared with Adults
In part, due to the challenge of performing clinical trials in children with sepsis-related critical illness85 there are a paucity of strong data from rigorous and appropriately-powered clinical trials to guide the management of children with severe sepsis. Consequently, published recommendations for sepsis management in infants and children closely mimic those for adult patients. The Surviving Sepsis Guidelines (2012 update) lists few differences in pediatric management recommendations from those in adults and these are summarized in Table 3.86 Of primary importance, as in adult patients with sepsis, is to provide empiric antimicrobial therapy that treats potential causative pathogens based on a patient’s age and exposure history.87 Full discussion of the details of managing sepsis in children is beyond the scope of this article, but we do note a few unique aspects of treating the pediatric septic patient. One major difference in how children with refractory septic shock and/or refractory hypoxemia from severe respiratory tract infection are clinically managed compared with adults is a higher use of extracorporeal life support (ECLS) as rescue therapy.86 ECLS survival rates in children with severe pneumonia and refractory septic shock reported by the ELSO registry are 50% and are above 80% in those who have single organ failure (refractory respiratory failure) triggered viral infection.88
Table 3. A capsule summary of pediatric-specific consensus recommendations for sepsis management from the 2012 Surviving Sepsis Guidelines86 and Pediatric Advanced Life Support Guidelines111.
| Initial Resuscitation: Pediatric Specific Considerations |
| 1. Infants anatomically have low pulmonary functional residual capacity and can desaturate very quickly. Supplemental oxygen should be delivered via face mask or nasal cannula or other devices to children with septic shock even if oxygen saturation levels appear normal with peripheral monitoring devices. |
| 2. Peripheral intravenous access is often difficult to obtain in hemodynamically unstable infants and young children. If unable to obtain peripheral intravenous access quickly, early use of intraosseus access is recommended for fluid resuscitation, inotrope infusion and delivery of antibiotics when central venous access is not easily obtainable. If mechanical ventilation is required then cardiovascular instability during intubation may be less likely after appropriate cardiovascular resuscitation. |
| 3. The American College of Critical Care Medicine-Pediatric Life Support (ACCM-PALS) guidelines112 are recommended for the management of septic shock in children. |
| Antibiotics and Source Control |
| 1. Empiric antibiotics should be administered within the first hour of determining that the patient has severe sepsis. Obtaining blood cultures prior to antibiotics is preferred, when possible, but should not delay antibiotic administration. |
| a. The empiric drug choice must be tailored to epidemic and endemic ecologies and consideration for treatment of resistant organisms is essential. |
| b. Clindamycin and anti-toxin therapies for toxic shock syndromes with refractory hypotension are recommended. |
| 2. Early and aggressive source control is essential. Because infants and young children have difficulty communicating the location of their pain, radiologic imaging is an essential part of the workup in children with severe sepsis. |
| Fluid Resuscitation |
| 1. In the industrialized world with access to inotropes and mechanical ventilation, initial resuscitation of hypovolemic shock begins with infusion of isotonic crystalloids (or albumin equivalent) with repeated boluses of up to 20 mL/kg of crystalloids (or albumin equivalent) over 5–10 min, titrated to reversing hypotension, increasing urine output, and attaining normal capillary refill, peripheral pulses, and level of consciousness. |
| a. In a child with hepatomegaly or rales, early inotropic support should be implemented, and fluid resuscitation carefully titrated. |
| 2. In children with compensated shock in resource-limited settings without access to inotropes or mechanical ventilation, fluid boluses may be harmful.92 Blood transfusion should be considered in patients with compensated shock who are profoundly anemic. |
| Extracorporeal Membrane Oxygenation (ECMO) |
| 1. Consider ECMO for refractory pediatric septic shock with respiratory failure. |
| Blood Products and Plasma Therapies |
| 1. Hemoglobin targets are similar in children as in adults. In hemodynamically unstable children in shock on vasopressor infusions, hemoglobin levels of ≥10 g/dL are targeted. In stable critically ill children, a lower hemoglobin target of ≥7.0 g/dL is recommended.113 |
| 2. Similar platelet transfusion targets in children as in adults. |
| 3. Consider plasma therapies in children to correct sepsis-induced thrombotic purpura disorders, including progressive disseminated intravascular coagulation, secondary thrombotic microangiopathy, and thrombotic thrombocytopenic purpura. |
Additionally, early use of goal-directed therapeutic targets, such as rapid and repeated fluid resuscitation, and early institution of vasopressor support when fluid resuscitation fails, are associated with decreased mortality in meningococcemia,89 This aggressive fluid resuscitation strategy has been extrapolated to pediatric treatment recommendations for other causes and presentations of pediatric sepsis.10,11,90,91 Recent data from the FEAST trial of fluid management in over 3000 African children showed markedly higher mortality those with compensated shock randomized to receive repeated boluses of saline or albumin compared with children who did not receive fluid boluses.92 Although these children had high rates of malaria and severe anemia, secondary analyses did not identify them as contributors to the increased mortality in the children receiving fluids.93 The results of the FEAST trial have raised concerns internationally as to whether the aggressive use of fluid boluses even in developed countries might harm children with compensated shock.94 Positive fluid balance has been associated with worse clinical outcome in other studies of critically ill children, many of whom had sepsis.95-98 Table 4 lists multiple pediatric sepsis resources that provide additional recommendations regarding management.
Table 4. Selected resources and guidelines for clinicians related to pediatric sepsis.
| Resource | Location | Comments |
|---|---|---|
| Infectious Diseases Society of America | http://www.idsociety.org | Provides links to clinical practice guidelines regarding the treatment of multiple bacteria and viruses for children including a guideline on management of pediatric community acquired pneumonia |
| Red Book | aapredbook.aappublications.org | In-depth pediatric infectious diseases resource published by the American Academy of Pediatrics |
| World Health Organization Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources | http://www.who.int/child-adolescent-health/publications/CHILD_HEALTH/PB.htm | Provides diagnostic and treatment guidelines for managing children suffering from various illnesses, including severe infections and sepsis. |
| Centers for Disease Control Healthcare Infection Control Practices Advisory Committee (HICPAC) | http://www.cdc.gov/hicpac/pubs.html | Publishes guidelines for prevention, surveillance, and treatment of nosocomial infections in multiple healthcare settings |
| UpToDate | http://www.uptodate.com | Evidence-based clinician support at the point of care electronic resource with many chapters devoted to pediatric sepsis and pediatric infections. |
| American College of Critical Care Medicine Guidelines for the Management of Pediatric Septic Shock | Currently integrated into the American Heart Association Pediatric Advanced Life Support guidelines as well as from original source112 | Algorithm for the management of septic shock in children. |
| Surviving Sepsis Campaign Guidelines for the Management of Sepsis | http://www.survivingsepsis.org | Evidence-based guidelines for the management of sepsis in adults and children. |
Factors Influencing Pediatric Sepsis-Related Mortality
Although children <12 mo old have the highest risk of death from sepsis, much of this mortality is driven by the high incidence of sepsis and the high rate of sepsis-related mortality in infants born very prematurely.3 Compared with older children, infants have the highest incidence of severe sepsis but much of it is viral and most will survive hospitalization. A higher mortality among male patients, suggested by studies in adult patients and animals, appears less prominent in children, although males are more likely to be hospitalized in infancy for severe infections.3,99,100 The incidence of malignancies and other chronic respiratory and cardiac conditions in children rises with age and contributes to sepsis-related mortality; the majority of older children hospitalized with sepsis have underlying conditions impairing their immune or cardiorespiratory systems.3
Site of infection also is related to likelihood of severe sepsis and death, with endocarditis and CNS infections associated with the highest mortality rates (21.1% endocarditis, 17.1% CNS infections).3 Certain organisms are associated with worse prognosis, particularly fungi, and infections with antibiotic-resistant bacteria, including MRSA, gram-negative bacilli, and nosocomial pathogens.101-103 The extent of systemic involvement is also very important, as children who develop multiple organ system failure from sepsis have the lowest likelihood of surviving.3,104 Survival from sepsis, after adjusting for sepsis severity, is usually higher in children compared with adults. In a large pediatric clinical trial of recombinant human-activated Protein C in pediatric septic shock,105 mortality in the cohort of the the septic children enrolled (who were all mechanically ventilated and on vasopressors) was approximately 17% compared with rates of 26–34% in clinical trials in adult sepsis and septic shock patients who did not have to be mechanically ventilated to gain entry.106,107
Although there are a paucity of national-level data on pediatric sepsis outcomes from pre-developed countries,108 the World Federation of Pediatric Intensive and Critical Care Societies (WFPICCS) is working to change this through their global sepsis initiative (http://www.wfpiccs.org). Sepsis mortality has been reported in studies of dengue fever and specific infections but few trials in pre-developed countries have enrolled a heterogeneous group of children with sepsis.109 Over half of African children in the multicenter FEAST trial that presented with hypotension and decompensated shock died.110 In the group that included mostly children with compensated septic shock, mortality was 7.3–10.6% in the first 48 h after presentation and 8.7–12.2% at 4 weeks depending on the study arm. In addition, approximately 2% of survivors had severe neurologic sequelae.92
Summary
In this brief review, we have highlighted some of the major differences between the diagnosis, epidemiology, host immune response, treatment, and outcome of infants and children compared with adults. As shown in Figure 1, decreasing the high burden of sepsis in the pediatric population requires a multi-tiered approach. Prevention of sepsis is paramount and public health initiatives have been shown to be high-impact, cost-effective interventions. Early recognition of sepsis and initial management in the outpatient and hospital wards are essential for preventing progression to more severe forms. Supportive intensive care unit interventions such as mechanical ventilation, vasopressor infusions, and continuous monitoring modalities are essential for preventing sepsis-related disability and death.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bahl R, Martines J, Ali N, Bhan MK, Carlo W, Chan KY, Darmstadt GL, Hamer DH, Lawn JE, McMillan DD, et al. Research priorities to reduce global mortality from newborn infections by 2015. Pediatr Infect Dis J. 2009;28(Suppl):S43–8. doi: 10.1097/INF.0b013e31819588d7. [DOI] [PubMed] [Google Scholar]
- 2.Carcillo JA. Reducing the global burden of sepsis in infants and children: a clinical practice research agenda. Pediatr Crit Care Med. 2005;6(Suppl):S157–64. doi: 10.1097/01.PCC.0000161574.36857.CA. [DOI] [PubMed] [Google Scholar]
- 3.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 4.Wiens MO, Kumbakumba E, Kissoon N, Ansermino JM, Ndamira A, Larson CP. Pediatric sepsis in the developing world: challenges in defining sepsis and issues in post-discharge mortality. Clin Epidemiol. 2012;4:319–25. doi: 10.2147/CLEP.S35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 7.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20:724–6. doi: 10.1097/00003246-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. American Heart Association Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126:e1361–99. doi: 10.1542/peds.2010-2972D. [DOI] [PubMed] [Google Scholar]
- 9.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP) PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–73. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 10.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, Orr RA. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira CF, Nogueira de Sá FR, Oliveira DS, Gottschald AF, Moura JD, Shibata AR, Troster EJ, Vaz FA, Carcillo JA. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. 2008;24:810–5. doi: 10.1097/PEC.0b013e31818e9f3a. [DOI] [PubMed] [Google Scholar]
- 12.Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatr. 2012;101:120–7. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 14.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–25. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 15.Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11:113–6. doi: 10.1179/096805105X37376. [DOI] [PubMed] [Google Scholar]
- 16.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 17.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleer A, Krediet TG. Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007;92:145–57. doi: 10.1159/000102054. [DOI] [PubMed] [Google Scholar]
- 19.Maródi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–44. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–12. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr. 2011;11:29. doi: 10.1186/1471-2431-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sautois B, Fillet G, Beguin Y. Comparative cytokine production by in vitro stimulated mononucleated cells from cord blood and adult blood. Exp Hematol. 1997;25:103–8. [PubMed] [Google Scholar]
- 23.Healy CM, Baker CJ. Prospects for prevention of childhood infections by maternal immunization. Curr Opin Infect Dis. 2006;19:271–6. doi: 10.1097/01.qco.0000224822.65599.5b. [DOI] [PubMed] [Google Scholar]
- 24.Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology. 2004;113:163–74. doi: 10.1111/j.1365-2567.2004.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, Hackell J, Paradiso PR, Siber G, Kohberger R, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–63. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: Review of evidence on indirect effects. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.05.005. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 27.Beeler JA, Eichelberger MC. Influenza and respiratory syncytial virus (RSV) vaccines for infants: safety, immunogenicity, and efficacy. Microb Pathog. 2013;55:9–15. doi: 10.1016/j.micpath.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119:487–94. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 29.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14:686–93. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 30.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60:367–89. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baltimore RS. Consequences of prophylaxis for group B streptococcal infections of the neonate. Semin Perinatol. 2007;31:33–8. doi: 10.1053/j.semperi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt P, Halasa N. Increasing rates of infants hospitalized with pertussis. Tenn Med. 2007;100:37–9, 42. [PubMed] [Google Scholar]
- 34.Hoppe JE. Neonatal pertussis. Pediatr Infect Dis J. 2000;19:244–7. doi: 10.1097/00006454-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, Wu KH, Goldsmith CS, Greer PW, Montague JL, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47:328–38. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 36.Berger JT, Carcillo JA, Shanley TP, Wessel DL, Clark A, Holubkov R, Meert KL, Newth CJ, Berg RA, Heidemann S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN) Critical pertussis illness in children: a multicenter prospective cohort study. Pediatr Crit Care Med. 2013;14:356–65. doi: 10.1097/PCC.0b013e31828a70fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 38.Cowgill KD, Ndiritu M, Nyiro J, Slack MP, Chiphatsi S, Ismail A, Kamau T, Mwangi I, English M, Newton CR, et al. Effectiveness of Haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–8. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenger JD. Epidemiology of Haemophilus influenzae type b disease and impact of Haemophilus influenzae type b conjugate vaccines in the United States and Canada. Pediatr Infect Dis J. 1998;17(Suppl):S132–6. doi: 10.1097/00006454-199809001-00008. [DOI] [PubMed] [Google Scholar]
- 40.Myint TT, Madhava H, Balmer P, Christopoulou D, Attal S, Menegas D, Sprenger R, Bonnet E. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30:127–51. doi: 10.1007/s12325-013-0007-6. [DOI] [PubMed] [Google Scholar]
- 41.Shibl AM, Memish ZA, Al-Kattan KM. Antibiotic resistance and serotype distribution of invasive pneumococcal diseases before and after introduction of pneumococcal conjugate vaccine in the Kingdom of Saudi Arabia (KSA) Vaccine. 2012;30(Suppl 6):G32–6. doi: 10.1016/j.vaccine.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC) Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62(RR-2):1–28. [PubMed] [Google Scholar]
- 43.Sabatini C, Bosis S, Semino M, Senatore L, Principi N, Esposito S. Clinical presentation of meningococcal disease in childhood. J Prev Med Hyg. 2012;53:116–9. [PubMed] [Google Scholar]
- 44.Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, Hop WC, Dekker I, Joosten KF, de Groot R, Hack CE. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost. 1996;76:932–8. [PubMed] [Google Scholar]
- 45.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, Yeh T, Kim SS, Cafaro DP, Scannon PJ, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356:961–7. doi: 10.1016/S0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC) Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Hasan MN, Huskins WC, Lahr BD, Eckel-Passow JE, Baddour LM. Epidemiology and outcome of Gram-negative bloodstream infection in children: a population-based study. Epidemiol Infect. 2011;139:791–6. doi: 10.1017/S0950268810001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CY, Chen PY, Huang FL, Lin CF. Microbiologic spectrum and susceptibility pattern of clinical isolates from the pediatric intensive care unit in a single medical center - 6 years’ experience. J Microbiol Immunol Infect. 2009;42:160–5. [PubMed] [Google Scholar]
- 50.Joram N, de Saint Blanquat L, Stamm D, Launay E, Gras-Le Guen C. Healthcare-associated infection prevention in pediatric intensive care units: a review. Eur J Clin Microbiol Infect Dis. 2012;31:2481–90. doi: 10.1007/s10096-012-1611-0. [DOI] [PubMed] [Google Scholar]
- 51.Hocevar SN, Edwards JR, Horan TC, Morrell GC, Iwamoto M, Lessa FC. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol. 2012;33:1200–6. doi: 10.1086/668425. [DOI] [PubMed] [Google Scholar]
- 52.Stover BH, Shulman ST, Bratcher DF, Brady MT, Levine GL, Jarvis WR, Pediatric Prevention Network Nosocomial infection rates in US children’s hospitals’ neonatal and pediatric intensive care units. Am J Infect Control. 2001;29:152–7. doi: 10.1067/mic.2001.115407. [DOI] [PubMed] [Google Scholar]
- 53.Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, Garrè ML, Moroni C, Conte M, Losurdo G, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1296–304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 54.Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, Likos AM, Posey DL, Klimov A, Lindstrom SE, et al. Influenza Special Investigations Team Influenza-associated deaths among children in the United States, 2003-2004. N Engl J Med. 2005;353:2559–67. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 55.Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;8:CD004879. doi: 10.1002/14651858.CD004879.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet. 2005;365:773–80. doi: 10.1016/S0140-6736(05)17984-7. [DOI] [PubMed] [Google Scholar]
- 57.Chong DC, Raboni SM, Abujamra KB, Marani DM, Nogueira MB, Tsuchiya LR, Neto HJ, Flizikowski FB, de Noronha L. Respiratory viruses in pediatric necropsies: an immunohistochemical study. Pediatr Dev Pathol. 2009;12:211–6. doi: 10.2350/07-02-0229.1. [DOI] [PubMed] [Google Scholar]
- 58.García García ML, Ordobás Gabin M, Calvo Reya C, González Alvarez M, Aguilar Ruiz J, Arregui Sierra A, Pérez Breña P. [Viral infection of the lower respiratory tract in hospitalized infants: etiology, clinical features and risk factors] An Esp Pediatr. 2001;55:101–7. [PubMed] [Google Scholar]
- 59.Wagner T. Bronchiolitis. Pediatr Rev. 2009;30:386–95. doi: 10.1542/pir.30-10-386. [DOI] [PubMed] [Google Scholar]
- 60.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 61.Law BJ, Carbonell-Estrany X, Simoes EA. An update on respiratory syncytial virus epidemiology: a developed country perspective. Respir Med. 2002;96(Suppl B):S1–7. doi: 10.1053/rmed.2002.1294. [DOI] [PubMed] [Google Scholar]
- 62.Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, Kauppila J, Leinonen M, McCracken GH., Jr. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–7. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 63.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122:805–11. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 64.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, et al. Pediatric Acute Lung Injury and Sepsis Investigator’s Network and the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–8. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network PICFlu Study Investigators Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–36. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis. 2005;16:271–81. doi: 10.1053/j.spid.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Sharp J, Harrison CJ, Puckett K, Selvaraju SB, Penaranda S, Nix WA, Oberste MS, Selvarangan R. Characteristics of young infants in whom human parechovirus, enterovirus or neither were detected in cerebrospinal fluid during sepsis evaluations. Pediatr Infect Dis J. 2013;32:213–6. doi: 10.1097/INF.0b013e318276b328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27:241–5. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 69.Hatherill M. Sepsis predisposition in children with human immunodeficiency virus. Pediatr Crit Care Med. 2005;6(Suppl):S92–8. doi: 10.1097/01.PCC.0000161579.39050.6B. [DOI] [PubMed] [Google Scholar]
- 70.Stanberry LR, Floyd-Reising SA, Connelly BL, Alter SJ, Gilchrist MJ, Rubio C, Myers MG. Herpes simplex viremia: report of eight pediatric cases and review of the literature. Clin Infect Dis. 1994;18:401–7. doi: 10.1093/clinids/18.3.401. [DOI] [PubMed] [Google Scholar]
- 71.Steiner I, Aebi C, Ridolfi Lüthy A, Wagner B, Leibundgut K. Fatal adenovirus hepatitis during maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50:647–9. doi: 10.1002/pbc.21120. [DOI] [PubMed] [Google Scholar]
- 72.Scheier E, Aviner S. Septicemia following rotavirus gastroenteritis. Isr Med Assoc J. 2013;15:166–9. [PubMed] [Google Scholar]
- 73.Singhi S, Kissoon N, Bansal A. Dengue and dengue hemorrhagic fever: management issues in an intensive care unit. J Pediatr (Rio J) 2007;83(Suppl):S22–35. doi: 10.2223/JPED.1622. [DOI] [PubMed] [Google Scholar]
- 74.Maitland K. Severe malaria: lessons learned from the management of critical illness in children. Trends Parasitol. 2006;22:457–62. doi: 10.1016/j.pt.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Summer AP, Stauffer WM, Fischer PR. Pediatric malaria in the developing world. Semin Pediatr Infect Dis. 2005;16:105–15. doi: 10.1053/j.spid.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Lumbiganon P, Viengnondha S. Clinical manifestations of melioidosis in children. Pediatr Infect Dis J. 1995;14:136–40. doi: 10.1097/00006454-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Kunze U, Asokliene L, Bektimirov T, Busse A, Chmelik V, Heinz FX, Hingst V, Kadar F, Kaiser R, Kimmig P, et al. Tick-borne encephalitis in childhood--consensus 2004. Wien Med Wochenschr. 2004;154:242–5. doi: 10.1007/s10354-004-0061-4. [DOI] [PubMed] [Google Scholar]
- 78.Rosanova MT, Paganini H, Bologna R, Lopardo H, Ensinck G. Risk factors for mortality caused by nontyphoidal Salmonella sp. in children. Int J Infect Dis. 2002;6:187–90. doi: 10.1016/S1201-9712(02)90109-8. [DOI] [PubMed] [Google Scholar]
- 79.Gaines NN, Patel B, Williams EA, Cruz AT. Etiologies of septic shock in a pediatric emergency department population. Pediatr Infect Dis J. 2012;31:1203–5. doi: 10.1097/INF.0b013e3182678ca9. [DOI] [PubMed] [Google Scholar]
- 80.Ballow M. Approach to the patient with recurrent infections. Clin Rev Allergy Immunol. 2008;34:129–40. doi: 10.1007/s12016-007-8041-2. [DOI] [PubMed] [Google Scholar]
- 81.Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(Suppl 2):S297–305. doi: 10.1016/j.jaci.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alarcón JO, Freimanis-Hance L, Krauss M, Reyes MF, Cardoso CA, Mussi-Pinhata MM, Cardoso E, Hazra R, NISDI Pediatric Study Group 2011 Opportunistic and other infections in HIV-infected children in Latin America compared to a similar cohort in the United States. AIDS Res Hum Retroviruses. 2012;28:282–8. doi: 10.1089/aid.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Álvaro-Meca A, Jensen J, Micheloud D, Díaz A, Gurbindo D, Resino S. Rate of candidiasis among HIV-infected children in Spain in the era of highly active antiretroviral therapy (1997-2008) BMC Infect Dis. 2013;13:115. doi: 10.1186/1471-2334-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, Seage GR., 3rd Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 85.Randolph AG, Meert KL, O’Neil ME, Hanson JH, Luckett PM, Arnold JH, Gedeit RG, Cox PN, Roberts JS, Venkataraman ST, et al. Pediatric Acute Lung Injury and Sepsis Investigators Network The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–40. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 86.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 87.O’Rourke J. Fear of excellence. Eye Ear Nose Throat Mon. 1971;50:1–2. [PubMed] [Google Scholar]
- 88.Jen HC, Shew SB. Hospital readmissions and survival after nonneonatal pediatric ECMO. Pediatrics. 2010;125:1217–23. doi: 10.1542/peds.2009-0696. [DOI] [PubMed] [Google Scholar]
- 89.Booy R, Habibi P, Nadel S, de Munter C, Britto J, Morrison A, Levin M, Meningococcal Research Group Reduction in case fatality rate from meningococcal disease associated with improved healthcare delivery. Arch Dis Child. 2001;85:386–90. doi: 10.1136/adc.85.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–75. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 91.Wills BA, Nguyen MD, Ha TL, Dong TH, Tran TN, Le TT, Tran VD, Nguyen TH, Nguyen VC, Stepniewska K, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–89. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- 92.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. FEAST Trial Group Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 93.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, et al. FEAST trial group Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Myburgh J, Finfer S. Causes of death after fluid bolus resuscitation: new insights from FEAST. BMC Med. 2013;11:67. doi: 10.1186/1741-7015-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–8. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 96.Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–25. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 98.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, Brett M, Convery M, Christie LM, Karamessinis L, et al. Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network. Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet) Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–9. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.García-Gómez E, González-Pedrajo B, Camacho-Arroyo I. Role of sex steroid hormones in bacterial-host interactions. Biomed Res Int. 2013;2013:928290. doi: 10.1155/2013/928290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–5. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 101.Burke RE, Halpern MS, Baron EJ, Gutierrez K. Pediatric and neonatal Staphylococcus aureus bacteremia: epidemiology, risk factors, and outcome. Infect Control Hosp Epidemiol. 2009;30:636–44. doi: 10.1086/597521. [DOI] [PubMed] [Google Scholar]
- 102.Chang PY, Hsueh PR, Wu PS, Chan PC, Yang TT, Lu CY, Chang LY, Chen JM, Lee PI, Lee CY, et al. Multidrug-resistant Acinetobacter baumannii isolates in pediatric patients of a university hospital in Taiwan. J Microbiol Immunol Infect. 2007;40:406–10. [PubMed] [Google Scholar]
- 103.Hounsom L, Grayson K, Melzer M. Mortality and associated risk factors in consecutive patients admitted to a UK NHS trust with community acquired bacteraemia. Postgrad Med J. 2011;87:757–62. doi: 10.1136/pgmj.2010.116616. [DOI] [PubMed] [Google Scholar]
- 104.Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Hubert P, Lacroix J. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348–53. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 105.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, et al. REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–43. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 106.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 107.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, et al. PROWESS-SHOCK Study Group Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 108.Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2:10404. doi: 10.7189/jogh.01.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaramillo-Bustamante JC, Marín-Agudelo A, Fernández-Laverde M, Bareño-Silva J. Epidemiology of sepsis in pediatric intensive care units: first Colombian multicenter study. Pediatr Crit Care Med. 2012;13:501–8. doi: 10.1097/PCC.0b013e31823c980f. [DOI] [PubMed] [Google Scholar]
- 110.Maitland K, Babiker A, Kiguli S, Molyneux E, FEAST Trial Group The FEAST trial of fluid bolus in African children with severe infection. Lancet. 2012;379:613–4, author reply 613-4. doi: 10.1016/S0140-6736(12)60260-8. [DOI] [PubMed] [Google Scholar]
- 111.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3):S876–908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 112.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, et al. TRIPICU Investigators. Canadian Critical Care Trials Group. Pediatric Acute Lung Injury and Sepsis Investigators Network Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 114.Principles and Practice of Pediatric Infectious Diseases. 4th ed. Eds: Long SS, Pickering LK, Prober CG. Edinburgh: Elsevier, 2012; pp 600-41. [Google Scholar]