Abstract
Multi-antibiotic drug-resistant (MDR) gram-negative bacilli are becoming a major threat to the standard care of septic patients. Empiric antimicrobial drug regimens to cover likely bacterial pathogens have to be altered in keeping with the spread of MDR pathogens in the health care setting and in the community. Reliable antibiotics for broad spectrum coverage for sepsis such as extended spectrum β-lactam antibiotics, carbapenems, and fluoroquinolones can no longer be counted upon to provide activity against a range of common, virulent pathogens that cause sepsis. In some regions of Asia, South America, and Eastern Europe in particular, MDR pathogens have become a major concern, necessitating the use of potentially toxic and costly antibiotic combinations as initial antibiotic therapy for septic shock. In this brief review, we will focus on the emergence of MDR gram-negative pathogens, resistance mechanisms, and suggest some management and prevention strategies against MDR pathogens.
Keywords: sepsis, septic shock, bacterial resistance, multidrug resistance, extended antibiotic resistance
Introduction
Medical care is an increasingly complex environment. Patients undergo ever more sophisticated surgeries, transplants, implanted devices, immunotherapies and chemotherapies, and their compromised immune defenses make them ever more vulnerable to infection. Unfortunately, the global spread of multidrug antimicrobial resistance and the paucity of novel antimicrobials in development are limiting clinicians’ ability to provide effective and safe treatments for these very susceptible patients. Resistance to antimicrobial agents in gram-negative bacilli is particularly challenging. Of the famous ESKAPE pathogens identified as the most important emerging threats in antimicrobial resistance (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter species, Pseudomonas aeruginosa, and Enterobacter species), the majority are gram-negative bacilli.1
In this review we will address the epidemiology, mechanisms of resistance, impact on clinical outcomes, treatment and prevention of the most important multidrug-resistant gram-negative pathogens encountered in clinical practice. Resistance mechanisms, phenotypes, and therapeutic options for the most common multidrug-resistant organisms encountered in clinical practice are summarized in Table 1.
Table 1. Resistance mechanisms, phenotypes, and therapeutic options for common multidrug-resistant organisms encountered in clinical practice.
| Pathogen | Resistance mechanisms | Resistance phenotype (antibiotics affected) | Therapy options |
|---|---|---|---|
| Escherichia coli | Extended-spectrum β-lactamases (ESBL)a | All penicillins, narrow spectrum cephalosporins, oxymino-β-lactams (cefotaxime, ceftazidime, cefepime), aztreonam | Carbapenems |
| Klebsiella pneumoniaea | Carbapenemases (i.e., KPC, NDM) | All penicillins, cephalosporins, carbapenems | Polymyxins,b tigecyclinec |
| Pseudomonas aeruginosa | Active efflux, porin loss, carbapenemases | Quinolones, aminoglycosides, anti-pseudomonal pencillins, cephalosporins, carbapenems | Polymyxinsd |
| Acinetobacter baumanii | Active efflux, porin loss, amp-C cephalosporinases, carbapenemases | Quinolones, penicillins, cephalosporins, carbapenems | Polymyxinse, Tigecyclinec |
a Genes conferring resistance to fluoroquinolones, aminoglycosides, and trimethoprim-sulfametoxazole are often co-transmitted with mobile β-lactamases. bFor serious infections, combination therapy (i.e., polymyxin with tigecycline or extended-infusion meropenem) is preferred over monotherapy.49cTigecyline monotherapy not recommended for bacteremia or nosocomial pneumonia. dPolymyxins in combination therapy with other antipseudomonal agents or rifampin were reportedly active in vitro, but clinical data are rare. ePolymyxins in combination with rifampicin reported successful in small, retrospective series.52,56
Epidemiology
The burden of multidrug antibiotic resistance has substantially increased worldwide,2 with important regional differences in prevalence. For example, in the Study for Monitoring Antimicrobial Resistance Trends which collected isolates from 28 countries during 2004, extended spectrum β-lactamase (ESBL) producing bacteria were highest in Latin America, the Middle East, Africa, and Asia.3 A recent survey of ICUs throughout the world found a similar distribution of ESBL producing gram negative bacilli and found that gram-negative bacilli now outnumber gram-positive pathogens in ICU infections,4 reversing a 20 year trend where gram-positive pathogens were the previously dominant, causative pathogens of sepsis.5 In the United States, the recent spread of carbapenem resistance is most troublesome. Data from CDC’s National Healthcare Safety Network (NHSN) shows that nosocomial Klebsiella pneumoniae isolates resistant to carbapenem increased from 2% in 2001 to 10% in 2011.6
The importation of multidrug-resistant pathogens through travel to endemic areas is increasingly being recognized. For example, in a prospective study of 100 Swedish volunteers screened prior and post-travel, the abroad acquisition of ESBL-producing organisms was 24%. The average trip duration was 2 weeks, and India was the most common destination.7 In the US and the UK, emergence of the NDM-1 producing bacteria was similarly associated with foreign travel and receipt of medical care in India and Pakistan.4,5 In this regard, the frequency of contamination with Enterobacteriaceae, aeromonads, and nonfermenters carrying the NDM-1 gene found in a New Delhi environmental point prevalence study is particularly telling,8 as is the 2011 report that 18% of the tap water samples in New Delhi showed evidence of fecal contamination.9
Multidrug-resistant pathogens make ideal nosocomial pathogens, as they can survive for a prolonged time in the hospital environment,10 and can be transferred easily between patients through the hands of healthcare workers.11 However, antimicrobial resistance is no longer a phenomenon restricted to the healthcare setting. Emergence of community-acquired urinary tract infections with E. coli organisms producing CTX-M extended spectrum β-lactamases and fluoroquinolones have been increasingly documented in the US, Europe, and Asia.12-14
Mechanisms of Antibiotic Resistance
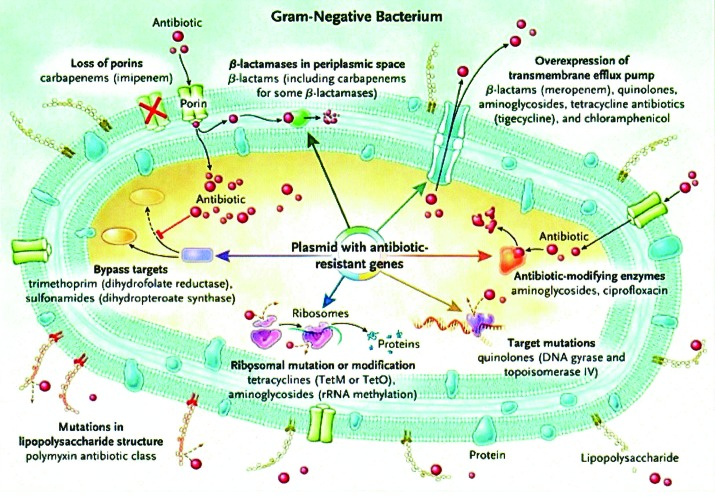
Gram-negative bacteria can employ a variety of mechanisms to achieve resistance to a single class or to multiple classes of antibiotics, as depicted in Figure 1. Mobile genetic elements such as plasmids can facilitate further spread of resistance genes between species.
Figure 1. Mechanisms of antimicrobial resistance in gram-negative bacteria. Re-printed with permission from reference 43.
β-lactam resistance
Penicillin, cephalosporin, and aztreonam
Penicillin, cephalosporin, and aztreonam resistance occurs through production of extended-β-lactamases (ESBL), which inactivate the antibiotics by splitting the amide bond of the β-lactam ring. Based on their molecular structure and amino-acid homology, ESBL are classified into Ambler classes A, C, and D (have serine at their active site), and class B (have zinc at their active site, and are thus called metallo-β-lactamases).15 Major ESBL families include TEM, SHV, CTX-M, (class A) found in Enterobacteriaceae, and OXA (class D), found in Pseudomonas isolates. They all hydrolyze oxyimino-β-lactam substrates, and are susceptible to β-lactamase inhibitors such as clavulanic acid and tazobactam. The CTX-M enzymes preferentially inactivate cefotaxime and ceftazidime, and are the most common ESBL worldwide.16 These plasmid-borne enzymes in Klebsiella and E. coli isolates have been recovered from both nosocomial as well as community-acquired isolates.17 In the United States and Europe, the increasing prevalence in CTX-M enzymes have been linked to the ST131 (0:25:H24) E. coli clone that accounts for most of the spread of isolates resistant to fluoroquinolone and broad-spectrum β-lactam antibiotics.18,19 Among class C ESBL, the most important clinically are the AmpC enzymes. They confer resistance to cephalothin, cefazolin, cefoxitin, most penicillins, and β-lactamase inhibitor-β-lactam combinations, and their production is induced in the presence of antibiotics. Many strains of Enterobacter have small populations of mutants that permanently overexpress AmpC enzymes.20 Consequently, exposure to third generation cephalosporins can select out these resistant sub-populations and lead to antibiotic resistance during therapy, as described in reports of treatment failure in patients with Enterobacter bacteremia treated with ceftriaxone.21
Carbapenem resistance
Carbapenem resistance is mainly achieved through the production of carbapenemases—ESBL capable of hydrolyzing a broad-spectrum of β-lactam antibiotics, including all penicillins, cephalosporins, and carbapenems. Among class A carbapenemases, Klebsiella pneumoniae carbapenemases (KPC) are clinically the most important. They are plasmid-based, confer resistance to all β-lactams, and can be transferred to other gram-negative species, such as E. coli,22 Enterobacter, Pseudomonas,23 and Salmonella,24 which have all been recovered from clinical isolates of hospitalized patients. Among the metallo-β-lactamases (class B), most clinically relevant enzymes are the IMP-type, VIM-type, SPM-type, and the NDM-type.15 The New Delhi metallo-β-lactamase (NDM-1) has received the most attention recently. Originally described in a Swedish patient hospitalized with K. pneumoniae infection in India in 2008, NDM-1 enzymes have since been reported in the US and UK primarily in connection with travel to India or Pakistan.4,25 These enzymes confer resistance to all β-lactams except aztreonam. However, most metallo-β-lactamases reside on mobile gene cassettes inserted into integrons which harbor additional antibiotic resistance genes to other antimicrobial classes. This multidrug resistance can be transferred to other species via transposons and plasmids, severely limiting therapeutic options in serious infections.15,24 Class D carbapenemases belong mostly to the OXA-type family, and are found primarily in P. aeruginosa and Acinetobacter species. Carbapenem resistance can also occur through other mechanisms, such as impermeability and efflux, especially in Pseudomonas isolates.26 Production of cephalosporinases such as AmpC enzymes combined with reduction in antimicrobial diffusion across bacterial membranes through alterations in the genes regulating porin channels can also confer carbapenem resistance in gram-negative bacteria.25
Fluoroquinolone resistance
Fluoroquinolone resistance is mediated primarily through alteration of drug targets (DNA gyrase and/or topoisomerase IV). In E. coli, the main mutations occur at specific hotspots associated with the quinolone resistance determining region within the genes gyrA and parC encoding the drug target DNA gyrase.27 Efflux pumps may also contribute to fluoroquinolone resistance in E. coli (acrAB–tolC system) and Pseudomonas aeruginosa (mex-encoded operons).27
Aminoglycoside resistance
Aminoglycoside resistance occurs through inactivating enzymes which modify the antibiotics through phosphorylation, adenylylation, or acetylation. Alternatively, methylation of the 16S rRNA drug target confers high-level resistance to the entire aminoglycoside class, including novel aminoglycosides in development.17,28 These plasmid-mediated methylases are often disseminated in association with carbapenemases, contributing to the spread of multidrug resistance among different species of gram-negative bacteria.29 In patients with cystic fibrosis harboring Pseudomonas aeruginosa isolates, resistance through decreased permeability by active efflux has also been described.30
Multidrug resistance
Multidrug resistance occurs through simultaneous activation of several resistance mechanisms such as production of chromosomally-encoded ESBL, and decreased permeability through loss of porin channels and activation of multidrug efflux pumps in response to antibiotic exposures. In addition, acquisition of plasmids and mobile elements carrying multiple resistance genes also contributes to the development of multidrug-resistant phenotypes. Pseudomonas aeruginosa and Acinetobacter baumannii are the two clinical pathogens most likely to become multidrug-resistant in this fashion.26
Clinical Impact of Antibiotic Resistance
Serious infections with gram-negative pathogens are associated with significant mortality, especially in immunocompromised hosts. For example, in studies of Pseudomonas aeruginosa bloodstream infections, mortality ranged from 39% to 42% in patients with stem-cell or solid organ transplants.31,32 Studies of patients with ICU infections with MDR Pseudomonas or Acinetobacter species have a significantly worse outcome than ICU patients with other types of infection.4,5 Multidrug resistance further increases the risk of death in gram-negative sepsis. For example, in severe nosocomial infections with Pseudomonas aeruginosa isolates producing metallo-β-lactamases, mortality ranged from 70% to 95%.32,33 In patients infected with carbapenem-resistant organisms from the KPC-producing family, mortality ranged from 24% to 65%.34-36 Receipt of antimicrobials that still retain activity against the multidrug-resistant isolates is crucial for survival. The impact of inadequate empiric antimicrobial therapy on mortality was illustrated in a study of 186 patients with bloodstream infections caused by ESBL producing organisms. The 3 week mortality associated with receipt of inadequate vs. adequate initial therapy was 60% vs. 19%, respectively.36
Prevention
The cornerstone of reducing infections with multidrug-resistant organisms relies on practicing high-quality antimicrobial stewardship and infection control. Avoiding unnecessary antimicrobial exposure, distinguishing colonization from true infection, dosing antimicrobials adequately, and stopping antimicrobial treatment as soon as possible after symptom resolution are all essential to preventing development of antimicrobial resistance.37 Active surveillance should strongly be considered in healthcare facilities caring for patients harboring multidrug-resistant organisms, since isolates recovered in clinical cultures often represent only a small fraction of the local antibiotic resistance burden. This is particularly true for carbapenemase-producing organisms, as illustrated in the study by Wiener-Well et al., where less than one-third of the patients identified as carriers through active surveillance had positive clinical cultures.38 Strict adherence to infection control strategies such as compliance with hand-hygiene and contact precautions in all healthcare settings is essential and cannot be over-emphasized.25
Treatment
Prompt initiation of antimicrobial therapy is critical for improving the clinical outcomes in severe sepsis, since the likelihood of survival in septic shock decreases by 8% for each hour of delay in antimicrobial administration.39,40 Ensuring that the empirical antimicrobial regimen prescribed is actually effective in the treatment of sepsis with a multidrug-resistant pathogen remains challenging. Access to local institution-wide antimicrobial biograms, and knowledge of local prevalence of multidrug resistance as well as prior antimicrobial receipt could help guide initial antimicrobial choices.41 Combination antimicrobial therapy initially can increase the chances that at least one drug retains activity against the suspected offending pathogen, and appears to improve survival in patients with severe sepsis.42-44 Obtaining appropriate cultures prior to instituting antimicrobial therapy will allow de-escalation once susceptibility results are known. The following antibiotics constitute possible options in the treatment of severe infections with multidrug-resistant pathogens, depending on isolate antibiogram results:
Carbapenems
Carbapenems remain the treatment of choice for severe infections with ESBL-producing organisms that do not have carbapenemase activity. This was illustrated in a prospective study of patients with ESBL-producing Klebsiella pneumoniae bacteremia: the 2 week mortality for patients treated with carbapenem monotherapy, other β-lactam therapy, and antibiotics without any activity against the offending organism was 3.7%, 44%, and 54%, respectively.45 Although some strains with ESBL from the TEM or SHV-type families may appear sensitive in vitro to cefepime and piperacillin–tazobactam, they are no longer susceptible when the inoculum is high,46 and therefore are not indicated in the treatment of sepsis or severe infections with a high bacterial burden (e.g., extensive pneumonia, peritonitis, or large abscesses).
Colistin
Colistin (Polymyxin E) is an old antibiotic with cationic detergent properties which binds to the lipid A component of bacterial lipopolysaccharide and disrupts the outer cell membrane of gram-negative bacteria, causing leakage of cytoplasmic contents.47 Some multidrug-resistant strains of Psudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas, and some Enterobacteriaceae (E. coli, Klebsiella, and Enterobacter) remain susceptible to colistin. The experience with colistin therapy is limited to mostly non-comparative, retrospective studies where colistin was administered in varying doses and durations, and was often given in combination with other antimicrobials.48 Prospective, randomized trials are needed before definitive recommendations regarding dosing and therapy duration can be made.
The literature to date suggests that colistin administered in combination with other antimicrobials appears to improve survival compared with monotherapy, at least for carbapenem-resistant Enterobacteriaceae.49 One of the most common combination therapies studied includes colistin–polymyxin B with carbapenem, which had a mortality rate of 12.5% (1/6) in patients with KPC-producing Klebsiella pneumoniae bacteremia. The mortality for colistin or carbapenem monotherapy in this study was 66.7% (8/12)50 Colistin combined with doripenem was also found to be bactericidal and synergistic in vitro for carbapenemase producing Klebsiella pneumonie isolates.51 The combination of tigegycline, colistin, and extended-infusion meropenem (2 g IV infused over 3 h every 8 h) was associated with the best outcomes in a multi-center retrospective study of 125 patients with sepsis from KPC-producing K. pneumoniae bacteremia.49 Intravenous colistin has also been used with some success in the treatment of nosocomial infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii isolates, especially when used in combination with rifampicin.52-56 Nephrotoxicity can develop in almost half of the patients receiving full doses of intravenous colistin, although it appears to be reversible for most patients.57
Tigecycline
Tigecycline has in vitro activity against ESBL-producing organisms, including some of the carbapenemase-producing Enterobacteriaceae, Acinetobacter, and Stenotrophomonas species. It is not active against Pseudomonas species, and Proteus isolates are intrinsically resistant. Tigecycline has been used with varying degrees of success in the treatment of infections with multidrug-resistant gram-negative organisms. For example, a retrospective analysis of 45 patients, primarily in the ICU with infections caused by multidrug-resistant Acinetobacter and Klebsiella species reported an 80% clinical success rate with tigecycline monotherapy.58 On the other hand, tigecycline is not recommended in the treatment of bacteremia. The serum concentration of this bacteriostatic, lipophilic drug is rather low with a large volume of distribution, and does not adequately exceed the MIC for most resistant gram-negative organisms.59 Development of resistance during therapy with tigecycline for initially susceptible isolates (carbapenemase and ESBL-producing Enterobacteriaceae and non-fermenters) raises further caution in the use of tigecycline as monotherapy for severe infections.60
Fosfomycin
Fosfomycin is another old broad-spectrum antibiotic that inhibits bacterial cell wall synthesis, and has in vitro activity against ESBL-producing organisms.61 Fosfomycin may also inhibit carbapenemase-producing organisms, as evidenced by a recent study where 60% of the 81 carbapenem-resistant Enterobacteriaceae strains tested were found to be susceptible to this antimicrobial.62 The experience with its use in severe infections is limited, as intravenous fosfomycin is not available in many countries, including the US. In a small prospective study of 11 critically ill patients with carbapenem-resistant Klebsiella pneumoniae infections, intravenous fosfomycin used in combination with other antimicrobials was reported to be associated with good clinical and bacteriological outcomes, and no significant adverse events.63
The additive benefit of intravenous fosfomycin in combination with colistin vs. colistin alone is currently being tested in patients with MDR Acinetobacter baumannii infections (clinicaltrials.gov NCT11297894). Fosfomycin aerosol, in combination with tobramycin aerosol administration, is also being studied in cystic fibrosis patients with difficult to treat lung infections with Pseudomonas aeruginosa. (NCT00794586)
Antimicrobials in the pipeline
There are currently several parenteral antimicrobial therapies under investigation for the treatment of multidrug-resistant gram-negative infections. Ceftolozane (CXA-101) in combination with tazobactam is active against ESBL-producing bacteria (CTX-M-15 β-lactamases specifically). It is currently undergoing phase 3 trials of complicated urinary tract and intra-abdominal infections. Ceftazidime in combination with avibactam (a new β-lactamase inhibitor) is active against P. aeruginosa and ESBL-producers including KPC type β-lactamases, but not against metallo-β-lactamases such as NDM and VIM. It is also in phase 3 studies for complicated urinary tract infection and intra-abdominal infections. Ceftaroline/avibactam is active in vitro against ESBL and KPC-producing organisms. The activity against resistant Acinetobacter species is limited for all these cephalosporin/β-lactamase inhibitor combinations. Imipenem in combination with another novel β-lactamase inhibitor, MK-7655, appears active in vitro against serine carbapenemases and against P. aeruginosa, but not against metallo-carbapenemases or A. baumannii. Plazomicin is a new generation aminoglycoside active against metallo-β-lactamases, carbapenemases, fluoroquinolone-resistant, and aminoglycoside-resistant gram-negative bacteria, but has limited activity against P. aeruginosa or A. baumannii. Eravacycline is a novel tetracycline that is not susceptible to the efflux or protection of the ribosomal target that render older tetracyclines ineffective. It has in vitro activity agasint KPC-producing bacteria, but not against Pseudomonas or Acinetobacter species. Carbavance (a β-lactam/β-lactamase inhibitor combination of biapenem and RPX7009), currently in phase I development, has activity against ESBL and KPC-producing organisms, and is also active against resistant Pseudomonas and Acinetobacter strains, but not against metallo-β-lactamases.64
Conclusion
None of the antimicrobials currently in development have activity against the entire spectrum of multidrug-resistant gram-negative bacteria, with the therapeutical options for infections with Acinetobacter, Pseudomonas, and other metallo-β-lactamase producing organisms particularly absent from both the current as well as the future drug armamentarium. Furthermore, none of the current drugs under investigation are in trials for the more severe sepsis syndromes like bloodstream infections, endocarditis, or invasive pneumonia that critical care clinicians are now increasingly being faced with. Therefore, death from sepsis caused by pathogens without therapeutic options is now an imminent threat. The best hope for the future is the development of a greater understanding of how antimicrobial resistance spreads globally, and whether the alarming trend in community-acquired multidrug resistance can be reversed. A concerted global commitment to intelligent use of antimicrobials, development of improved bacterial vaccines, better antibiotic stewardship, and the implementation of effective infection control strategies are urgently needed.65
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N Engl J Med. 2009;360:439–43. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 3.Rossi F, Baquero F, Hsueh PR, Paterson DL, Bochicchio GV, Snyder TA, Satishchandran V, McCarroll K, DiNubile MJ, Chow JW. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends) J Antimicrob Chemother. 2006;58:205–10. doi: 10.1093/jac/dkl199. [DOI] [PubMed] [Google Scholar]
- 4.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect Dis. 2011;11:381–93. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Tängdén T, Cars O, Melhus A, Löwdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–8. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 9.Anon. Municipal Council of Delhi: 18% DJB water not potable. Reported in Times of India 11 March 2011. http://artciles.timesofindia.indiatimes/com/2011-03-11/delhi/28679651_1_djb-water-drinking-water-samples
- 10.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan DJ, Liang SY, Smith CL, Johnson JK, Harris AD, Furuno JP, Thom KA, Snyder GM, Day HR, Perencevich EN. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol. 2010;31:716–21. doi: 10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi Y, Adams J, O’Keefe A, Quereshi Z, Ewan L, Paterson DL. Community-acquired extended-spectrum beta-lactamase producers, United States. Emerg Infect Dis. 2007;13:1121–3. doi: 10.3201/eid1307.070094. [DOI] [PubMed] [Google Scholar]
- 13.Freeman JT, McBride SJ, Heffernan H, Bathgate T, Pope C, Ellis-Pegler RB. Community-onset genitourinary tract infection due to CTX-M-15-Producing Escherichia coli among travelers to the Indian subcontinent in New Zealand. Clin Infect Dis. 2008;47:689–92. doi: 10.1086/590941. [DOI] [PubMed] [Google Scholar]
- 14.Woodford N, Ward ME, Kaufmann ME, Turton J, Fagan EJ, James D, Johnson AP, Pike R, Warner M, Cheasty T, et al. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J Antimicrob Chemother. 2004;54:735–43. doi: 10.1093/jac/dkh424. [DOI] [PubMed] [Google Scholar]
- 15.Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med. 2005;352:380–91. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 16.Rice LB. The clinical consequences of antimicrobial resistance. Curr Opin Microbiol. 2009;12:476–81. doi: 10.1016/j.mib.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39:283–94. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010;51:286–94. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J Infect Dis. 2013;207:919–28. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–82. doi: 10.1128/CMR.00036-08. [Table of Contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 22.Navon-Venezia S, Chmelnitsky I, Leavitt A, Schwaber MJ, Schwartz D, Carmeli Y. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob Agents Chemother. 2006;50:3098–101. doi: 10.1128/AAC.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP, Colombian Nosocomial Resistance Study Group First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob Agents Chemother. 2007;51:1553–5. doi: 10.1128/AAC.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miriagou V, Tzouvelekis LS, Rossiter S, Tzelepi E, Angulo FJ, Whichard JM. Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob Agents Chemother. 2003;47:1297–300. doi: 10.1128/AAC.47.4.1297-1300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan LK. Carbapenem-resistant enterobacteriaceae: an emerging problem in children. Clin Infect Dis. 2012;55:852–9. doi: 10.1093/cid/cis543. [DOI] [PubMed] [Google Scholar]
- 26.Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S49–56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 27.Piddock LJ. Mechanisms of fluoroquinolone resistance: an update 1994-1998. Drugs. 1999;58(Suppl 2):11–8. doi: 10.2165/00003495-199958002-00003. [DOI] [PubMed] [Google Scholar]
- 28.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45:88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Yu H, Guo Q, Xu X, Ye X, Wu S, Guo Y, Wang M. Distribution of 16S rRNA methylases among different species of Gram-negative bacilli with high-level resistance to aminoglycosides. Eur J Clin Microbiol Infect Dis. 2010;29:1349–53. doi: 10.1007/s10096-010-1004-1. [DOI] [PubMed] [Google Scholar]
- 30.Westbrock-Wadman S, Sherman DR, Hickey MJ, Coulter SN, Zhu YQ, Warrener P, Nguyen LY, Shawar RM, Folger KR, Stover CK. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–83. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcin F, Leone M, Antonini F, Charvet A, Albanèse J, Martin C. Non-adherence to guidelines: an avoidable cause of failure of empirical antimicrobial therapy in the presence of difficult-to-treat bacteria. Intensive Care Med. 2010;36:75–82. doi: 10.1007/s00134-009-1660-8. [DOI] [PubMed] [Google Scholar]
- 32.Zavascki AP, Barth AL, Gonçalves AL, Moro AL, Fernandes JF, Martins AF, Ramos F, Goldani LZ. The influence of metallo-beta-lactamase production on mortality in nosocomial Pseudomonas aeruginosa infections. J Antimicrob Chemother. 2006;58:387–92. doi: 10.1093/jac/dkl239. [DOI] [PubMed] [Google Scholar]
- 33.Carmeli Y, Akova M, Cornaglia G, Daikos GL, Garau J, Harbarth S, Rossolini GM, Souli M, Giamarellou H. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin Microbiol Infect. 2010;16:102–11. doi: 10.1111/j.1469-0691.2009.03115.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabuda DM, Laupland K, Pitout J, Dalton B, Rabin H, Louie T, Conly J. Utilization of colistin for treatment of multidrug-resistant Pseudomonas aeruginosa. Can J Infect Dis Med Microbiol. 2008;19:413–8. doi: 10.1155/2008/743197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–7. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 36.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D’Inzeo T, Fadda G, Cauda R, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51:1987–94. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice LB. The Maxwell Finland Lecture: for the duration-rational antibiotic administration in an era of antimicrobial resistance and clostridium difficile. Clin Infect Dis. 2008;46:491–6. doi: 10.1086/526535. [DOI] [PubMed] [Google Scholar]
- 38.Wiener-Well Y, Rudensky B, Yinnon AM, Kopuit P, Schlesinger Y, Broide E, Lachish T, Raveh D. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect. 2010;74:344–9. doi: 10.1016/j.jhin.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine. Society of Hospital Medicine. Surgical Infection Society. World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 41.Leekha S, Standiford HC. Empiric antimicrobial therapy for Gram-negative sepsis: back to the future. Crit Care Med. 2011;39:1995–6. doi: 10.1097/CCM.0b013e318223b94b. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–85. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 43.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–13. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38:1651–64. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 45.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–7. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 46.Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2001;45:3548–54. doi: 10.1128/AAC.45.12.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton J, Pankey GA. Polymyxin B, colistin, and sodium colistimethate. Med Clin North Am. 1982;66:135–42. doi: 10.1016/s0025-7125(16)31447-x. [DOI] [PubMed] [Google Scholar]
- 48.Hanberger H, Giske CG, Giamarellou H. When and how to cover for resistant gram-negative bacilli in severe sepsis and septic shock. Curr Infect Dis Rep. 2011;13:416–25. doi: 10.1007/s11908-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 49.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–50. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 50.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–13. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56:3395–8. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motaouakkil S, Charra B, Hachimi A, Nejmi H, Benslama A, Elmdaghri N, Belabbes H, Benbachir M. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. J Infect. 2006;53:274–8. doi: 10.1016/j.jinf.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Levin AS, Barone AA, Penço J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–11. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 54.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–9. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Rahal JJ. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S95–9. doi: 10.1086/504486. [DOI] [PubMed] [Google Scholar]
- 56.Bassetti M, Repetto E, Righi E, Boni S, Diverio M, Molinari MP, Mussap M, Artioli S, Ansaldi F, Durando P, et al. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother. 2008;61:417–20. doi: 10.1093/jac/dkm509. [DOI] [PubMed] [Google Scholar]
- 57.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48:1724–8. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 58.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, Nicolaou C, Yphantis D, Antoniadou A, Trikka-Graphakos E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58:273–84. doi: 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Curcio D. Tigecycline for treating bloodstream infections: a critical analysis of the available evidence. Diagn Microbiol Infect Dis. 2008;61:358–9, author reply 360-1. doi: 10.1016/j.diagmicrobio.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46:567–70. doi: 10.1086/526775. [DOI] [PubMed] [Google Scholar]
- 61.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 62.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37:415–9. doi: 10.1016/j.ijantimicag.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect. 2010;16:184–6. doi: 10.1111/j.1469-0691.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 64.Boucher HW, Talbot GH, Benjamin DK, Jr., Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D, Infectious Diseases Society of America 10 x ’20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–94. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diazgranados CA, Cardo DM, McGowan JE., Jr. Antimicrobial resistance: international control strategies, with a focus on limited-resource settings. Int J Antimicrob Agents. 2008;32:1–9. doi: 10.1016/j.ijantimicag.2008.03.002. [DOI] [PubMed] [Google Scholar]