Abstract
An important role for bioenergetic dysfunction is increasingly emerging to potentially explain the paradox of clinical and biochemical organ failure in sepsis yet minimal cell death, maintained tissue oxygenation and recovery in survivors. Associations are well-recognized between the degree of mitochondrial dysfunction and outcomes. While this does not confirm cause-and-effect, it does nevertheless suggest a new route for therapeutic intervention focused on either mitochondrial protection or acceleration of the recovery process through stimulation of mitochondrial biogenesis (new protein turnover). This is particularly pertinent in light of the multiple trial failures related to immunomodulatory therapies. This overview will provide insights into mitochondrial biology, the relevance to sepsis, and therapeutic opportunities that possibly emerge.
Keywords: sepsis, mitochondria, multi-organ failure, nitric oxide, reactive oxygen species, biogenesis, mitophagy
Introduction
Sepsis represents a deranged and exaggerated systemic inflammatory response to infection that can progress to multi-organ dysfunction (severe sepsis) including shock. Sepsis-related organ failure still carries a significant morbidity and mortality,1,2 with long-term physical and neurocognitive problems affecting many survivors or critical illness.3,4 While an excessive degree of inflammation in response to the infectious insult is a clear trigger for activation of multiple downstream pathways, the precise pathophysiologic mechanisms underlying the development of multi-organ failure (MOF) remain elusive.5
While the presence of an impaired circulation leading to tissue hypoperfusion makes a well-recognized contribution to the development of MOF, organ dysfunction can still occur even in the absence of gross macrovascular abnormality. Some authorities propose intraorgan redistribution of blood flow with consequent shunting of blood away from nutrient capillaries while others suggest an obstructed/constricted microcirculation may impair regional perfusion. However, these claims need to be set in the context of a remarkably preserved histology in most organs affected by the septic process. While many organs manifest evidence of inflammation with migration of inflammatory cells (neutrophils and macrophages), increased interstitial fluid related to a greater degree of capillary leak, and some epithelial disruption, there is remarkably little cell death, either apoptotic or necrotic. The degree of cell death is disproportionately minor in comparison to the severe clinical and biochemical presentation of organ dysfunction. Even at postmortem, a small increase in apoptosis was noted in immune tissues such as spleen, lymph nodes, lymphocytes, and gut epithelium, whereas minimal change was noted in multiple other organs like the heart, lung, brain, muscle, and kidney.6,7
Acute tubular necrosis within the kidney is a relative misnomer in both human and laboratory model sepsis,7,8 while histological injury is often more traceable to the therapy rather than the underlying septic condition. For example, contraction band necrosis is a common finding within the heart on necropsy of septic shock patients and this is pathognomonic of excess catecholamine levels, particular related to high-dose administration of norepinephrine, epinephrine, and dobutamine.9
A novel paradigm needs to be embraced that can explain (1) the clinical manifestations of organ failure yet an absence of significant cell injury, (2) the relatively rapid recovery of function in survivors, even in organs that are poorly regenerative, and (3) the finding that tissue oxygen tensions are preserved or even elevated within failed organs in resuscitated established sepsis.10-12 As the predominant utilizer of oxygen within the body are mitochondria, primarily for generation of ATP but also for other roles including heat production, a metabolic shutdown akin to hibernation, is a plausible option worth exploring. This would maintain tissue oxygen levels by decreasing demand, and protect against cell death.
Mitochondrial Function in Health
The physiological roles of mitochondria
Apart from erythrocytes, all cell types possess mitochondria. These organelles provide (in most cell types) the bulk of the energy (in the currency of ATP) required for normal cellular functioning, including the ability to respond to any (patho)physiological stress. Mitochondria utilize approximately 98% of total body oxygen consumption. They have many other roles other than ATP production including heat generation, intracellular calcium regulation, thermoregulation, and production of reactive oxygen species (ROS).13-16 ROS are required for signaling, maintenance of vascular tone, and oxygen sensing. Other than carbon dioxide, the body generates three other endogenous gases, nitric oxide, carbon monoxide, and hydrogen sulfide, which are all important regulators of mitochondrial signaling in health.17-19 The higher concentrations of these gases generated in disease states such as sepsis have progressively greater inhibitory effects on mitochondrial respiration and ROS generation.17-19
Mitochondria are also the site of production (e.g., cortisol) or action (e.g., triiodothyronine, estrogen) of many hormones,20-22 and the biosynthesis of heme and iron-sulfur clusters.23 Mitochondria also trigger cell death pathways—necrosis when ATP levels fall below a certain threshold and apoptosis through release of mitochondrial cytochrome c into the cytoplasm.24
Energy generation
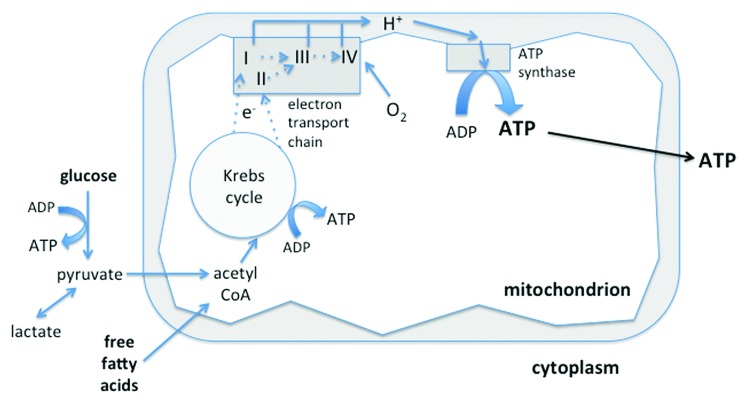
ATP is generated either by glycolysis in the cytosol but, to a much greater degree, by oxidative phosphorylation (Fig. 1). This process occurs in both human mitochondria and bacteria—signifying their shared lineage—initially by transfer of electrons from the Krebs cycle to the electron transport chain via NADH and FADH2. The chain consists of four enzyme complexes (Complexes I–IV) and two transporters (ubiquinone and cytochrome c). While electrons are being moved down the chain, protons are pumped across the inner mitochondrial membrane, creating an electrical potential. This “chemiosmotic gradient” provides the energy (proton-motive force) for ATP synthase (Complex V) to phosphorylate ADP to ATP. ATP is then transported out of the mitochondria via the specialized adenine nucleotide translocase (ANT) transporter.
Figure 1. Sources of ATP production. Glucose is metabolized to pyruvate via the glycolytic pathway. Pyruvate (and free fatty acids) enter the mitochondria where they are converted to acetyl CoA. This enters the Krebs cycle that, via NADH and FADH2, donates electrons to Complexes I and II of the electron transport chain, respectively. The electrons pass down the chain to Complex III and thence to Complex IV, where oxygen is the terminal electron acceptor. Protons cross the mitochondrial membrane and, in so doing, create an electrochemical gradient to enable ATP synthase to generate ATP from ADP. The ATP is then transported to the cytosol to fuel energy-requiring cellular processes. A relatively small amount of ATP is also produced by glycolysis and the Krebs cycle.
The substrate for electron transfer and eventual ATP production comes primarily from glucose (via glycolysis) or β-oxidation of fat (which enters as fatty acids via the Krebs cycle or succinate into the electron transport chain). The carnitine pathway is an intermediary step for transport of long-chain fatty acids.
Oxygen is the terminal electron acceptor of the chain at Complex IV, being reduced to water. Premature or incomplete reduction of oxygen will increase superoxide radical production, mainly at Complex III but also Complex I. The mitochondrion has intrinsic defense mechanisms to protect against damage induced by ROS through its large array of antioxidants (e.g., superoxide dismutase, glutathione, thioredoxin).25 However, these can be overwhelmed in pathological processes generating large amounts of ROS.
Importantly, the process of ATP production is by no means 100% “efficient” in terms of oxygen utilization. In health approximately 1–2% of oxygen consumed is directed toward ROS production whereas much greater amounts of oxygen are “uncoupled” from energy production and lost as heat. This varies from tissue to tissue, being low in heart and much higher in liver and skeletal muscle.26 Uncoupling is due to various mechanisms, including specialized uncoupling proteins (UCPs) within the inner mitochondrial membrane, the best known of which is UCP-1 present in brown fat that is a vital source of heat generation for neonates and hibernating mammals
The mitochondrial life cycle
Mitochondrial biogenesis involves the production of mitochondrial proteins encoded either by nuclear DNA, with subsequent import and integration into the mitochondria, or via mitochondrial DNA which encodes 13 proteins mainly situated within the oxidative phosphorylation pathway. Biogenesis thus replaces damaged proteins and improves the capacity for energy production if energy demands increase over time.
PPARgamma-coactivator-1α (PGC-1α) is a key player that orchestrates mitochondrial biogenesis.27 It activates transcription factors such as nuclear respiratory factors 1 and 2 (NRF-1 and -2) that upregulate nuclear production of mitochondrial proteins and subsequent expression of transcription factors such as Tfam (transcription factor A for the mitochondrion) that stimulate transcription of mitochondrial DNA. PGC-1α expression varies in response to many stimuli, e.g., physiological (e.g., exercise), pathophysiological (e.g., hypoxia), and hormonal (through interaction with thyroid, glucocorticoid, estrogen, and estrogen-related receptors.27 Endogenous nitric oxide also upregulates expression of PGC-1α.28 While NO (and its metabolites such as peroxynitrite) can, in the short term inhibit mitochondrial respiration through direct inhibition of the electron transport chain, competing with oxygen at Complex IV, or nitrosylating/nitrating mitochondrial Complex proteins,29 NO may also be responsible for generating new, healthy mitochondria by stimulating mitochondrial biogenesis.28
Mitochondria also undergo numerous morphological changes during fusion and fission events. This plays an important role in cell division and proliferation, as well as in the self-directed removal of damaged or surplus mitochondria, a process known as mitophagy.30 Proteins driving fusion events (e.g., mitofusin-2) and fission events (e.g., DRP-1) have been associated with altered mitochondrial membrane potential and reduced oxygen consumption.30 Fission and fusion increase in stress conditions, playing critical roles in removing damaged mitochondria and augmenting repair processes.
Mitochondrial Dysfunction in Sepsis and Multi-Organ Failure
Systemic inflammation
A systemic inflammatory response can be triggered by microbial antigens (sepsis) or, in a similar fashion, intrinsic factors released into the circulation as a result of trauma or other injury. These host-derived danger associated molecular patterns (DAMPs) are released in response to stress, tissue injury or cell death and include intracellular constituents such as heat shock proteins, histones, DNA, and mitochondria themselves.31,32
Specialized receptors (e.g., the Toll-like system) recognize these microbial and host molecular patterns and modulate (either up- or downregulating) the transcription of genes encoding for proteins not only involved in inflammation (such as cytokines and cytokine receptors), but also in multiple pathways involving cardiovascular, immune, hormonal, coagulation, metabolic, and bioenergetic systems.5 The cytokines themselves have signaling effects on most cell types, triggering activation or suppression of multiple intracellular pathways thus modulating their function. Other products of inflammation include reactive species such as nitric oxide and superoxide that are produced in supranormal quantities, the degree of which relates to outcomes.33,34 These may directly affect protein functionality through posttranscriptional effects, e.g., oxidation, nitrosylation, nitration, and acetylation, and may cause direct damage to other cell constituents, e.g., lipid peroxidation.35
Hormonal activation is an early component of the inflammatory response to sepsis.36 Acute phase hormones such as adrenaline and cortisol prepare the body for stress, e.g., increasing cardiac output, diverting blood flow to appropriate organs involved in flight and fight (e.g., brain, heart, and muscle), modifying hepatic protein production toward acute phase proteins involved with defense and transport, and modulating metabolic activity. For example, they are counter-regulatory against insulin to increase glucose availability while, at the same time, altering cellular substrate utilization. Lactate release from muscle is enhanced by catecholamines to provide a ready fuel substrate for other organs such as brain, liver, and heart.37 At the same time, non-essential activities, i.e., those not immediately involved in dealing with the stressor such as general anabolic activities and DNA repair, are downscaled.
A very early activation of the cardiovascular system involves a combination of macro- and microvascular responses with increases in overall cardiac output and redistribution of blood flow to those organs needing a greater delivery of oxygen and energy substrates to supply the greater metabolic requirements of their cells. Changes in vascular tone and alterations in the endothelial barrier allow an increased egress of fluid containing substrate and white cells out of the circulation to deal with infected or damaged tissue. Coagulation increases in inflamed areas to wall off areas of damage and to occlude draining blood vessels, thus preventing spread of bacteria, toxins, and DAMPs to other parts of the body.38 However, while appropriate in contained areas, an excessive and uncontrolled response will affect body organs initially untouched by the original insult. Excessive systemic capillary leak will result in large amounts of interstitial fluid and loss of circulating intravascular volume with a resulting decrease in cardiac output. This may be compounded by exogenous loss of fluid (related to pyrexia-related sweating, vomiting, diarrhea, ileus, etc.), suppression of myocardial contractility through high levels of inflammatory mediators (including NO) and other mechanisms,39 and an excessive loss of vascular tone critically affecting organ perfusion.40 In addition to myocardial depression, as the organ dysfunction progresses, other factors also contribute to a reduction in the hyperdynamic circulation seen at the onset of sepsis. This may include a direct negative feedback resulting from a reduction in metabolic demand, in part caused by a decreased mitochondrial requirement for oxygen.
Impact of an exaggerated inflammatory response on mitochondria
Mitochondria can be affected in various ways through the systemic inflammatory process:
1) Impaired perfusion early in the septic process, due to intrinsic and extrinsic fluid losses and decreased intake, myocardial depression, microcirculatory redistributions of blood flow and loss of vascular tone, can lead to tissue hypoxia, i.e., insufficient oxygen at the mitochondrial level to drive oxidative phosphorylation of ADP to ATP.41,42 While the particular enzyme characteristics of Complex IV allows it to function effectively at low oxygen concentrations in health, critically low levels may compromise ATP generation and potentially trigger cell death pathways.42
2) Generation of excess amounts of NO, carbon monoxide, hydrogen sulfide, and other ROS directly inhibit mitochondrial respiration, and cause direct damage to mitochondrial protein and other structures such as the lipid membrane.17-19,35 We reported a decrease in mitochondrial complex I activity that was associated with the degree of nitric oxide production in the skeletal muscle of patients admitted to intensive care with septic shock43 and, subsequently, in muscle and liver in a long-term rodent model of fecal peritonitis.44 Others have shown similar findings e.g., Vanasco et al.45 In a recent paper looking at rapid postmortem liver and kidney samples, gross tissue histology showed minimal cell death however, on electron microscopy, widespread mitochondrial injury was apparent with hydropic mitochondria and membrane injury.7
3) Hormonal alterations in sepsis affect mitochondrial function and efficiency. For example, thyroid hormone is thought to predominantly exert its effects via modulating mitochondrial activity.20 Early in the septic process there is a rise in thyroid activity however, in established sepsis, the “sick euthyroid” or “low T3” syndrome is a well-recognized phenomenon46,47 that may impact mitochondrial function.48
4) Genes transcribing mitochondrial proteins are downregulated early in the inflammatory response. This was first recognized in human volunteers receiving endotoxin49 and subsequently described by us in critically ill patients.50
The majority of the literature does support the above findings though variation should be acknowledged. Whether this relates to species differences, inter-organ differences or methodological limitations requires further elucidation.
Metabolic consequences
The above effects on mitochondria—inhibition, damage, and decreased turnover of new mitochondrial protein—will affect generation of ATP. This will be compounded by the mitochondrial inhibition/damage induced by the many drugs given as part of patient management in the critical care setting, including antibiotics, catecholamine inotropes, and sedatives. If cellular metabolic activity continued in the face of insufficient energy, then ATP levels will drop and cell death pathways will be activated. Notably, as described earlier, this does not appear to be a major feature of sepsis-induced organ failure so clearly the cell must adapt to cope with the falling energy supply. One option available is an increase in non-mitochondrial ATP production through enhanced glycolytic activity.51 However, this is only designed to be a partial and relatively short-term solution and cannot completely replace mitochondrial ATP production. Second, and more likely, a decrease in metabolic activity will reduce energy requirements and generate a new steady-state whereby the cell does not function normally yet, at the same time, does not allow ATP levels to drop to trigger cell death. This is akin to hibernation52,53 or many other biological equivalents such as bacterial dormancy or to estivation, the summer equivalent of hibernation whereby cells belonging to multiple species ranging from plants to fish to snakes to some mammals, enter a state of torpor until the hot, arid conditions subside.54,55 Hibernation is a well-recognized phenomenon in the human heart when a low level of myocardial perfusion persists following an ischemic event. In such cases, cardiomyocytes decrease their activity, i.e., contraction, until adequate perfusion is regained and normal function is then restored.56
If this hypothesis is correct, there are likely to be switches that decrease metabolism in response to a diminished availability of energy and an inflammatory milieu. Whether these relate in sepsis to levels of tissue oxygen or ROS, levels of ATP or to other factors requires elucidation.
A decrease in cell functionality will be manifest, if sufficiently severe, as altered physiological and biochemical functioning of the organ. This is then described as organ “dysfunction” or “failure” but may actually represent a late-stage adaptive process by the cell/organ/body to deal with the onslaught of a prolonged and severe inflammatory response.57 Other energy-sparing mechanisms may also come into play. For instance, oliguria is arguably a consequence of a decrease in glomerular filtration that spares the kidney from its major energy-utilizing activity, namely reabsorption of 98% of the salt and water filtered into the tubules. Akin to being unwell from any moderate or severe illness that will reduce general everyday activity and encourage the sufferer to rest, sleep, and decrease their food intake, so a similar parallel can be drawn to organ and cellular function. This is likely driven by proinflammatory cytokines, modulations in autonomic activity and hormonal changes. Support for a bioenergetic-metabolic shutdown as a plausible explanation for sepsis-induced MOF comes from the corroborative findings of minimal cell death,6,7 maintained (or elevated) tissue oxygen levels,10-12 reduced oxygen consumption in line with increasing severity,58 and the ability to recover function within days to weeks after resolution of the deranged inflammatory process. Notably, incubation of cells in plasma taken from septic patients results in a marked depression of mitochondrial respiration with associated oxidative stress.59,60
Clinical relevance
Various preclinical and clinical studies have demonstrated an association between the degree of mitochondrial impairment or histological damage and either clinical severity, organ dysfunction or poor outcomes. For example, we showed that low skeletal muscle Complex I levels in critically ill patients were associated with higher organ failure scores while higher levels of ATP were seen in survivors compared with non-survivors.43 Similar results were seen in our long-term animal model.44
Recovery processes
Haden et al. used a long-term mouse model of S. aureus peritonitis to demonstrate that organ dysfunction and clinical illness were accompanied by decreases in metabolic rate and mitochondrial mass.61 Recovery of metabolic activity and organ function, accompanied by clinical improvement, were preceded by an upregulation of markers of mitochondrial biogenesis such as PGC-1α, Tfam, and NRF-1, and suppression of RIP140, an endogenous co-repressor.61
Mitochondrial biogenesis thus seems critical in the recovery process. We showed in intensive care patients suffering from multi-organ failure that eventual survivors had, early in their disease process, higher levels of PGC-1α and better-maintained levels of Complex protein levels alongside a greater protective antioxidant (manganese superoxide) response.50 This was measured from vastus lateralis thigh muscle biopsy specimens. Of note, a recent study of endotoxic mice found that locomotor muscles were more susceptible to mitochondrial injury compared with ventilatory muscles, with decreased biogenesis and an increase in autophagy.62
Thus, biogenetic responses may not only vary with disease severity but also anatomical location. Timing of recovery may also vary between species, is likely to be age-dependent, affected by the type of insult (e.g., a bacterial vs. an endotoxic insult), and may be enhanced or delayed by therapeutic interventions. Bacteriostatic antibiotics inhibit biogenesis while a variety of agents stimulate this process. For example, transgenic mice producing more PPARγ and wild type mice treated with the PPARγ agonist rosiglitazone both showed increased PGC-1α levels; this was associated with mitochondrial protection, less myocardial dysfunction and improved survival following lipopolysaccharide administration.63 Likewise, treatment with resveratrol, a stimulant for PGC-1α production via sirtuin activation, improved mitochondrial injury and cardiac function, though not survival in another septic murine model.64
A parallel interest is growing in the ability to clear damaged mitochondria.65 Mitophagy (autophagic degradation) and mitoptosis (programmed destruction) are the processes by which cells deal with impaired mitochondria. The efficiency of these processes may be an important contributory factor to pathogenesis of various disease states. Mitophagy involves selective sequestration with subsequent degradation of damaged mitochondria before they can activate cell death pathways and cause death of the cell as a whole. Mitophagy thus functions as an early protective response. In contrast, increased oxidative stress and apoptotic proteases can inactivate mitophagy and trigger further inflammation,66 so a fine balance exists. Gunst et al. recently demonstrated that impaired autophagy contributes to mitochondrial dysfunction and organ failure in a rabbit burn model.67 Deficiency of the inducible form of NO synthase or pharmacological inhibition of NO production enhanced inflammasome-dependent cytokine production and decreased stabilization of mitochondria, thereby increasing mortality in a murine endotoxin model.68 Using a septic mouse model, Crouser et al. suggested an increase of mitophagy occurs early in sepsis with subsequent repopulation by healthy mitochondrial populations.69
Putative therapies
A variety of strategies are available that can either protect mitochondria from injury, or increase biogenesis with the aim of accelerating recovery.70 The challenge with any such approach is to find a middle ground between abrogating the harm induced by excessive damage/inhibition/reduced turnover of mitochondria, yet without significantly impairing any adaptive and protective process that may compromise host recovery. As an exemplar, reactive oxygen species are damaging in excess yet also offer important signaling, immune-modulating and other roles that are vital not only in health but also in stress states. The role of energy metabolism in immune cell activation and suppression is increasingly recognized;71,72 the impact of altered bioenergetic function on innate immunity needs to be placed in context with functionality in other organs.
Antioxidants
Antioxidants can protect mitochondria against oxidative/nitrosative stress. For example, melatonin has antioxidant effects and improved redox state and mortality in animal models of sepsis.73,74 Antioxidants targeted specifically to mitochondria (e.g., MitoQ and MitoE) have also shown improved mitochondrial activity and reduced severity of organ failure in animal models.74,75
Decreasing metabolic rate
Decreasing metabolic rate is well established in clinical practice through inducing therapeutic hypothermia in cardiac arrest survivors,75 with possible utility in other neurological injuries.76 A recent study in rats with pneumococcal pneumonia demonstrated that hypothermia was associated with increased adenosine triphosphate availability and turnover.77
Carbon monoxide and hydrogen sulfide have similar effects that can induce the hibernation state alluded to earlier. While high levels of either are toxic to mitochondria, lower concentrations may be tissue-protective. Protection has been demonstrated with a water-soluble carbon monoxide releasing agent given to a mouse model post-induction of sepsis. Survival rates improved and accompanied by an increase in mitochondrial respiration, in PGC-1α expression and mitochondrial DNA copy number.78 Hydrogen sulfide, also an inhibitor of complex IV, reduces oxygen consumption in mice and induces a reversible state of “suspended animation”.79 Its potential utility in sepsis has been demonstrated in several animal studies with improvements in organ function and survival.80-83 However, in these studies the drug has been administered early, almost as a prophylactic treatment. Its benefits may be derived from its anti-inflammatory actions although, arguably, it may act predominantly through promoting a protective metabolic shutdown triggered by decreased energy availability akin to the intrinsic adaptive process argued previously.
Stimulating mitochondrial biogenesis
Carbon monoxide is released endogenously after activation of heme oxygenase (HO)-1. Induction of HO-1 in sepsis models has been shown to have an action through NRF-2, linking it to mitochondrial biogenesis, and improving survival.84,85 Thomas et al. recently reported use of a recombinant human TFAM in cultured mouse fibroblasts and a murine model of sepsis with improved redox and mitochondrial activity profiles, and survival rates.86 Their murine model of Parkinson disease also showed rhTFAM improved motor function.86 This may have implications in severe sepsis as muscle wasting occurs early,87 and with significant subsequent impact on return to normal function.4
Conclusion
In summary, there is significant evidence that implicates mitochondrial dysfunction in sepsis-induced organ dysfunction. Whether this is causative or epiphenomenal is less clear. However, survivors have better preservation of ATP, mitochondrial function, and biogenesis markers. Multi-organ failure may however represent a mechanism through which the likelihood of eventual survival is enhanced in those hardy enough to survive as cells may enter a “hibernating” state in the face of overwhelming inflammation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- ADP
adenosine diphosphate
- ANT
adenine nucleotide translocase
- ATP
adenosine triphosphate
- DAMP
danger associated molecular pattern
- FADH2
flavin adenine dinucleotide
- MOF
multi-organ failure
- NO
nitric oxide
- NADH
nicotinamide adenine dinucleotide
- PGC-1ɑ
PPARɣ-coactivator-1ɑ
- ROS
reactive oxygen species
- UCP
uncoupling protein
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–23. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JC, Girard TD, Gordon SM, Thompson JL, Shintani AK, Thomason JWW, Pun BT, Canonico AE, Dunn JG, Bernard GR, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182:183–91. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–16. doi: 10.1097/01.CCM.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–17. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langenberg C, Bagshaw SM, May CN, Bellomo R. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmittinger CA, Dünser MW, Torgersen C, Luckner G, Lorenz I, Schmid S, Joannidis M, Moser P, Hasibeder WR, Halabi M, et al. Histologic pathologies of the myocardium in septic shock: a prospective observational study. Shock. 2013;39:329–35. doi: 10.1097/SHK.0b013e318289376b. [DOI] [PubMed] [Google Scholar]
- 10.Boekstegers P, Weidenhöfer S, Pilz G, Werdan K. Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection. 1991;19:317–23. doi: 10.1007/BF01645355. [DOI] [PubMed] [Google Scholar]
- 11.Rosser DM, Stidwill RP, Jacobson D, Singer M. Oxygen tension in the bladder epithelium rises in both high and low cardiac output endotoxemic sepsis. J Appl Physiol (1985) 1995;79:1878–82. doi: 10.1152/jappl.1995.79.6.1878. [DOI] [PubMed] [Google Scholar]
- 12.Dyson A, Rudiger A, Singer M. Temporal changes in tissue cardiorespiratory function during faecal peritonitis. Intensive Care Med. 2011;37:1192–200. doi: 10.1007/s00134-011-2227-z. [DOI] [PubMed] [Google Scholar]
- 13.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–8. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 16.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26:711–23. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen FJ, Schiffer TA, Weitzberg E, Lundberg JO. Regulation of mitochondrial function and energetics by reactive nitrogen oxides. Free Radic Biol Med. 2012;53:1919–28. doi: 10.1016/j.freeradbiomed.2012.08.580. [DOI] [PubMed] [Google Scholar]
- 18.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 19.Bauer I, Pannen BH. Bench-to-bedside review: Carbon monoxide--from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper ME, Seifert EL. Thyroid hormone effects on mitochondrial energetics. Thyroid. 2008;18:145–56. doi: 10.1089/thy.2007.0250. [DOI] [PubMed] [Google Scholar]
- 21.Psarra AM, Sekeris CE. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life. 2008;60:210–23. doi: 10.1002/iub.37. [DOI] [PubMed] [Google Scholar]
- 22.Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- 23.Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Mühlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta. 2012;1823:1491–508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin F, Sancheti H, Cadenas E. Mitochondrial thiols in the regulation of cell death pathways. Antioxid Redox Signal. 2012;17:1714–27. doi: 10.1089/ars.2012.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 27.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 29.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–90. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 31.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–75. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojeda Ojeda M, Larrondo Muguercia H, Magdariaga Figuerola A, Sánchez Valdivia A, Rodríguez Alonso I, Valenzuela Silva C, García Iglesias E, Domínguez Alonso E, Buurman WA, Araña Rosaínz MdeJ. Temporal trends of circulating nitric oxide and pro-inflammatory cytokine responses ex vivo in intra-abdominal sepsis: results from a cohort study. Inflamm Res. 2011;60:289–97. doi: 10.1007/s00011-010-0267-4. [DOI] [PubMed] [Google Scholar]
- 34.Santos SS, Brunialti MKC, Rigato O, Machado FR, Silva E, Salomão R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock. 2012;38:18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 35.Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34(Suppl 1):4–14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losser MR, Damoisel C, Payen D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit Care. 2010;14:231. doi: 10.1186/cc9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy B, Gibot S, Franck P, Cravoisy A, Bollaert P-E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. 2005;365:871–5. doi: 10.1016/S0140-6736(05)71045-X. [DOI] [PubMed] [Google Scholar]
- 38.Dixon B. The role of microvascular thrombosis in sepsis. Anaesth Intensive Care. 2004;32:619–29. doi: 10.1177/0310057X0403200502. [DOI] [PubMed] [Google Scholar]
- 39.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 40.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–95. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 41.Stidwill RP, Rosser DM, Singer M. Cardiorespiratory, tissue oxygen and hepatic NADH responses to graded hypoxia. Intensive Care Med. 1998;24:1209–16. doi: 10.1007/s001340050746. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–63. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 44.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–7. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 45.Vanasco V, Magnani ND, Cimolai MC, Valdez LB, Evelson P, Boveris A, Alvarez S. Endotoxemia impairs heart mitochondrial function by decreasing electron transfer, ATP synthesis and ATP content without affecting membrane potential. J Bioenerg Biomembr. 2012;44:243–52. doi: 10.1007/s10863-012-9426-3. [DOI] [PubMed] [Google Scholar]
- 46.Chinga-Alayo E, Villena J, Evans AT, Zimic M. Thyroid hormone levels improve the prediction of mortality among patients admitted to the intensive care unit. Intensive Care Med. 2005;31:1356–61. doi: 10.1007/s00134-005-2719-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, Pan W, Wang H, Wang S, Pan S, Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Crit Care. 2012;16:R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32:670–93. doi: 10.1210/er.2011-0007. [DOI] [PubMed] [Google Scholar]
- 49.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. Inflamm and Host Response to Injury Large Scale Collab. Res. Program A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 50.Carré JE, Orban J-C, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–51. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–9. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Chung DJ, Szyszka B, Brown JCL, Hüner NPA, Staples JF. Changes in the mitochondrial phosphoproteome during mammalian hibernation. Physiol Genomics. 2013;45:389–99. doi: 10.1152/physiolgenomics.00171.2012. [DOI] [PubMed] [Google Scholar]
- 53.Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–9. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- 54.Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Camb Philos Soc. 2004;79:207–33. doi: 10.1017/S1464793103006195. [DOI] [PubMed] [Google Scholar]
- 55.Reilly BD, Schlipalius DI, Cramp RL, Ebert PR, Franklin CE. Frogs and estivation: transcriptional insights into metabolism and cell survival in a natural model of extended muscle disuse. Physiol Genomics. 2013;45:377–88. doi: 10.1152/physiolgenomics.00163.2012. [DOI] [PubMed] [Google Scholar]
- 56.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–14. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 57.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–8. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 58.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med. 1993;21:1012–9. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Boulos M, Astiz ME, Barua RS, Osman M. Impaired mitochondrial function induced by serum from septic shock patients is attenuated by inhibition of nitric oxide synthase and poly(ADP-ribose) synthase. Crit Care Med. 2003;31:353–8. doi: 10.1097/01.CCM.0000050074.82486.B2. [DOI] [PubMed] [Google Scholar]
- 60.Garrabou G, Morén C, López S, Tobías E, Cardellach F, Miró O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 61.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosi CA. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2007;176:768–77. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mofarrahi M, Sigala I, Guo Y, Godin R, Davis EC, Petrof B, Sandri M, Burelle Y, Hussain SN. Autophagy and skeletal muscles in sepsis. PLoS One. 2012;7:e47265. doi: 10.1371/journal.pone.0047265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drosatos K, Khan RS, Trent CM, Jiang H, Son N-H, Blaner WS, Homma S, Schulze PC, Goldberg IJ. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6:550–62. doi: 10.1161/CIRCHEARTFAILURE.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smeding L, Leong-Poi H, Hu P, Shan Y, Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS, et al. Salutary effect of resveratrol on sepsis-induced myocardial depression. Crit Care Med. 2012;40:1896–907. doi: 10.1097/CCM.0b013e31824e1370. [DOI] [PubMed] [Google Scholar]
- 65.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–21. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 67.Gunst J, Derese I, Aertgeerts A, Ververs E-J, Wauters A, Van den Berghe G, Vanhorebeek I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41:182–94. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 68.Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23:201–12. doi: 10.1038/cr.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med. 2006;34:2439–46. doi: 10.1097/01.CCM.0000230240.02216.21. [DOI] [PubMed] [Google Scholar]
- 70.Protti A, Singer M. Bench-to-bedside review: potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Crit Care. 2006;10:228. doi: 10.1186/cc5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wahl DR, Byersdorfer CA, Ferrara JL, Opipari AW, Jr., Glick GD. Distinct metabolic programs in activated T cells: opportunities for selective immunomodulation. Immunol Rev. 2012;249:104–15. doi: 10.1111/j.1600-065X.2012.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. ScientificWorldJournal. 2011;11:2509–29. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Escames G, López LC, Ortiz F, López A, García JA, Ros E, Acuña-Castroviejo D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007;274:2135–47. doi: 10.1111/j.1742-4658.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- 74.Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110:472–80. doi: 10.1093/bja/aes577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1095–102. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 77.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 78.Beurskens CJP, Aslami H, Kuipers MT, Horn J, Vroom MB, van Kuilenburg ABP, Roelofs JJ, Schultz MJ, Juffermans NP. Induced hypothermia is protective in a rat model of pneumococcal pneumonia associated with increased adenosine triphosphate availability and turnover. Crit Care Med. 2012;40:919–26. doi: 10.1097/CCM.0b013e3182373174. [DOI] [PubMed] [Google Scholar]
- 79.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329:641–8. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 80.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 81.Spiller F, Orrico MIL, Nascimento DC, Czaikoski PG, Souto FO, Alves-Filho JC, Freitas A, Carlos D, Montenegro MF, Neto AF, et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am J Respir Crit Care Med. 2010;182:360–8. doi: 10.1164/rccm.200907-1145OC. [DOI] [PubMed] [Google Scholar]
- 82.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aslami H, Pulskens WP, Kuipers MT, Bos AP, van Kuilenburg ABP, Wanders RJA, Roelofsen J, Roelofs JJ, Kerindongo RP, Beurskens CJ, et al. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS One. 2013;8:e63497. doi: 10.1371/journal.pone.0063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem. 2011;286:16374–85. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, Piantadosi CA. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185:851–61. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas RR, Khan SM, Portell FR, Smigrodzki RM, Bennett JP., Jr. Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion. 2011;11:108–18. doi: 10.1016/j.mito.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahlbeck K, Fredriksson K, Rooyackers O, Måbäck G, Remahl S, Ansved T, Eriksson L, Radell P. Signs of critical illness polyneuropathy and myopathy can be seen early in the ICU course. Acta Anaesthesiol Scand. 2009;53:717–23. doi: 10.1111/j.1399-6576.2009.01952.x. [DOI] [PubMed] [Google Scholar]