Abstract
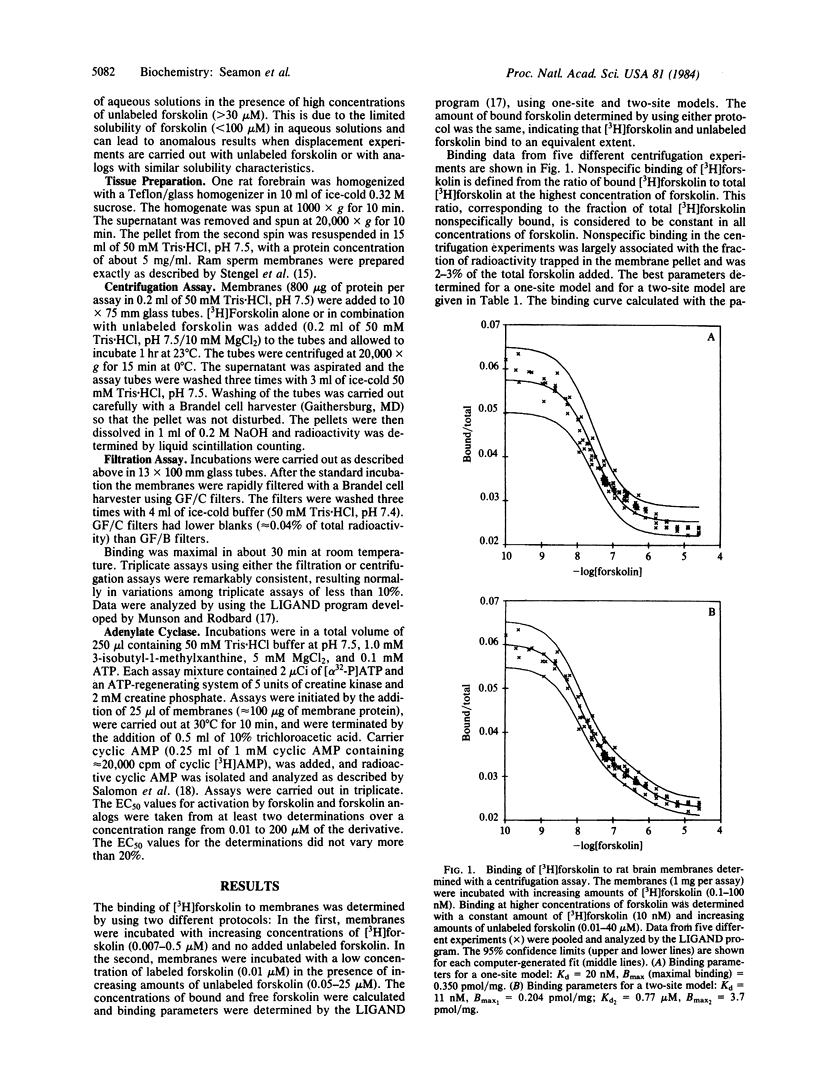
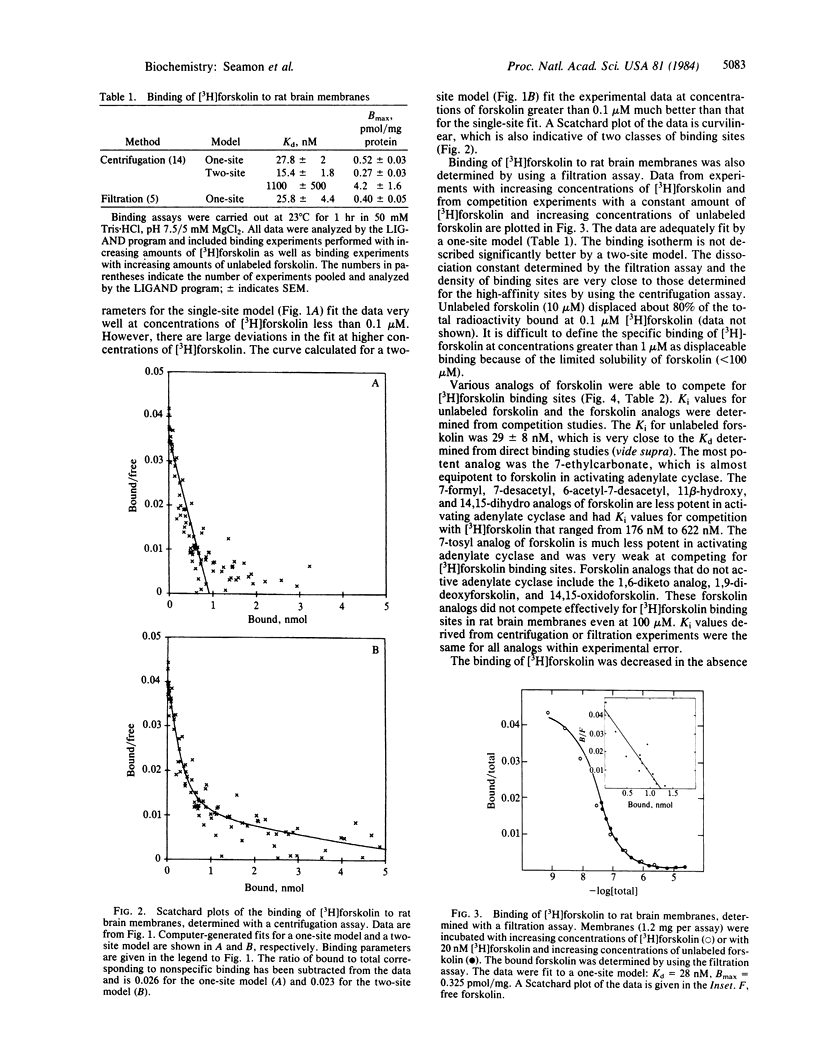
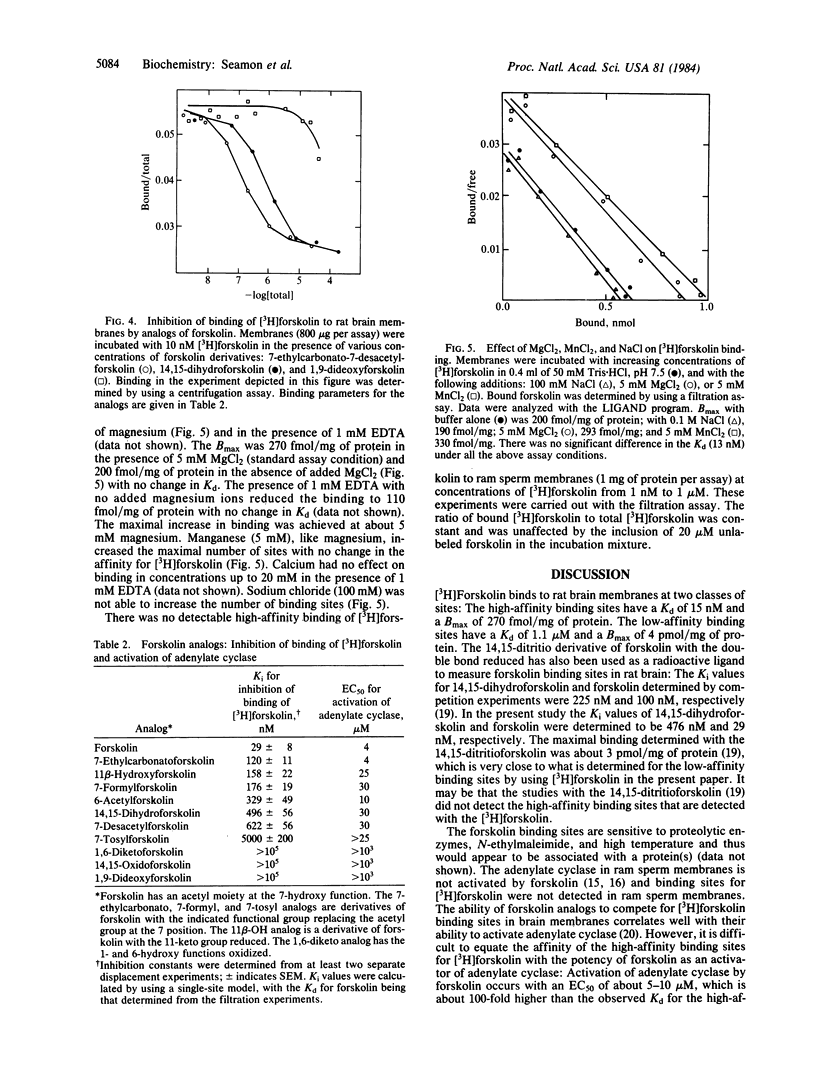
[12-3H]Forskolin (27 Ci/mmol) has been used to study binding sites in rat brain tissue by using both centrifugation and filtration assays. The binding isotherm measured in the presence of 5 mM MgCl2 by using the centrifugation assay is described best by a two-site model: Kd1 = 15 nM, Bmax1 (maximal binding) = 270 fmol/mg of protein; Kd2 = 1.1 microM; Bmax2 = 4.2 pmol/mg of protein. Only the high-affinity binding sites are detected when the binding is determined by using a filtration assay; Kd = 26 nM, Bmax = 400 fmol/mg of protein. Analogs of forskolin that do not activate adenylate cyclase (EC 4.6.1.1) do not compete effectively for [3H]forskolin binding sites. Analogs of forskolin that are less potent than forskolin in activating adenylate cyclase are also less potent in competing for forskolin binding sites. The presence of 5 mM MgCl2 or MnCl2 was found to enhance binding. In the presence of 1 mM EDTA the amount of high-affinity binding is reduced to 110 fmol/mg of protein with no change in Kd. There is no effect of CaCl2 (20 mM) or NaCl (100 mM) on the binding. No high-affinity binding can be detected in membranes from ram sperm, which contains an adenylate cyclase that is not activated by forskolin. It is proposed that the high-affinity binding sites for forskolin are associated with the activated complex of catalytic subunit and stimulatory guanine nucleotide binding protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender J. L., Neer E. J. Properties of the adenylate cyclase catalytic unit from caudate nucleus. J Biol Chem. 1983 Feb 25;258(4):2432–2439. [PubMed] [Google Scholar]
- Clark R. B., Goka T. J., Green D. A., Barber R., Butcher R. W. Differences in the forskolin activation of adenylate cyclases in wild-type and variant lymphoma cells. Mol Pharmacol. 1982 Nov;22(3):609–613. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., Manclark C. R. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M. Bimodal regulation of adenylate cyclase. FEBS Lett. 1982 Feb 22;138(2):157–163. doi: 10.1016/0014-5793(82)80431-6. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:81–89. [PubMed] [Google Scholar]
- Forte L. R., Bylund D. B., Zahler W. L. Forskolin does not activate sperm adenylate cyclase. Mol Pharmacol. 1983 Jul;24(1):42–47. [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Direct evidence for the role of the coupling proteins in forskolin activation of adenylate cyclase. J Cyclic Nucleotide Res. 1982;8(5):337–346. [PubMed] [Google Scholar]
- Jefferson L. S., Liao W. S., Peavy D. E., Miller T. B., Appel M. C., Taylor J. M. Diabetes-induced alterations in liver protein synthesis. Changes in the relative abundance of mRNAs for albumin and other plasma proteins. J Biol Chem. 1983 Jan 25;258(2):1369–1375. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Baer H. P. Forskolin binding sites in rat liver and brain membranes. Eur J Pharmacol. 1983 Oct 28;94(3-4):337–340. doi: 10.1016/0014-2999(83)90424-7. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W., Metzger H., de Souza N. J., Reden J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983 Mar;26(3):436–439. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Stengel D., Guenet L., Desmier M., Insel P., Hanoune J. Forskolin requires more than the catalytic unit to activate adenylate cyclase. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):681–690. doi: 10.1016/0303-7207(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]


