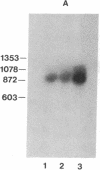
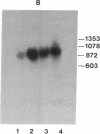
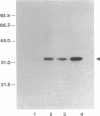
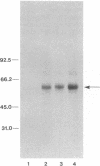
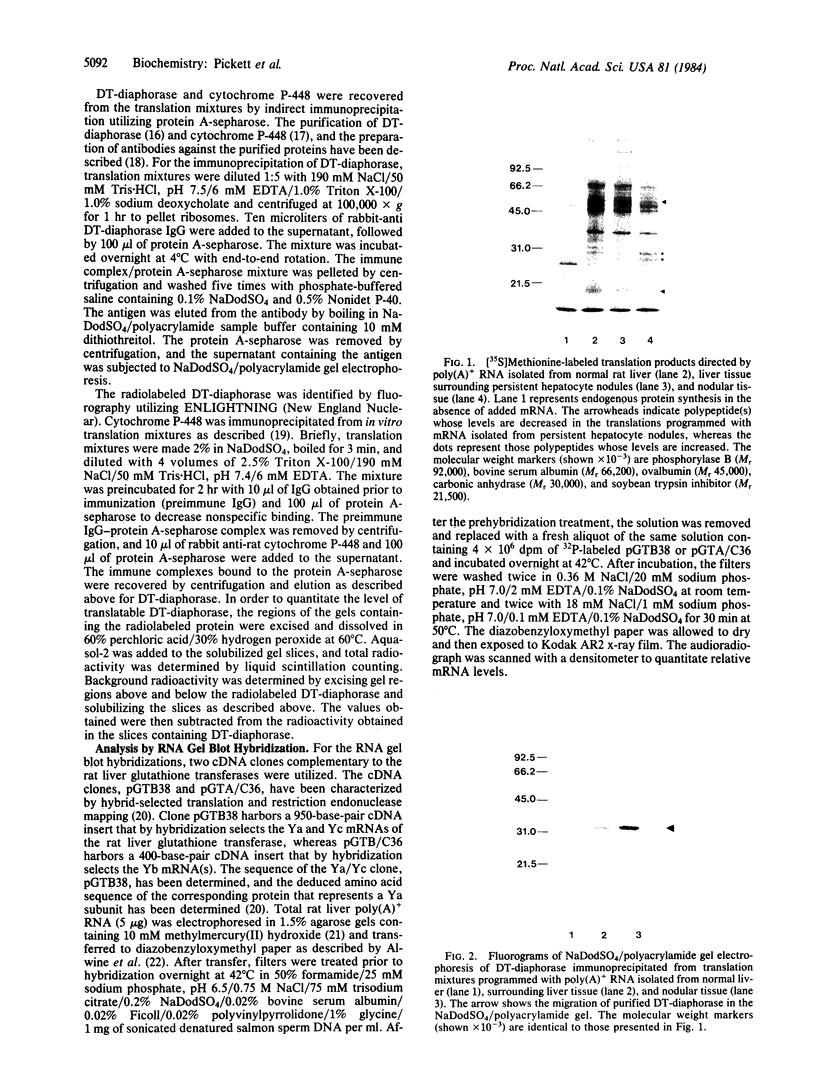
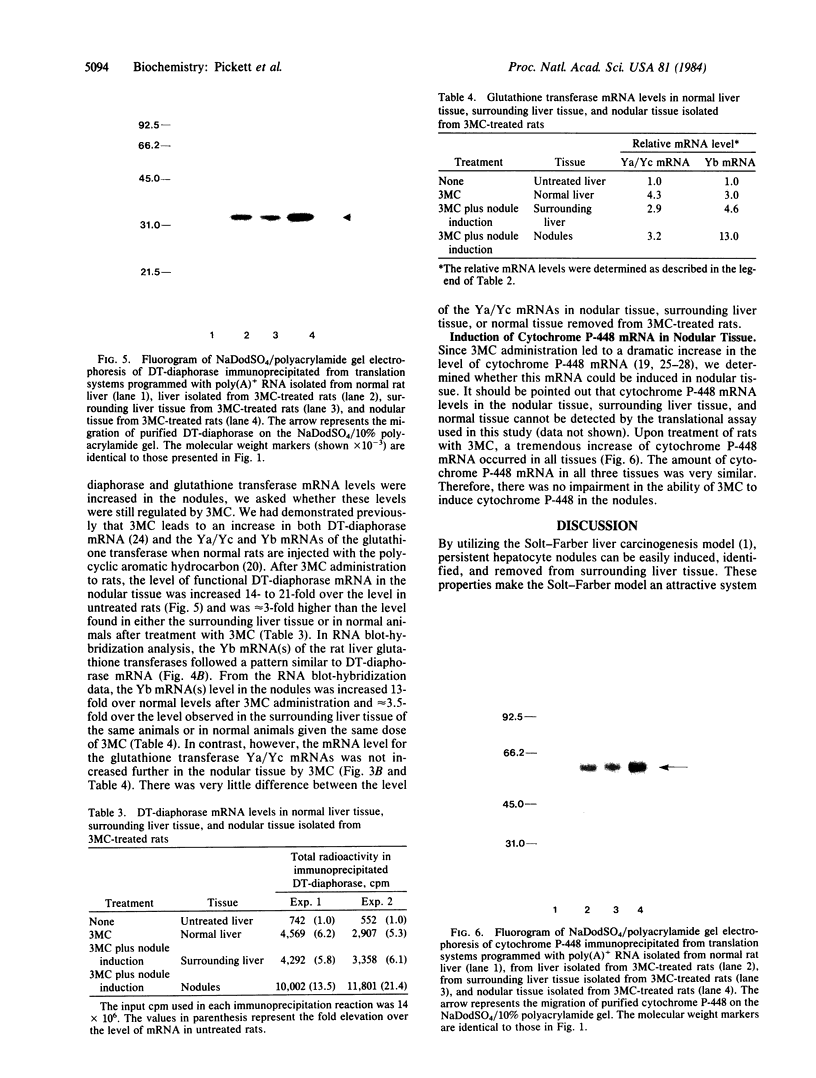
Abstract
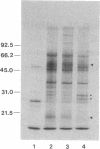
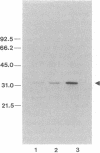
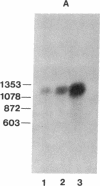
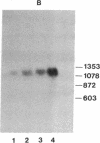
We have utilized cDNA probes and in vitro translation analysis to quantitate the levels of rat liver glutathione transferase (glutathione S-aralkyltransferase; RX:glutathione R-transferase, EC 2.5.1.18) and DT-diaphorase [NAD-(P)H:quinone-acceptor oxidoreductase, EC 1.6.99.2] mRNAs in persistent hepatocyte nodules induced by chemical carcinogens. Our results indicate that within the nodules, glutathione transferase mRNAs specific for the Ya/Yc and Yb subunits are increased 3-fold and 5-fold, respectively, over the levels observed in normal liver or in the liver tissue surrounding the nodules. Similarly, the level of DT-diaphorase mRNA is increased 5- to 7-fold within the nodules as compared to surrounding liver tissue or normal liver. When animals were administered 3-methylcholanthrene, a typical inducer of these mRNAs in normal animals, a further increase in the glutathione transferase Yb mRNA(s) and DT-diaphorase mRNA was observed in the nodules; however, the Ya/Yc mRNA levels remained unaffected. Our data indicate that during chemically induced neoplastic transformation, the mRNA levels for the Yb subunit of glutathione transferase and DT-diaphorase are increased in the nodules but still retain the capacity to be regulated by 3-methylcholanthrene. Although the glutathione transferase Ya/Yc mRNAs are also increased in the nodules, they lost their ability to be regulated by 3-methylcholanthrene. These latter data suggest that within the nodules there is a specific defect in the regulatory mechanism(s) that leads to an induction of the Ya/Yc mRNAs in normal tissue by xenobiotics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Aström A., DePierre J. W., Eriksson L. Characterization of drug-metabolizing systems in hyperplastic nodules from the livers of rats receiving 2-acetylaminofluorene in their diet. Carcinogenesis. 1983;4(5):577–581. doi: 10.1093/carcin/4.5.577. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P., Waechter F., Oesch F., Stäubli W. Immunochemical localization of epoxide hydratase in rat liver: effects of 2-acetylaminofluorene. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1101–1108. doi: 10.1016/0006-291x(79)91994-6. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Brosseau M., Levin W., Reik L., Ryan D. E., Thomas P. E. Administration of 3-methylcholanthrene to rats increases the specific hybridizable mRNA coding for cytochrome P-450c. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4083–4087. doi: 10.1073/pnas.78.7.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R., Sweeney G. D., Jones K., Lee G., Farber E. A relative deficiency of cytochrome P-450 and aryl hydrocarbon [benzo(a)pyrene] hydroxylase in hyperplastic nodules induced by 2-acetylaminofluorene in rat liver. Cancer Res. 1976 Nov;36(11 Pt 1):3888–3893. [PubMed] [Google Scholar]
- Cayama E., Tsuda H., Sarma D. S., Farber E. Initiation of chemical carcinogenesis requires cell proliferation. Nature. 1978 Sep 7;275(5675):60–62. doi: 10.1038/275060a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fagan J. B., Pastewka J. V., Park S. S., Guengerich F. P., Gelboin H. V. Identification and quantitation of a 2.0-kilobase messenger ribonucleic acid coding for 3-methylcholanthrene-induced cytochrome P-450 using cloned cytochrome P-450 complementary deoxyribonucleic acid. Biochemistry. 1982 Dec 7;21(25):6574–6580. doi: 10.1021/bi00268a039. [DOI] [PubMed] [Google Scholar]
- Farber E. The sequential analysis of liver cancer induction. Biochim Biophys Acta. 1980 May 6;605(2):149–166. doi: 10.1016/0304-419x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Griffin M. J., Kizer D. E. Purification and quantitation of preneoplastic antigen from hyperplastic nodules and normal liver. Cancer Res. 1978 Apr;38(4):1136–1141. [PubMed] [Google Scholar]
- Huang M. T., Miwa G. T., Lu A. Y. Rat liver cytosolic azoreductase. Purification and characterization. J Biol Chem. 1979 May 25;254(10):3930–3934. [PubMed] [Google Scholar]
- Hunt S. W., 3rd, Hoffee P. A. Amplification of adenosine deaminase gene sequences in deoxycoformycin-resistant rat hepatoma cells. J Biol Chem. 1983 Nov 10;258(21):13185–13192. [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Sato K. Properties of the increased glutathione S-transferase A form in rat preneoplastic hepatic lesions induced by chemical carcinogens. Biochem Biophys Res Commun. 1983 Apr 15;112(1):20–28. doi: 10.1016/0006-291x(83)91791-6. [DOI] [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Thomas P. E., Ryan D., Kizer D. E., Griffin M. J. Identification of epoxide hydrase as the preneoplastic antigen in rat liver hyperplastic nodules. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3240–3243. doi: 10.1073/pnas.75.7.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morville A. L., Thomas P., Levin W., Reik L., Ryan D. E., Raphael C., Adesnik M. The accumulation of distinct mRNAs for the immunochemically related cytochromes P-450c and P-450d in rat liver following 3-methylcholanthrene treatment. J Biol Chem. 1983 Mar 25;258(6):3901–3906. [PubMed] [Google Scholar]
- Okita K., Noda K., Fukumoto Y., Takemoto T. Cytochrome P-450 in hyperplastic liver nodules during hepatocarcinogenesis with N-2-fluorenylacetamide in rats. Gan. 1976 Dec;67(6):899–902. [PubMed] [Google Scholar]
- Pickett C. B., Jeter R. L., Morin J., Lu A. Y. Electroimmunochemical quantitation of cytochrome P-450, cytochrome P-448, and epoxide hydrolase in rat liver microsomes. J Biol Chem. 1981 Aug 25;256(16):8815–8820. [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Effect of phenobarbital on the level of translatable rat liver epoxide hydrolase mRNA. Proc Natl Acad Sci U S A. 1981 Feb;78(2):893–897. doi: 10.1073/pnas.78.2.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Donohue A. M., Lu A. Y., Hales B. F. Differential induction of rat hepatic cytochrome P-448 and glutathione S-transferase B messenger RNAs by 3-methylcholanthrene. Biochem Biophys Res Commun. 1982 Jan 29;104(2):611–619. doi: 10.1016/0006-291x(82)90681-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schor N. A. The use of the D-T diaphorase for the detection of foci of early neoplastic transformation in rat liver. Cancer Lett. 1978 Sep;5(3):167–171. doi: 10.1016/s0304-3835(78)80034-2. [DOI] [PubMed] [Google Scholar]
- Sharma R. N., Gurtoo H. L., Farber E., Murray R. K., Cameron R. G. Effects of hepatocarcinogens and hepatocarcinogenesis on the activity of rat liver microsomal epoxide hydrolase and observations on the electrophoretic behavior of this enzyme. Cancer Res. 1981 Sep;41(9 Pt 1):3311–3319. [PubMed] [Google Scholar]
- Tukey R. H., Nebert D. W., Negishi M. Structural gene product of the [Ah] complex. Evidence for transcriptional control of cytochrome P1-450 induction by use of a cloned DNA sequence. J Biol Chem. 1981 Jul 10;256(13):6969–6974. [PubMed] [Google Scholar]
- West S. B., Huang M. T., Miwa G. T., Lu A. Y. A simple and rapid procedure for the purification of phenobarbital-inducible cytochrome P-450 from rat liver microsomes. Arch Biochem Biophys. 1979 Mar;193(1):42–50. doi: 10.1016/0003-9861(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Wang R., Lu A. Y., Pickett C. B. Rat liver DT-diaphorase: regulation of functional mRNA levels by 3-methylcholanthrene, trans-stilbene oxide, and phenobarbital. Arch Biochem Biophys. 1984 Jul;232(1):408–413. doi: 10.1016/0003-9861(84)90556-3. [DOI] [PubMed] [Google Scholar]