Abstract
Paragonimiasis is an important food-borne parasitic zoonosis caused by infection with lung flukes of the genus Paragonimus. Of the 7 members of the genus known in Thailand until recently, only P. heterotremus has been confirmed as causing human disease. An 8th species, P. pseudoheterotremus, has recently been proposed from Thailand, and has been found in humans. Molecular data place this species as a sister species to P. heterotremus, and it is likely that P. pseudoheterotremus is not specifically distinct from P. heterotremus. In this study, we collected metacercariae of both nominal species (identification based on metacercarial morphology) from freshwater crabs from Phetchabun Province in northern Thailand, Saraburi Province in central Thailand, and Surat Thani Province in southern Thailand. In addition, we purchased freshwater crabs imported from Myanmar at Myawaddy Province, western Thailand, close to the Myanmar-Thailand border. The DNAs extracted from excysted metacercariae were PCR-amplified and sequenced for ITS2 and cox1 genes. The ITS2 sequences were nearly identical among all samples (99-100%). Phylogenies inferred from all available partial cox1 sequences contained several clusters. Sequences from Indian P. heterotremus formed a sister group to sequences from P. pseudoheterotremus-type metacercariae. Sequences of P. heterotremus from Thailand, Vietnam, and China formed a separate distinct clade. One metacercaria from Phitsanulok Province was distinct from all others. There is clearly considerable genetic variation in the P. heterotremus complex in Thailand and the form referred to as P. pseudoheterotremus is widely distributed in Thailand and the Thai-Myanmar border region.
Keywords: Paragonimus heterotremus, Paragonimus pseudoheterotremus, molecular epidemiology, internal transcribed spacer 2 (ITS2), cytochrome c oxidase subunit 1 (cox1)
INTRODUCTION
Paragonimiasis is an important food-borne zoonosis, especially in Asian countries, caused by infection with lung flukes of the genus Paragonimus. More than 50 species of Paragonimus have been described from Asia, America, and Africa [1]. Seven species, namely, P. heterotremus, P. westermani, P. siamensis, P. bangkokensis, P. harinasutai, P. paishuihoensis, and P. macrorchis have been reported from Thailand. Of these, only P. heterotremus was regarded until recently as causing human disease in Thailand [2,3], as it also does in India [4-6], Vietnam [7-9], and Laos [10]. Recently, an 8th nominal species, P. pseudoheterotremus, was described from Thailand [11], and human pulmonary paragonimiasis caused by this species has been identified using DNA sequence data of the internal transcribed spacer 2 (ITS2) and cytochrome c oxidase subunit 1 (cox1) from eggs in the sputum of a patient [12]. This species was distinguished from P. heterotremus by the small size of its metacercariae and by details of adult surface spination. P. pseudoheterotremus appears, on the basis of DNA sequence data, to be a very close sister species to P. heterotremus. In Vietnam, metacercariae resembling those of both of these species have been found, but cannot be separated using molecular data (Doanh, pers. comm.). P. pseudoheterotremus may thus not be specifically distinct from P. heterotremus. However, both names will be used in this paper, simply to indicate the size differences in metacercariae.
The discovery of a human case attributed to P. pseudoheterotremus [12] prompted us to study the distributions and relationships of metacercariae of P. heterotremus and P. pseudoheterotremus types in freshwater crabs in Thailand. Moreover, neither of these types were reported from Myanmar, which shares a long border with Thailand. Here, we report ITS2 and cox1 gene sequences of metacercariae of P. heterotremus and P. pseudoheterotremus types from several regions in Thailand and an area in Myanmar, and place these in a phylogenetic context using new and published sequences.
MATERIALS AND METHODS
Collection of Paragonimus metacercariae
Paragonimus metacercariae (Fig. 1) were collected from naturally infected freshwater crabs which were collected in 4 provinces of Thailand (Fig. 2); Phitsanulok Province and Phetchabun Province in the north, Saraburi Province in the center, and Surat Thani Province in the south of Thailand. In addition, crabs were also collected in Myawaddy Province, Myanmar, bordering western Thailand. The crabs were digested with artificial gastric juice (1% pepsin in HCl solution). Digested samples were filtered through wire sieves (pore size; 300 and 500 µm), and the filtrate was allowed to stand for sedimentation. The sediments were examined under a stereomicroscope. The metacercariae found were excysted using a method previously described [13]. Each freshly excysted Paragonimus metacercaria was morphologically identified using microscopy before DNA extraction.
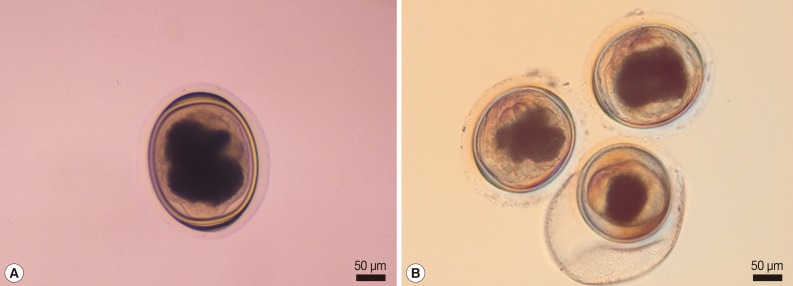
Fig. 1.
Representative metacercariae morphologically resembling those of P. heterotremus (A) and P. pseudoheterotremus (B). The P. heterotremus-type metacercaria (A) was collected in Phisanulok Province, northern Thailand, whereas the P. pseudoheterotremus-type metacercariae (B) were collected in Surat Thani Province, southern Thailand.
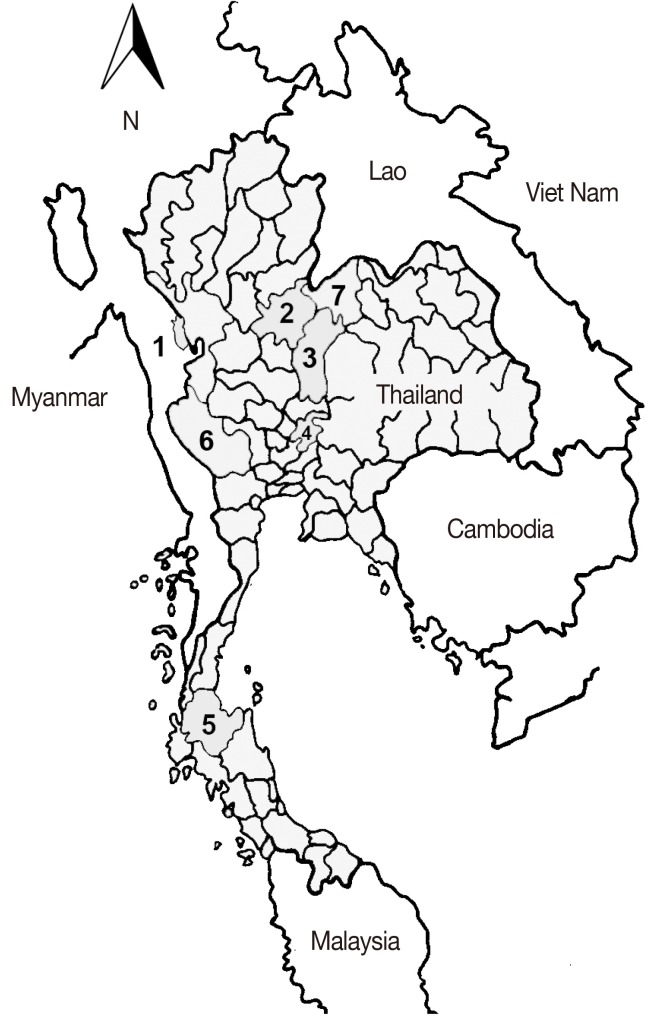
Fig. 2.
Locations of provinces in Thailand and Myanmar from which freshwater crabs were sampled for this study. Myawaddy: Myanmar (1; present study), Thailand: Phitsanulok (2; present study), Phetchabun (3; present study), Saraburi (4; present study and Thaenkham and Waikagul, 2008), and Surat Thani (5; present study). In addition, provinces from which P. pseudoheterotremus has been reported in previous studies are shown: Kanchanaburi (6; Thaenkham and Waikagul, 2008) and Loei (7; Intapan et al., 2012).
DNA extraction
To extract genomic DNA, each excysted Paragonimus metacercaria was homogenized separately with a disposable polypropylene pestle (Bellco Glass Inc., Vineland, New Jersy, USA). Then, DNA was extracted using a NucleoSpin Tissue Kit (Macherey-Nagel GmbH & Co., Düren, Germany). The DNA was eluted in 100 µl of distilled water and stored at -20℃ until used.
PCR, sequencing, and molecular phylogenetic analysis
The ITS2 sequence was amplified using primers PhITS2-F (5'-CTG TGT GAA TTA ATG TGA ACT GC-3') and PhITS2-R (5'-AGT GAT ATG CTT AAG TTC AGC G-3') [12], which were designed from the known ITS2 sequence of P. heterotremus from northeastern India (GenBank accession no. AB308377). A fragment of the cox1 gene was amplified using primers, PphCOI-F (5'-CCG GGT TTG GTG TTG TG-3') and PphCOI-R (5'-ACA ACG AAC CAA GTG TCA TG-3') [12], which were designed from a region of P. pseudoheterotremus cox1 gene (GenBank accession no. EF446315).
PCR was conducted using a GeneAmp® PCR System 9700 (Applied Biosystems, Singapore). The reaction was carried out in a 25 µl volume containing PCR 1× FastStart High Fidelity Reaction Buffer (Roche Applied Science, Mannheim, Germany), 1.8 mM MgCl2, 0.2 mM of each deoxyribonucleotide triphosphate, 0.2 µM of each primer, and 0.625 units of FastStart High Fidelity Enzyme Blend (Roche Applied Science). The DNA template was initially denatured at 94℃ for 5 min. The amplification procedure comprised 35 cycles at 95℃ for 30 sec (denaturation), 55℃ for 30 sec (annealing), and 72℃ for 30 sec (extension), with a final extension at 72℃ for 10 min. The amplified product was subjected to electrophoresis on a 1.5% agarose gel. The PCR product was then cut and purified for DNA sequencing, which was performed using the MegaBACE 1,000 DNA Analysis System (GE Healthcare, Piscataway, New Jersy, USA).
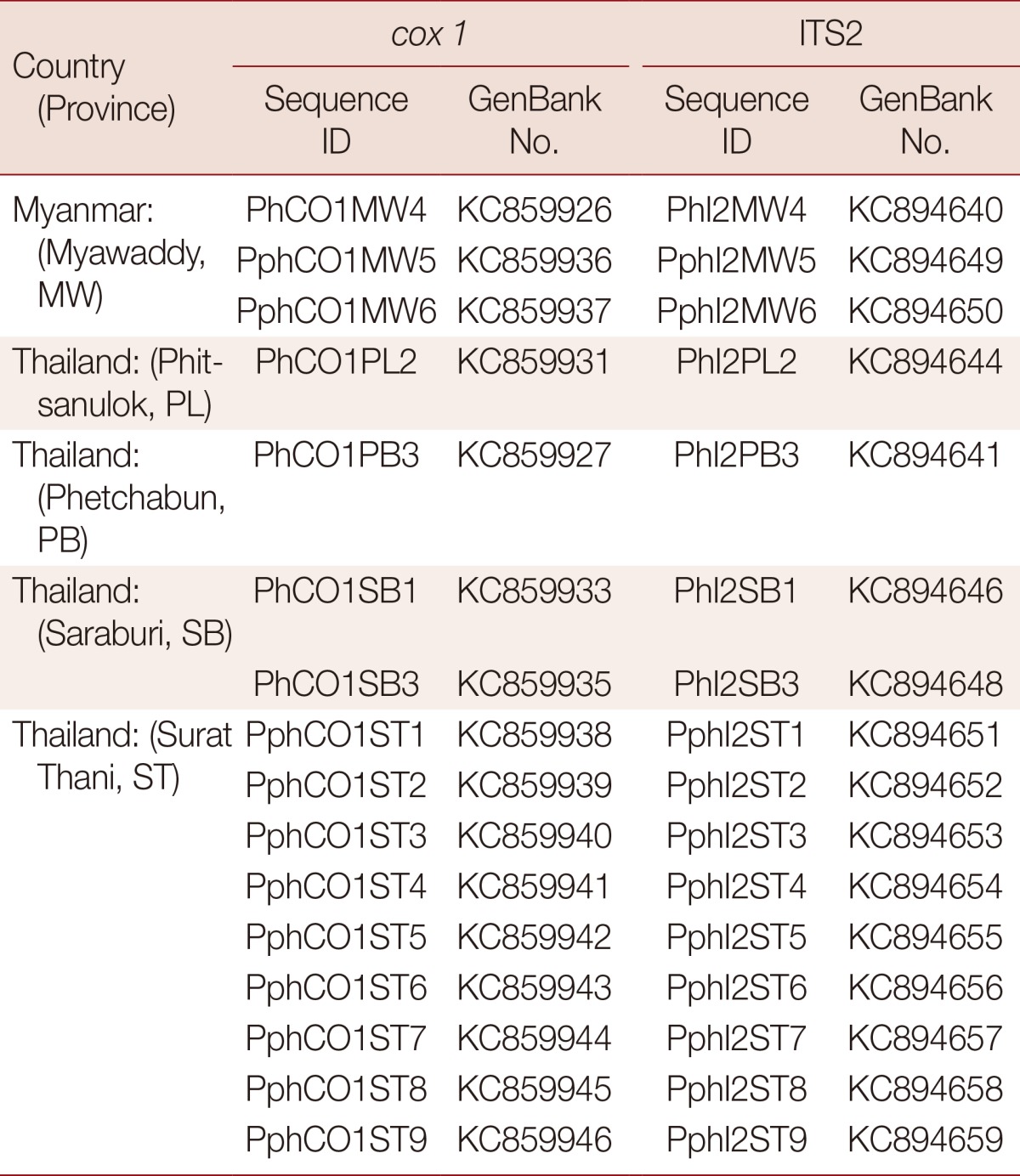
The partial ITS2 and cox1 gene sequences of individual Paragonimus metacercariae from each locality were deposited in GenBank under accession numbers presented in Table 1. They were analyzed using the BLAST-N search (National Center for Biotechnology Information, Bethesda, Maryland, USA). Published cox1 sequences from P. heterotremus and P. pseudoheterotremus were aligned with our new sequences (alignment length 309 bp when trimmed to the length of the shortest sequence) using ClustalW [14] and a maximum likelihood tree constructed using MEGA 5.2 [15]. The best-fit substitution model was determined in MEGA to be the Hasegawa-Kishino-Yano (HKY) model with uniform rates among sites, but assuming a proportion (0.65) of invariant sites. Sequences from P. westermani were used to provide an outgroup.
Table 1.
Accession numbers for new Paragonimus sequences deposited in GenBank
RESULTS
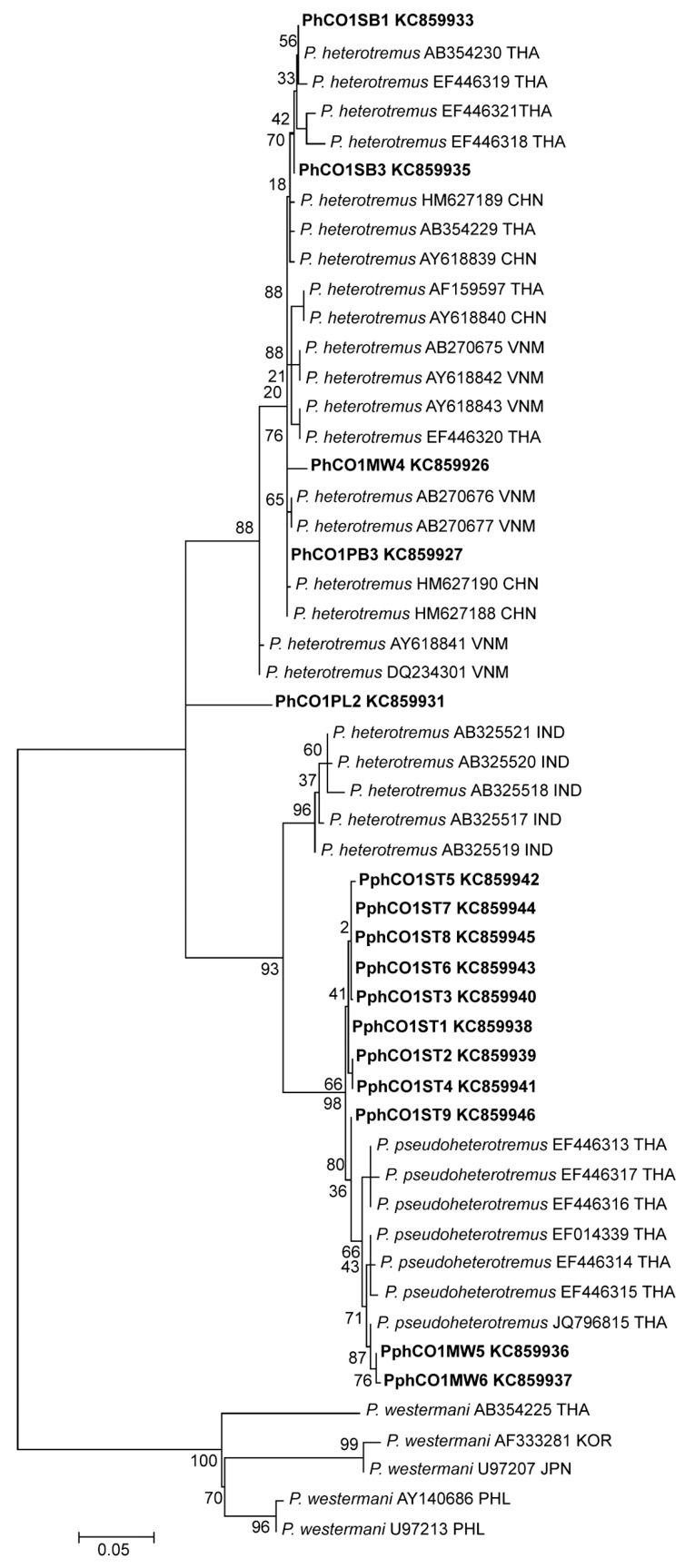
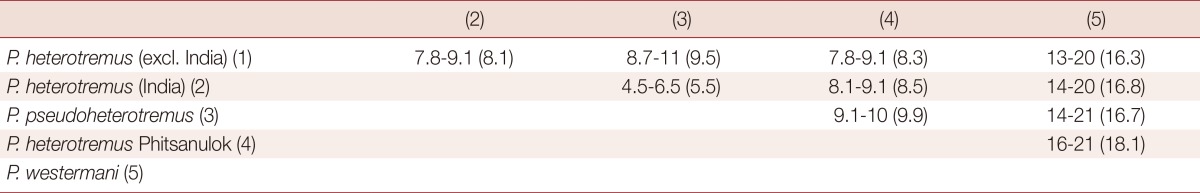
The partial ITS2 sequences of all metacercariae from different localities in Thailand and Myanmar were almost completely (98-99%) identical. Phylogenies inferred from all available partial cox1 sequences contained several well-supported clusters (Fig. 3). Sequences from Indian P. heterotremus formed a sister group to sequences from P. pseudoheterotremus-type metacercariae. Sequences of P. heterotremus from Thailand, Vietnam, and China formed a separate distinct clade. A metacercaria from Phitsanulok Province was distinct from all others. Table 2 shows the ranges and means of percentage differences between these clusters, and that P. heterotremus from northeastern India are closest to sequences from metacercariae of the P. pseudoheterotremus-type from Thailand.
Fig. 3.
Maximum likelihood tree based on partial cytochrome c oxidase subunit I (cox1) gene sequences. Sequences of P. heterotremus, P. pseudoheterotremus, and P. westermani (outgroup) obtained from GenBank are indicated with accession number and country code (ISO 3166-1 alpha-3 codes). Paragonimus sequences of this study are presented in bold (KC859926, KC859936, KC859937, KC859931, KC859927, KC859933, KC859935, KC859938-KC859946). The sequences were deposited in GenBank numbers as shown in Table 1.
Table 2.
Pairwise percentage differences in range (mean) between clusters on the tree
DISCUSSION
In this study, we explored the molecular relationships of metacercariae corresponding morphologically with those described for P. heterotremus and P. pseudoheterotremus. Samples used were collected in different parts of Thailand including the Thai-Myanmar border area. Metacercariae corresponding to the P. heterotremus type were found in northern and central parts of Thailand and Myawaddy Province, Myanmar. Those of the P. pseudoheterotremus type were found in Surat Thani Province, Thailand and in Myawaddy Province, Myanmar. Members of this complex are clearly widespread in Thailand and the Thai-Myanmar border region, and this must alert us to possible human infections in those areas. The ITS2 sequences were near-identical among all metacercariae sampled, and with sequences available from GenBank. This reflects the situation seen in the P. skrjabini complex, which exhibits far less variation in ITS2 sequences than in mitochondrial genes [16].
Although P. pseudoheterotremus was proposed as a species distinct from P. heterotremus, the phylogeny in Fig. 3, based on mitochondrial cox1 sequences, shows that the true situation is complicated. P. heterotremus from northeastern India is sister to Thai metacercariae of the P. pseudoheterotremus type, and 1 metacercaria from Phitsanulok Province in Thailand is distinct from all others. The considerable variation in cox1 sequences found in Thailand indicates that further work is required to demonstrate the full diversity of P. heterotremus and related forms in Thailand.
ACKNOWLEDGMENTS
This research was funded by grants from the National Science and Technology Development Agency (Discovery Based Development Grant); the Higher Education Research Promotion National Research University Project of Thailand, Office of the Higher Education Commission through the Health Cluster (SHep-GMS), Thailand; the Faculty of Medicine, Khon Kaen University. Wanchai Maleewong, Pewpan M. Intapan, and Tongjit Thanchomnang are supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. We wish to acknowledge the support of the Khon Kaen University Publication Clinic, Research and Technology Transfer Affairs, Khon Kaen University, for their assistance.
References
- 1.Blair D, Wu B, Chang ZS, Gong X, Agatsuma T, Zhang YN, Chen SH, Lin JX, Chen MG, Waikagul J, Guevara AG, Feng Z, Davis GM. A molecular perspective on the genera Paragonimus Braun, Euparagonimus Chen and Pagumogonimus Chen. J Helminthol. 1999;73:295–299. [PubMed] [Google Scholar]
- 2.Vanijanonta S, Radomyos P, Bunnag D, Harinasuta T. Pulmonary paragonimiasis with expectoration of worms: a case report. Southeast Asian J Trop Med Public Health. 1981;12:104–106. [PubMed] [Google Scholar]
- 3.Sugiyama H, Morishima Y, Rangsiruji A, Binchai S, Ketudat P, Kawanaka M. Application of multiplex PCR for species discrimination using individual metacercariae of Paragonimus occurring in Thailand. Southeast Asian J Trop Med Public Health. 2006;37:48–52. [PubMed] [Google Scholar]
- 4.Devi KR, Narain K, Bhattacharya S, Negmu K, Agatsuma T, Blair D, Wickramashinghe S, Mahanta J. Pleuropulmonary paragonimiasis due to Paragonimus heterotremus: molecular diagnosis, prevalence of infection and clinicoradiological features in an endemic area of northeastern India. Trans R Soc Trop Med Hyg. 2007;101:786–792. doi: 10.1016/j.trstmh.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Lall M, Sahni AK, Rajput AK. Pleuropulmonary paragonimiasis: mimicker of tuberculosis. Pathog Glob Health. 2013;107:40–42. doi: 10.1179/2047773212Y.0000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh TS, Sugiyama H, Rangsiruji A. Paragonimus and paragonimiasis in India. Indian J Med Res. 2012;136:192–204. [PMC free article] [PubMed] [Google Scholar]
- 7.Doanh PN, Dung do T, Thach DT, Horii Y, Shinohara A, Nawa Y. Human paragonimiasis in Viet Nam: epidemiological survey and identification of the responsible species by DNA sequencing of eggs in patients' sputum. Parasitol Int. 2011;60:534–537. doi: 10.1016/j.parint.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Doanh PN, Shinohara A, Horii Y, Habe S, Nawa Y, The DT, Le NT. Morphological and molecular identification of 2 Paragonimus spp., of which metacercariae concurrently found in a land crab, Potamiscus tannanti, collected in Yenbai Province, Vietnam. Parasitol Res. 2007;100:1075–1082. doi: 10.1007/s00436-006-0411-9. [DOI] [PubMed] [Google Scholar]
- 9.Le TH, Van De N, Blair D, McManus DP, Kino H, Agatsuma T. Paragonimus heterotremus Chen and Hsia, 1964 in Vietnam: a molecular identification and relationships of isolates from different hosts and geographical origins. Acta Trop. 2006;98:25–33. doi: 10.1016/j.actatropica.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Yahiro S, Habe S, Duong V, Odermatt P, Barennes H, Strobel M, Nakamura S. Identification of the human paragonimiasis causative agent in Lao People's Democratic Republic. J Parasitol. 2008;94:1176–1177. doi: 10.1645/GE-1457.1. [DOI] [PubMed] [Google Scholar]
- 11.Waikagul J. A new species of Paragonimus (Trematoda: Troglotrematidae) from a cat infected with metacercariae from mountain crabs Larnaudia larnaudii. J Parasitol. 2007;93:1496–1500. doi: 10.1645/GE-1054.1. [DOI] [PubMed] [Google Scholar]
- 12.Intapan PM, Sanpool O, Thanchomnang T, Imtawil K, Pongchaiyakul C, Nawa Y, Maleewong W. Molecular identification of a case of Paragonimus pseudoheterotremus infection in Thailand. Am J Trop Med Hyg. 2012;87:706–709. doi: 10.4269/ajtmh.2012.12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Intapan PM, Maleewong W. In vitro excystation of Paragonimus heterotremus metacercariae. J Parasitol. 2001;87:1184–1186. doi: 10.1645/0022-3395(2001)087[1184:IVEOPH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doanh PN, Hien HV, Nonaka N, Horii Y, Nawa Y. Discovery of Paragonimus skrjabini in Vietnam and its phylogenetic status in the Paragonimus skrjabini complex. J Helminthol. 2013;87:450–456. doi: 10.1017/S0022149X1200065X. [DOI] [PubMed] [Google Scholar]