Abstract
Opisthorchis viverrini and Clonorchis sinensis are parasites known to be carcinogenic and causative agents of cholangiocarcinoma in Asia. The standard method for diagnosis for those parasite infections is stool examination to detect parasite eggs. However, the method has low sensitivity, and eggs of O. viverrini and C. sinensis are difficult to distinguish from each other and from those of some other trematodes. Here, we report a multiplex real-time PCR coupled with high resolution melting (HRM) analysis for the differentiation of O. viverrini and C. sinensis eggs in fecal samples. Using 2 pairs of species-specific primers, DNA sequences from a portion of the mitochondrial NADH dehydrogenase subunit 2 (nad 2) gene, were amplified to generate 209 and 165 bp products for O. viverrini and C. sinensis, respectively. The distinct characteristics of HRM patterns were analyzed, and the melting temperatures peaked at 82.4±0.09℃ and 85.9±0.08℃ for O. viverrini and C. sinensis, respectively. This technique was able to detect as few as 1 egg of O. viverrini and 2 eggs of C. sinensis in a 150 mg fecal sample, which is equivalent to 7 and 14 eggs per gram of feces, respectively. The method is species-specific, rapid, simple, and does not require fluorescent probes or post-PCR processing for discrimination of eggs of the 2 species. It offers a new tool for differentiation and detection of Asian liver fluke infections in stool specimens.
Keywords: Opisthorchis viverrini, Clonorchis sinensis, high resolution melting analysis, multiplex real-time PCR, differentiation, detection
INTRODUCTION
Opisthorchis viverrini and Clonorchis sinensis are 2 small human liver flukes. They are amongst the most pathogenic food-borne trematodes [1,2]. Infections with these species cause serious public health problems in various endemic countries in Asia [3,4]. Both flukes are recognized as carcinogenic, chronic infections being associated with development of a cancer of the bile duct (cholangiocarcinoma) and also of the liver (hepatocellular carcinoma) [5,6]. Both species have similar life cycles, involving 2 intermediate hosts which are snails and cyprinoid fishes. Adult worms mature in the bile ducts of definitive hosts (piscivorous mammals) and produce eggs which pass through the bile ducts and exit in feces to the environment. O. viverrini is widespread over the Greater Mekong Basin, including Lao PDR, Cambodia, central Vietnam, and Thailand, whereas C. sinensis is endemic in People's Republic of China, Korea, Taiwan, and northern Vietnam. Importantly, some areas such as An Giang Province, Vietnam [7] and Sanamchaikaet district, Chachoengsao Province, Thailand [8], were presented the overlapping of these 2 parasite infections. An estimated 700 million people throughout the world are at risk of infection and up to 15 million clonorchiasis cases exist in China and 10 million opisthorchiasis cases exist in Thailand and Lao PDR [1,9,10]. In addition, the numbers of reported cases of clonorchiasis and opisthorchiasis in the USA have been increasing along with the influx of Asian immigrants [11,12] and importation of undercooked cyprinoid fishes [13].
Parasitological methods based on egg detection in stools are time-consuming and need professional expertise. Although stool examination is the standard diagnostic method, it has some definite weak points. Eggs of O. viverrini and C. sinensis are not easy to distinguish from each other or from eggs of minute intestinal flukes. Furthermore, stool examination has low sensitivity, especially in lightly infected cases [14].
Multiplex real-time PCR with high resolution melting (HRM) analysis has been used for detection or differentiation of various pathogenic organisms such as viruses, bacteria, or parasites [15-17]. Recently, the method was used for differentiation of human hookworms (Necator americanus, Ancylostoma duodenale, A. ceylanicum, A. caninum, and A. braziliense) [18] and of filarial nematodes (Brugia malayi, B. pahangi, and Dirofilaria immitis) [19]. However, multiplex real-time PCR with HRM for differentiation of O. viverrini and C. sinensis eggs has not been reported yet. Here, we report a multiplex real-time PCR with HRM to detect and differentiate the eggs of O. viverrini and C. sinensis in patient stool samples. We also report the diagnostic specificity and sensitivity of the approach.
MATERIALS AND METHODS
O. viverrini and C. sinensis materials
O. viverrini adults (Khon Kaen isoline, Thailand) were obtained from experimentally infected hamsters. Human stool specimens infected with O. viverrini (n=12) were the leftover specimens from patients who visited Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand. C. sinensis adults were obtained from infected cats from Thai Binh Province, Vietnam, whereas C. sinensis-infected human stool specimens (n=8) were portions of the leftover specimens received from clonorchiasis patients in Nghia Hong-Nghia Hung, Nam Dinh Province, Vietnam. These samples were used for sensitivity evaluation.
Stool specimens were examined for parasite eggs by the quantitative formalin ethyl acetate concentration technique [20]. The intensity of O. viverrini eggs was presented as eggs per gram (EPG) of feces with the geometric mean of 157.0 EPG, and that of C. sinensis eggs was presented with the geometric mean of 2,330.7 EPG. DNA was extracted from specimens using the QIAamp® DNA stool mini kit (Qiagen, Hilden, Germany). The DNA samples were stored at-70℃ until used. This study was approved by the Khon Kaen University Ethics Committee for Human Research (reference no. HE541243).
Primer design and positive control plasmids
Two pairs of species-specific primers [21] were designed to bind to the mitochondrial NADH dehydrogenase subunit 2 (nad 2) gene of O. viverrini and C. sinensis. The primers were OV-F (5'-ATG TAG TGT TGG TTG GAG TT-3') and OV-R (5'-CAC AAT TAC CGC CGT AGC-3') for O. viverrini, and CS-F (5'-GTC TGT TGA GCT TTC TCC T-3') and CS-R (5'-TAA AGA CCC TGG AAA CGA GAT-3') for C. sinensis. The PCR products were obtained using these primer pairs and conventional PCR. Positive control plasmids of O. viverrini and C. sinensis were constructed by cloning of the relevant PCR products into the pGEM-T Easy vector (Promega, Madison, Wisconsin, USA), according to the manufacturer's instructions. The resultant plasmids were propagated in Escherichia coli strain JM109. Sequences obtained from these completely matched the sequences of genes from which the primers were designed.
Sensitivity and specificity determination
For determination of analytical sensitivity, batches of 50, 20, 10, 5, 2, or 1 O. viverrini or C. sinensis eggs obtained from each adult fluke recovered from infected animals, were added to separate aliquots of 150 mg of stool from non-infected humans. Genomic DNA was then extracted from these "spiked" stool samples and subjected to the multiplex PCR (see below). Ten-fold serial dilutions of O. viverrini or C. sinensis positive control plasmids (107-103 copies) were also subjected to multiplex real-time PCR.
The analytical specificity was evaluated using DNA extracted from human feces known to contain propagules of parasite species other than O. viverrini and C. sinensis (5 samples for each of the followings: Capillaria philippinensis, Trichuris trichiura, Taenia spp., minute intestinal flukes, Isospora belli, Giardia duodenalis, hookworms, Ascaris lumbricoides, Strongyloides stercoralis, Trichostrongylus spp., Schistosoma mekongi, or S. japonicum). Five human fecal samples from healthy controls were also evaluated as negative controls. The diagnostic values were calculated using standard methods [22].
Multiplex real-time PCR with HRM assay
LightCycler® PCR detection and analysis systems were used for amplification and quantification. For amplification detection, a LightCycler 480 High Resolution Melting Master Kit (Roche Applied Science, Mannheim, Germany) was used as recommended by the manufacturer. After the amplicon of specific region was amplified, the temperature was increased for melting double strand DNA (dsDNA) to single strand DNA (60℃ to 95℃). The incorporated-HRM fluorescent dye in dsDNA was decreased, and fluorescent signals were reduced corresponding to the amount of remained dsDNA after melting. The melting temperature was monitored, and the melting curve was analyzed real-time. The kit contained 3 contents, including Master Mix (FastStart Taq DNA Polymerase, reaction buffer, dNTP mix and HRM dye), MgCl2, and H2O PCR-grade. The PCR mixture was prepared to contain 1×LightCycler 480 HRM Master Mix, 2.25 mM MgCl2, and 0.4 µM of each 4 primers (OV-F, OV-R, CS-F, and CS-R). The total reaction volume was 20 µl. The PCR cycling for HRM curve acquisition was run under the following conditions: 1 cycle for pre-incubation at 95℃ for 10 min, 45 cycles of amplification step at 95℃ for 10 sec, 55℃ for 8 sec, and 72℃ for 15 sec. After amplification, the PCR products were melted by raising the temperature from 60℃ to 95℃, with an increment of 0.1℃/sec, in order to obtain information on melting profiles. Melting temperature (Tm) was determined by melting curve analysis. Distilled water was used as a negative control for these assays.
RESULTS
Standardization of the HRM real-time PCR
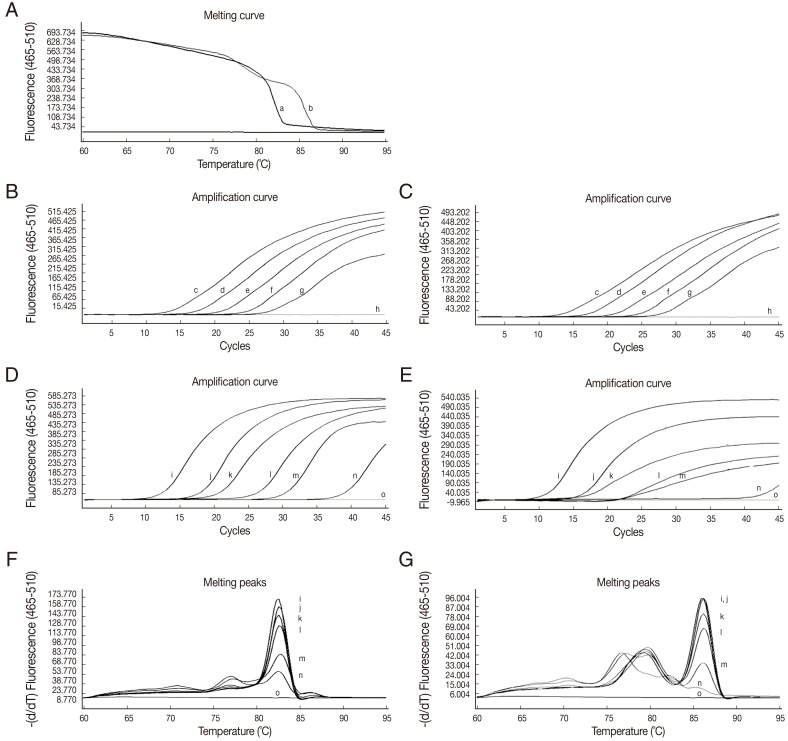
The analytical sensitivity of HRM real-time PCR was determined using 5 µl of 10-fold serial dilutions (107-103 copies) of the mixture of O. viverrini and C. sinensis positive control plasmids in distilled water. Representative melting temperatures were 82.3±0.09℃ for O. viverrini and 85.9±0.08℃ for C. sinensis (Fig. 1A). DNA of both O. viverrini and C. sinensis could be detected at the lowest concentration used (103 copies) for each positive control plasmid (Fig. 1B, C). This is equivalent to the genomic DNA of O. viverrini (3.50×10-6 ng) and of C. sinensis (3.42×10-6 ng) when considering 40 cycles as the cut-off detection limit (data not shown). As few as 1 O. viverrini egg or 2 C. sinensis eggs in 150 mg of uninfected human feces (Fig. 1D, E) could be detected. DNA from eggs of O. viverrini and C. sinensis could be clearly distinguished by their specific melting temperatures (Fig. 1F, G).
Fig. 1.
Multiplex real-time PCR with HRM analyses of amplification products of mitochondrial nad 2 DNA sequences. (A) The melting curves of the positive control plasmids (107 copies) of O. viverrini (a) and C. sinensis (b). (B, C) Amplification plots of fluorescence (y-axis) and cycle numbers (x-axis) showing the analytical sensitivity of HRM real-time PCR for detecting 10-fold dilutions of O. viverrini (B) and C. sinensis (C) plasmids from 107 to 103 copies/reaction (c-g) and distilled water (h). (D, E) Amplification plot showing the analytical sensitivity of HRM real-time PCR for detecting O. viverrini (D) and C. sinensis (E) eggs, 50 eggs (i); 20 eggs (j); 10 eggs (k); 5 eggs (l); 2 eggs (m); 1 egg (n); distilled water (o). (F, G) Melting peaks showing the different melting temperatures of DNA from O. viverrini (F) and C. sinensis (G) eggs. Lower case letters correspond to egg counts (i-n) and distilled water (o) in figure (D) and (E), respectively.
HRM real-time PCR for detection of O. viverrini and C. sinensis eggs in fecal samples
The HRM real-time PCR yielded positive results for all the 12 O. viverrini-infected and 8 C. sinensis-infected fecal samples (for O. viverrini detection, cycle number (Cn) ranged 18.2-27.7;mean±SD=20.9±2.5; median=20.7 and for C. sinensis detection, Cn ranged 15.2-22.1; mean±SD=17.0±2.3; median= 16.4). The range, mean±SD, and the median of the Tm values of the O. viverrini-infected human feces were 82.0-82.4℃, 82.3±0.1℃, and 82.3℃, respectively, and those of the C. sinensis-infected human fecal samples were 85.4-86.3℃, 86.1±0.3℃, and 86.1℃, respectively.
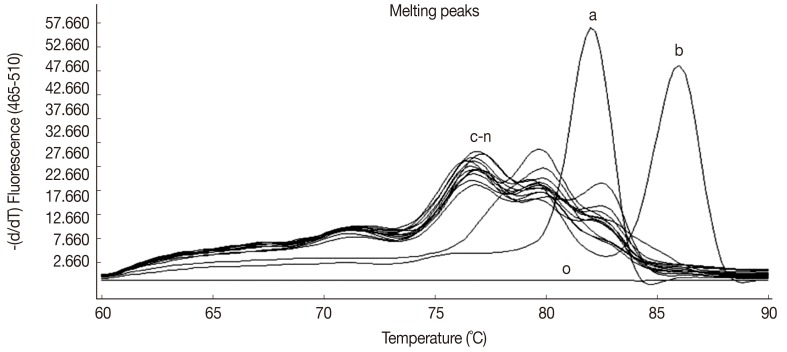
No positive results were perceived when various DNAs from feces containing propagules of other parasites (see Materials and Methods) were analyzed (Fig. 2). For each of these samples, no amplicon was observed on agarose gel electrophoresis, which demonstrates the specificity of the technique. The sensitivity, specificity, and positive and negative predictive values were all 100%.
Fig. 2.
Melting peak analyses showing the specificity of HRM real-time PCR for differential detection of O. viverrini (a) and C. sinensis (b) eggs in fecal samples. Curves for DNA from fecal samples containing propagules of other parasites (c-n; see Materials and Methods), or distilled water (o), were clearly distinct from these.
DISCUSSION
Multiplex fluorescent resonance energy transfer (FRET) real-time PCR has been reported for the simultaneous detection and differentiation of C. sinensis and O. viverrini eggs in human fecal samples [21]. However, the FRET probe-based real-time PCR requires 2 different fluorophore-labeled hybridization probes for generating melting curves. The present study explored another format, the HRM real-time PCR without fluorophore-labeled probes to differentiate O. viverrini and C. sinensis amplicons by melting temperature analysis. This is the first report of HRM multiplex real-time PCR to distinguish between O. viverrini and C. sinensis eggs in fecal samples in a single assay. Therefore, the method is much cheaper than the real-time FRET PCR on a cost-per-sample basis. The HRM real-time PCR offers a new alternative for rapid, sensitive, and species-specific for differentiation and detection of Asian liver fluke infections in stool specimens.
The analytical sensitivity in this study was 1 O. viverrini egg or 2 C. sinensis eggs in 150 mg feces, which is quite similar to that reported previously [21,23-25]. Moreover, multiplex real-time PCR with HRM produced no cross reactions between C. sinensis and O. viverrini or various fecal DNAs from other human parasitoses, indicating high specificity. The high sensitivity and specificity suggest the present test will be of diagnostic value. The difference between melting temperatures (more than 3℃) was easy to detect.
In conclusion, a reliable single-tube multiplex HRM real-time PCR for the simultaneous differentiation and detection of O. viverrini and C. sinensis eggs in infected human feces was successfully developed. The method can potentially be used as a tool for species-specific epidemiological surveys of liver flukes in Asian populations, especially in countries where both O. viverrini and C. sinensis infections are reported [7,8,26].
ACKNOWLEDGMENTS
This research was supported by the National Science and Technology Development Agency (Discovery-Based Development Grant); the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission through the Health Cluster (SHeP-GMS) and Khon Kaen University, Thailand. Wanchai Maleewong, Pewpan M. Intapan, and Tongjit Thanchomnang are supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. Worasak Kaewkong is supported by the National Research University Project of Thailand, Khon Kaen University, Thailand.
References
- 1.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia-epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 2.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- 4.Fürst T, Duthaler U, Sripa B, Utzinger J, Keiser J. Trematode infections: liver and lung flukes. Infect Dis Clin North Am. 2012;26:399–419. doi: 10.1016/j.idc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Infection with liver fluke (Opisthorchis viverrini, Opisthorchis felineus, Clonorchis sinensis) IARC Monogr Eval Carcinog Risks Hum. 1994;61:121–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Fried B, Reddy A, Mayer D. Helminths in human carcinogenesis. Cancer Lett. 2011;305:239–249. doi: 10.1016/j.canlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Thu ND, Dalsgaard A, Loan LT, Murrell KD. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in An Giang Province, Vietnam. Korean J Parasitol. 2007;45:45–54. doi: 10.3347/kjp.2007.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traub RJ, Macaranas J, Mungthin M, Leelayoova M, Cribb T, Murrell KD, Thompson RC. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand. PLoS Negl Trop Dis. 2009;3:e367. doi: 10.1371/journal.pntd.0000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol. 2009;163:196–206. doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24. doi: 10.1016/j.parint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Stauffer WM, Sellman JS, Walker PF. Biliary liver flukes (opisthorchiasis and clonorchiasis) in immigrants in the United States: often subtle and diagnosed years after arrival. J Travel Med. 2004;11:157–159. [PubMed] [Google Scholar]
- 12.Fried B, Abruzzi A. Food-borne trematode infections of humans in the United States of America. Parasitol Res. 2010;106:1263–1280. doi: 10.1007/s00436-010-1807-0. [DOI] [PubMed] [Google Scholar]
- 13.Yossepowitch O, Gotesman T, Assous M, Marva E, Zimlichman R, Dan M. Opisthorchiasis from imported raw fish. Emerg Infect Dis. 2004;10:2122–2126. doi: 10.3201/eid1012.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003;49:853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- 16.Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8:597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- 17.Tong SY, Giffard PM. Microbiological applications of high-resolution melting analysis. J Clin Microbiol. 2012;50:3418–3421. doi: 10.1128/JCM.01709-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngui R, Lim YA, Chua KH. Rapid detection and identification of human hookworm infections through high resolution melting (HRM) analysis. PLoS One. 2012;7:e41996. doi: 10.1371/journal.pone.0041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wongkamchai S, Monkong N, Mahannol P, Taweethavonsawat P, Loymak S, Foongladda S. Rapid detection and identification of Brugia malayi, B. pahangi, and Dirofilaria immitis by high-resolution melting assay. Vector Borne Zoonotic Dis. 2013;13:31–36. doi: 10.1089/vbz.2012.0971. [DOI] [PubMed] [Google Scholar]
- 20.Elkins DB, Haswell-Elkins M, Anderson RM. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India I Study design and pre-and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg. 1986;80:774–792. doi: 10.1016/0035-9203(86)90384-6. [DOI] [PubMed] [Google Scholar]
- 21.Sanpool O, Intapan PM, Thanchomnang T, Janwan P, Lulitanond V, Doanh PN, Van Hien H, Dung do T, Maleewong W, Nawa Y. Rapid detection and differentiation of Clonorchis sinensis and Opisthorchis viverrini eggs in human fecal samples using a duplex real-time fluorescence resonance energy transfer PCR and melting curve analysis. Parasitol Res. 2012;111:89–96. doi: 10.1007/s00436-011-2804-7. [DOI] [PubMed] [Google Scholar]
- 22.Galen RS. Predictive value and efficiency of laboratory testing. Pediatr Clin North Am. 1980;27:861–869. doi: 10.1016/s0031-3955(16)33930-x. [DOI] [PubMed] [Google Scholar]
- 23.Intapan PM, Thanchomnang T, Lulitanond V, Pongsaskulchoti P, Maleewong W. Rapid molecular detection of Opisthorchis viverrini in human fecal samples by real-time polymerase chain reaction. Am J Trop Med Hyg. 2009;81:917–920. doi: 10.4269/ajtmh.2009.09-0275. [DOI] [PubMed] [Google Scholar]
- 24.Cai XQ, Yu HQ, Bai JS, Tang JD, Hu XC, Chen DH, Zhang RL, Chen MX, Ai L, Zhu XQ. Development of a TaqMan based real-time PCR assay for detection of Clonorchis sinensis DNA in human stool samples and fishes. Parasitol Int. 2012;61:183–186. doi: 10.1016/j.parint.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Rahman SM, Bae YM, Hong ST, Choi MH. Early detection and estimation of infection burden by real-time PCR in rats experimentally infected with Clonorchis sinensis. Parasitol Res. 2011;109:297–303. doi: 10.1007/s00436-011-2253-3. [DOI] [PubMed] [Google Scholar]
- 26.De NV, Murrell KD, Cong le D, Cam PD, Chau le V, Toan ND, Dalsgaard A. The food-borne trematode zoonoses of Vietnam. Southeast Asian J Trop Med Public Health. 2003;34:12–34. [PubMed] [Google Scholar]