Abstract
To increase public health awareness for prevention of opisthorchiasis caused by eating raw freshwater fish, the distribution and abundance of Opisthorchis viverrini metacercariae (OV MC) was investigated in freshwater fish obtained from 20 provinces in northeastern Thailand between April 2011 and February 2012. A cross-sectional survey was conducted on 12,890 fish consisting of 13 species randomly caught from 26 rivers, 10 dams, and 38 ponds/lakes. Fish, were collected in each of the rainy and winter seasons from each province. Fish were identified, counted, weighed, and digested using pepsin-HCl. Samples were examined for OV MC by a sedimentation method, and metacercariae were identified under a stereomicroscope. OV MC were found in 6 species of fish; i.e., Cyclocheilichthys armatus, Puntius orphoides, Hampala dispar, Henicorhynchus siamensis, Osteochilus hasselti, and Puntioplites proctozysron from localities in 13 provinces. Among the sites where OV MC-infected fish were found, 70.0% were dams, 23.7% were ponds/lakes, and 7.7% were rivers. The mean intensity of OV MC ranged from 0.01 to 6.5 cysts per fish (or 1.3-287.5 cysts per kg of fish). A high mean intensity of OV MC per fish (>3 cysts) was found in 5 provinces: Amnat Charoen (6.5 cysts), Nakhon Phanom (4.3), Mukdahan (4.1), Khon Kaen, (3.5) and Si Sa Ket (3.4). In conclusion, OV MC are prevalent in natural cyprinid fish, with the infection rate varying according to fish species and habitats.
Keywords: Opisthorchis viverrini, metacercaria, foodborne trematode, cyprinid fish, distribution, intensity
INTRODUCTION
Human small liver flukes are Clonorchis sinensis, Opisthorchis felineus, and Opisthorchis viverrini. O. felineus is found in some parts of the European Union and the former Soviet Union [1]; C. sinensis is seen in eastern Asia, including China, the Republic of Korea, Japan, and northern Vietnam; while O. viverrini is found in Southeast Asia, including Thailand, Laos, Cambodia, and southern Vietnam [1]. These flukes are among the fishborne zoonotic trematodes (FZT), so-called because the primary source of transmission is by eating freshwater fish, and the parasites can cycle through animal hosts, with or without human involvement. At least 10 million people in endemic areas are infected with O. viverrini and thus remain at risk for hepatobiliary disease and cholangiocarcinoma (CCA) [2]. In Thailand, the highest prevalence of opisthorchiasis is found in the northeastern region, where the incidence of CCA is also high [2]. Widespread infection in humans in this region is due to the people's fondness for eating raw, semi-cooked, or fermented fish dishes, which may be contaminated with metacercariae [3]. After ingestion an infective stage, metacercariae excyst and migrate into the biliary system, causing hepatobiliary disease during long-term infection and increasing the potential risk for CCA [1,2].
Cyprinid freshwater fish are abundant and easily caught in Southeast Asian countries. They are a primary source of protein among rural residents, and are usually consumed raw or as inadequately cooked dishes such as koi-pla, lab-pla, pla-som, and pla-ra [4,5]. Every day, millions of people eat infected fish prepared in these ways. Moreover, the lack of hygienic toilet facilities completes the epidemiological cycle by facilitating the return of O. viverrini eggs to waterways, where they are first consumed by Bithynia snails and cercariae emerging from the snails subsequently encyst in cyprinid fish species. Recently, it has been reported that the prevalence of FZT metacercariae in aquacultured fish in Khon Kaen Province, Thailand is high (46.9%) [6], which may further increase the possibility of transmission to humans who consume them raw or under improperly cooked conditions. Although opisthorchiasis is still persistent in northeastern Thailand due to raw fish consumption, no survey has yet attempted to identify high-risk regions in all 20 provinces. A previous survey in 2 provinces in northeastern Thailand revealed that the seasonal variation of O. viverrini infection in cyprinid fish with maximal distribution of metacercarial intensity was seen during February and December [7].
In this survey, natural freshwater fish were randomly caught from large bodies of water not subject to drought, including dams, ponds/lakes, and rivers in 20 provinces of northeastern Thailand, over a 10-month period (April 2011 to February 2012). Fish were identified, counted, weighed, and digested by pepsin-HCl. O. viverrini metacercariae (OV MC) were collected using a sedimentation method and identified under a stereomicroscope. The present results may have a significant impact on food quality and safety, and provide data to assist the development of an effective strategy for the targeted prevention and control of foodborne diseases in high-risk regions in northeastern Thailand.
MATERIALS AND METHODS
Fish collection
Freshwater fish were caught from 74 bodies of water, including 26 rivers, 10 dams, and 38 ponds/lakes, located in 20 provinces of northeastern Thailand between April 2011 and February 2012. Ponds/lakes not subject to conditions of drought were included in selection criteria. Five to 10 kg (except, 0.9 kg for Loei and 3 kg for Udon Thani) of fish were collected in each of the rainy and winter seasons. Fish were transferred on ice to the laboratory of the Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, where they were identified by comparison with the FishBase online at http://www.fishbase.org/home.htm.
O. viverrini metacercariae isolation and identification
Pools of the same fish species from each sampling site were counted, weighed, and then homogenized in a blender with 0.25% pepsin-1.5% HCl (Wako Pure Chemical Industries, Osaka, Japan) in 0.85% NaCl solution. Each mixture was incubated in a shaking water bath at 37℃ for 1 hr. The digested materials were filtered through a series of sieves (1,000, 300, 106, and 250 µm mesh) and then washed with 0.85% NaCl in a sedimentation jar until the supernatant became clear. Finally, the metacercariae in the sediment were isolated and identified under a stereomicroscope. The metacercariae were identified based on morphological criteria such as the size of cysts, folded body displaying vigorous movement within the cyst, and prominent and clearly visible oral and ventral suckers as described previously [8]. Also, the mean intensity [9] of OV MC was calculated by counting the number of OV MC detection divided by the kilogram of fish or by the number of fish.
Statistical analysis
Data were expressed as the mean±SD. The statistical significance of the frequency of positive sites among dams, rivers, and ponds/lakes was analyzed by the Mann-Whitney U test. Statistical analysis was performed using SPSS version 15 (SPSS Inc., Chicago, Illinois, USA). A P-value less than 0.05 was considered statistically significant.
RESULTS
Distribution of O. viverrini metacercariae in freshwater fish
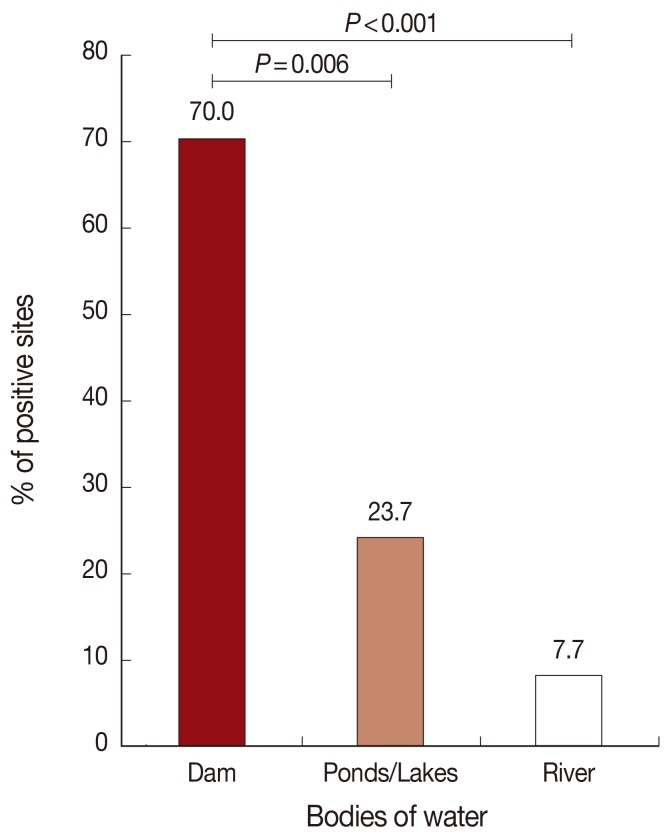
A total of 214.5 kg of fish were collected from 74 bodies of water, including dams, rivers, and ponds/lakes, in 20 provinces in northeastern Thailand. OV MC-infected fish were found in 24.3% (18/74) of sites. Among these 18 sites, 70.0% (7/10) were dams, 23.7% (9/38) were ponds/lakes, and 7.7% (2/26) were rivers (Table 1; Fig. 1). The prevalence of positive sites of dam was significantly higher than ponds/lakes, and ponds/lakes was significantly higher than river, in the order (Fig. 1). In the case of river sites, infected fish were found only in branches of the main river; OV MC were not identified in any fish collected from the main stream of any river. OV MC were detected in 13 provinces: Yasothon, Ubon Ratchathani, Nakhon Ratchasima, Khon Kaen, Sakon Nakhon, Si Sa Ket, Surin, Buri Ram, Amnat Charoen, Mukdahan, Chaiyaphum, Nakhon Phanom, and Nong Khai (Table 1). Notably, OV MC were detected in freshwater fish from all provinces in 2 of the 3 Departments of Prevention and Control of Disease (DPC), i.e., DPC5 and DPC7 (Fig. 2). In contrast, OV MC were not detected in fish collected from Roi Et, Maha Sarakham, Kalasin, Loei, Nong Bua Lam Phu, Bueng Kan, and Udon Thani, all located in DPC6. To confirm the results, fish were re-collected from the same sites in a different season (rainy or winter season as appropriate). However, the second collection yielded results similar to the first for all sampling sites (data not shown). The prevalence of OV MC in freshwater fish for each province is shown in Fig. 2.
Table 1.
The number of positive regions for O. viverrini metacercariae-infected fish per sampling region in 20 provinces of northeastern Thailand
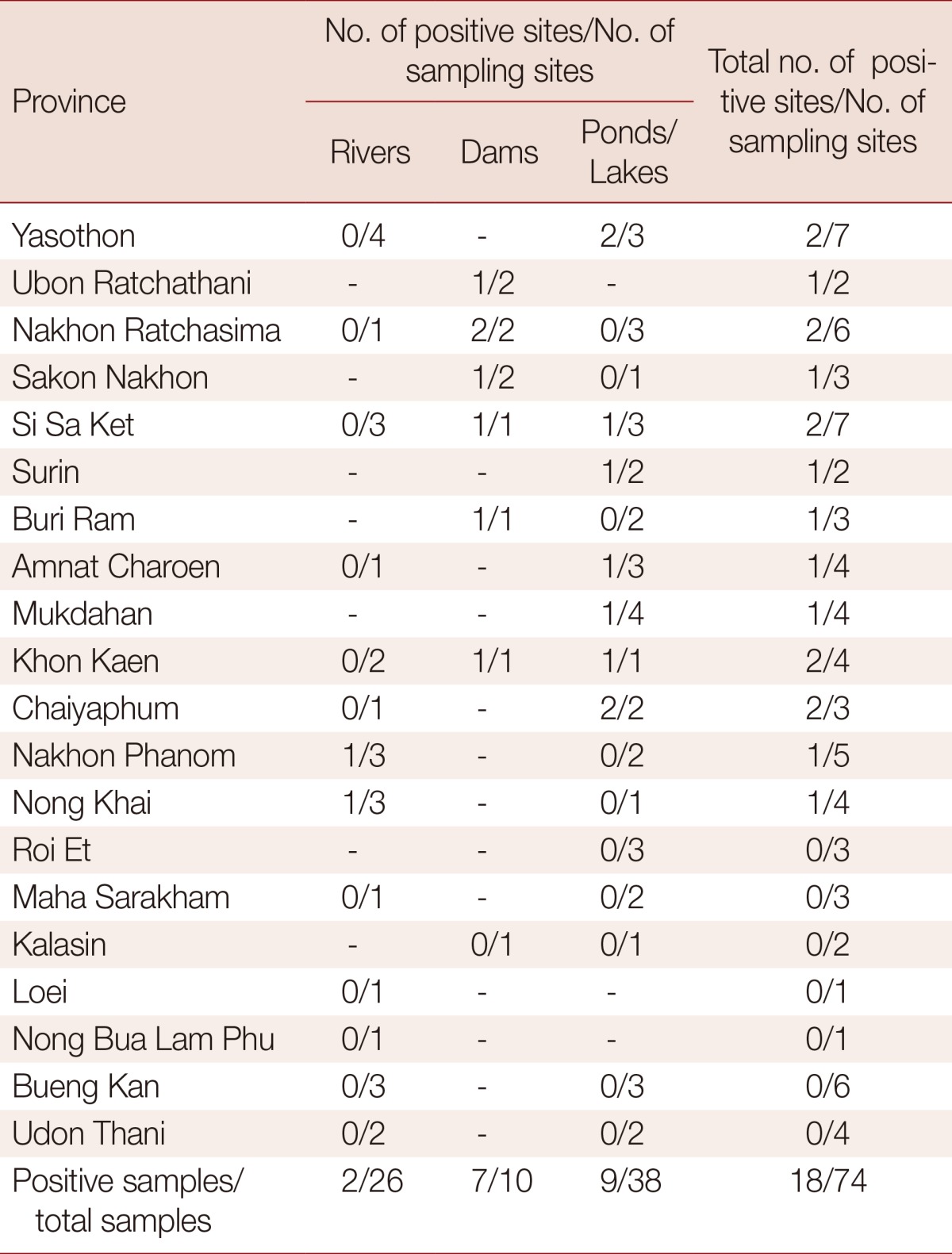
Fig. 1.
Distribution of O. viverrini metacercaria (OV MC)-infected fish. Natural fish were obtained from 26 rivers, 10 dams, and 38 ponds/lakes located in 20 provinces in northeastern Thailand. The percentage of positive sites was calculated as described in Materials and Methods. The distribution of OV MC was found to occur in the following order: dams>ponds/lakes>rivers.
Fig. 2.
Prevalence of OV MC-infected fish in northeastern Thailand. Natural fish were obtained from 74 bodies of water in various areas of 20 provinces in northeastern Thailand according to the Departments of Prevention and Control of Disease (DPC), DPC5, DPC6, and DPC7. Fish, 5-10 kg, were collected from each province at 2 different seasons of the year, rainy and winter. Fish were digested with 0.25% artificial pepsin and examined for the presence of OV MC. The percentage of OV MC prevalence was calculated from the number of positive sites per number of sites sampled.
Freshwater fish specific for O. viverrini metacercarial infection
A total of 12,890 freshwater fish were collected, which comprised of 13 species; Cyclocheilichthys armatus, Hampala dispar, Puntioplites proctozysron, Puntius orphoides, Oreochromis niloticus, Pristolepis fasciatus, Devario regina, Labeo chrysophekadion, Barbonymus gonionotus, Rasbora tornieri, Henicorhynchus siamensis, Osteochilus hasselti, and Chitala ornata. Of these, 6 species were infected with OV MC: C. armatus, P. orphoides, H. dispar, H. siamensis, O. hasselti, and P. proctozysron. C. armatus was found in 9 provinces and H. dispar in 4 provinces, while the other 4 species (P. orphoides, H. siamensis, O. hasselti, and P. proctozysron) were each detected in only 1 province. The fish most commonly contaminated with OV MC were C. armatus and H. dispar. The distribution of OV MC infection in freshwater fish collected from 13 positive provinces is shown in Table 2.
Table 2.
Species of fish, habitat of collection, weight, number of fish, and number of O. viverrini metacercariae (OV MC)-infected fish in 13 positive provinces in Thailand
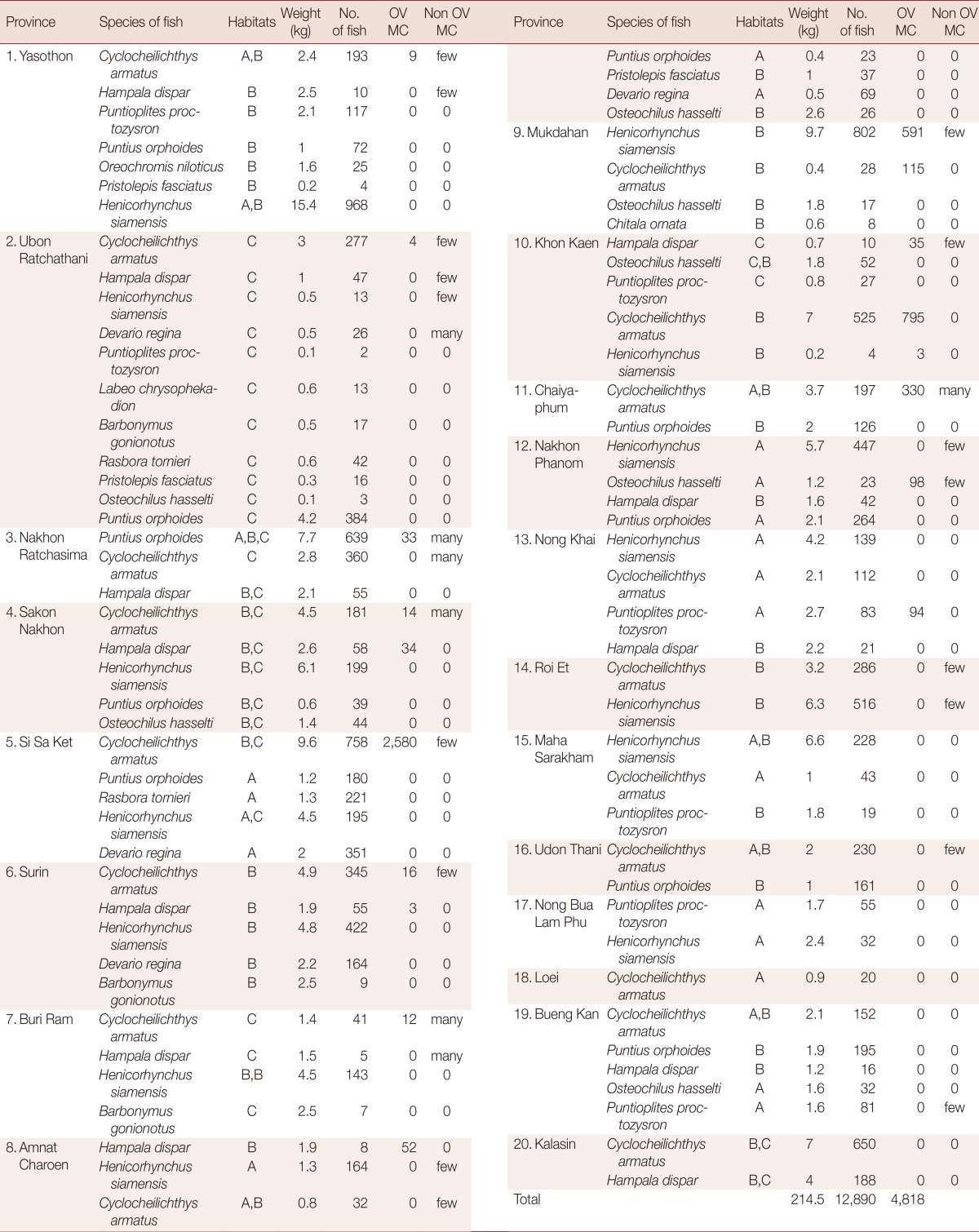
A, B, and C refer to river, ponds/lakes, and dam, respectively.
Few=less than 100 cysts; Many=more than 100 cysts.
O. viverrini metacercarial intensity in cyprinid fish
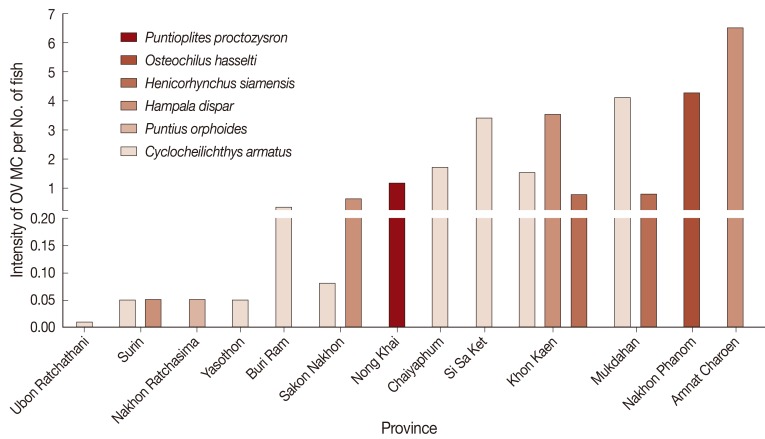
The mean intensity of OV MC per fish was classified as low (less than 1 cyst), moderate (1-3 cysts), and high intensity (more than 3 cysts). Data were presented as the average of OV MC per fish of any given species (Fig. 3). Fish with high intensity (>3 cysts) of infection were found in Amnat Charoen (6.5 cysts), followed by Nakhon Phanom (4.3), Mukdahan (4.1), Khon Kaen (3.5), and Si Sa Ket (3.4). A moderate intensity of OV MC per fish was found in Chaiyaphum (1.7 cysts) and Nong Khai (1.1). A low intensity of OV MC per fish was found in Sakon Nakhon (0.6 cysts), followed by Buri Ram (0.3), Yasothon (0.1), Nakhon Ratchasima (0.1), Surin (0.1), and Ubon Ratchathani (0.0). In addition, the intensities of OV MC per fish among 3 DPC regions were analyzed. Fish species in the order of OV MC intensity were C. armatus (2.0), H. dispar (0.1), and P. orphoides (0.1) in DPC5; P. orphoides (3.5), C. armatus (1.5), and H. dispar (0.8) in DPC6; and C. armatus (8.5), P. orphoides (7.8), and H. dispar (4.3) in DPC7.
Fig. 3.
Intensity of OV MC infection in cyprinid fish, by province of Thailand.
In Yasothon, Ubon Ratchathani, Si Sa Ket, Buri Ram, and Chaiyaphum, C. armatus was the only OV MC-infected species. Also, only 1 fish species was found to be infected in Nakhon Ratchasima, Amnat Charoen, Nakhon Phanom, and Nong Khai; P. orphoides, H. dispar, O. hasselti, and P. proctozysron, respectively. Notably, in Khon Kaen, Sakon Nakhon, Surin, and Mukdahan, more than 1 species of fish were infected with OV MC.
DISCUSSION
Freshwater cyprinid fish are the second intermediate host of the human liver flukes O. viverrini. Although infected fish are found throughout many parts of southern Vietnam [10], Cambodia [11,12], Laos [13], and Thailand [14], the fish species most likely to be contaminated are different in each region. For example, in Savannakhet Province in Laos, OV MC were found in 6 species of fish: Puntius brevis, H. dispar, Esomus metallicus, Mystacoleucus marginatus, Puntioplites falcifer, and C. armatus [13]. In Vientiane, Laos, OV MC were found in 7 species of fish: Onychostoma elongatum, C. armatus, H. dispar, P. brevis, Cyclocheilichthys repasson, O. hasselti, and Hypsibarbus lagleri [15]. Similarly, we found differences across provinces in northeastern Thailand in the identities of the most heavily infected fish species. This may due to the difference of water reservoirs, fish biodiversity in the Mekong basins, and the Anthropocene alteration of these ecosystems [16]. Moreover, other ecological systems such as snail and snail-fish interaction [17], as well as people's defecation habits and prevalence in reservoir host, may differ.
In Thailand, cyprinid fish species infected with OV MC are mostly in the genus Cyclocheilichthys, Puntius, and Hampala [14,18-20]. In a survey conducted in 2012, at least 5 additional fish species were reported to be infected with OV MC, namely Barbonymus gonionotus (Puntius gonionotus), Cirrhinus mrigala, Cyprinus carpio, Ctenopharyngodon idellus, and Hypophthalmichthys molitrix [21]. In the present study, out of 13 different freshwater fish species collected from rivers, dams, and ponds/lakes, OV MC were detected in 6 species; C. armatus, H. siamensis, H. dispar, O. hasselti, P. proctozysron, and P. orphoides.
The different species of OV MC-infected fish in Thailand may be due to the different numbers of fish identified and the period of the study. For example, during our recent survey from April 2011 to February 2012 at Nakhon Ratchasima, 3 species of freshwater fish consisting of 639 P. orphoides, 360 C. armatus, and 55 H. dispar were collected from rivers, dams, and ponds/lakes; 33 OV MC were detected only in P. orphoides. In a previous survey in 2010 in the same province, a total of 640 cyprinid fish comprising 5 species were collected from different study sites. Of these, 12.3% were positive for OV MC infection. These infected fish were predominantly C. armatus, C. repasson, P. proctzysron, Hampala macrolepidota, and H. dispar [22]. In contrast, no OV MC-infected fish were found in 3 species (52 Puntius leiacanthus, 22 C. armatus, and 5 H. dispar) in a study conducted in the year 2000 in the same province [23]. The negative result for fish caught in the main stream of rivers [23] is in agreement with our present findings. This result can be explained by the shoaling effect similar to other trematode cercariae via behaviour-mediated differences in exposure between shoaling and non-shoaling fishes [24]. Moreover, the negative result of OV MC identification from Loei and Udon Thani provinces may due to the low number of fish collection than other province.
OV MC intensities may differ depending on the site and time of year of fish collection. The intensity in infected fish was 1.0-15.0 per infected fish in southern Cambodia [11], while it was 252 per infected fish in Laos [13]. In Thailand, OV MC intensity in infected fish in the northeastern area (8-88 cysts per fish) was higher than that in the northern area (1.4 cysts per fish) [19], in accordance with the prevalence of O. viverrini infection in humans. In northeastern Thailand, there appears to have been a dramatic reduction in OV MC intensity: from intensities of 8-88 cysts per fish between April 1980 and March 1981 [20] to 0.01-6.5 cysts per fish between April 2011 and February 2012 in the present study. Although the intensity of OV MC in fish has decreased markedly over the last 30 years, a high intensity of OV MC per fish (>3 cysts) is still found in some provinces, i.e. Amnat Charoen, Nakhon Phanom, Mukdahan, Khon Kaen, and Si Sa Ket. For example, in Khon Kaen, the average number of OV MC per fish (0.8-3.5 cysts) in this survey was similar to the average number (1.7 cysts or 127.4 per kg) found in the survey conducted in 1991-1992 [7]. In contrast to the results of a previous survey in 1992-1996 in which OV MC were found in fish from Udon Thani [14], in the present study, no OV MC were detected in 391 fishes obtained from 2 rivers and 2 ponds/lakes in Udon Thani. Moreover, no OV MC infection was observed in fish collected from some DPC6 provinces, including Roi Et, Maha Sarakham, Kalasin, Loei, Nong Bua Lam Phu, and Bueng Kan. The contradictory result may be due to the flooding effect that happened in 2011 in these regions leading to the habitats and population dynamics of the 2 intermediate hosts, Bithynia snails and cyprinid fish [7,25]. Nevertheless, the increase number of fish collection (>5 kg of fish) and sampling in the hot or dry season in these negative provinces might reveal some infected fish, something requiring further research. In addition, the species of fish infected with OV MC in DPC5, DPC6, and DPC7 regions were similar (C. armatus, H. dispar, and P. orphoides), while the numbers of OV MC per fish were different, suggesting the population genetics of Bithynia snails, the first intermediate hosts of O. viverrini in Thailand, might influence the transmission of cercariae to fish [26].
The metacercarial load in fish was positively associated with infection levels among humans. A high prevalence of positive locations in DPC7 (28.1%) and a high intensity of OV MC in fish in Amnat Charoen, Nakhon Phanom, and Mukdahan Provinces may account for these areas having the highest rate of human infections (32.6% for Amnat Charoen, 60.8% for Nakhon Phanom, and 29.5% for Mukdahan, based on a recent survey in northeastern Thailand by the DPC during 2009) [27]. Likewise, a high infection rate in natural hosts, such as cats and dogs, in Khon Kaen [28] was found to be related to the high intensity of OV MC in fish in that province. Moreover, the low intensity of OV MC per fish in Nakhon Ratchasima is in keeping with the low prevalence of human opisthorchiasis there (2.5%) [29]. In contrast, in Yasothon Province, although aquaculture fish were found to have low infective potential, the infection rate in humans was high (38.7%) [30]. This result implies that eating raw fish behavior of people living in this region might occur more frequently. To prevent a relative risk of opisthorchiasis-associated CCA in this endemic area [2], education program should be made for more awareness of safe eating behavior. Nevertheless, the transmission of OV MC from fish to humans might vary, and is the net result of a complex interplay between the host and parasite [31]. Therefore, further research should investigate the correlation of OV MC intensity in fish, eating behavior, and the infection rate in humans in this region.
In conclusion, OV MC infection is endemic in natural freshwater cyprinid fish in northeastern Thailand. Fish obtained from dams had a higher frequency of OV MC infection than fish from ponds/lakes and rivers, respectively. C. armatus, H. siamensis, H. dispar, O. hasselti, P. proctozysron, and P. orphoides were found to be infected with OV MC. Fish collected from water bodies in Si Sa Ket, Chaiyaphum, Mukdahan, Khon Kaen, Nakhon Phanom, and Nong Khai Province had a high prevalence of OV MC infection. Education and health promotion programs are necessary for urgent prevention of the spread of O. viverrini infection to humans in areas with a high intensity of infection.
ACKNOWLEDGMENTS
This work was supported by the Higher Education Research Promotion and National Research Universities Project, Office of the Higher Education Commission, Thailand, through the Health Cluster (SHeP-GMS681), Khon Kaen University. We thank the research assistants at the Faculty of Medicine (AS53101, AS54302, AS55201), Khon Kaen University, for their technical support. We also extend our thanks to TRF Senior Researcher Scholar Grant (RTA5580004) to Prof. Wanchai Maleewong.
References
- 1.IARC. A review of human carcinogens: Opisthorchis viverrini and Clonorchis sinensis. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:341–370. [Google Scholar]
- 2.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy-Warr C, Andrews RH, Sithithaworn P, Petney TN, Sripa B, Laithavewat L, Ziegler AD. Raw attitudes, wetland cultures, life-cycles: socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2012;61:65–70. doi: 10.1016/j.parint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Chuboon S, Wongsawad C, Ruamsuk A, Nithikathkul C. Survival of Haplorchis taichui metacercariae in Lab-Pla, Thai traditional food preparation. Southeast Asian J Trop Med Public Health. 2005;36(suppl 4):110–111. [PubMed] [Google Scholar]
- 5.Prasongwatana J, Laummaunwai P, Boonmars T, Pinlaor S. Viable metacercariae of Opisthorchis viverrini in northeastern Thai cyprinid fish dishes-as part of a rational program for control of O. viverrini-associated cholangiocarcinoma. Parasitol Res. 2013;112:1323–1327. doi: 10.1007/s00436-012-3154-9. [DOI] [PubMed] [Google Scholar]
- 6.Pitaksakulrat O, Sithithaworn P, Laoprom N, Laha T, Petney TN, Andrews RH. A cross-sectional study on the potential transmission of the carcinogenic liver fluke Opisthorchis viverrini and other fishborne zoonotic trematodes by aquaculture fish. Foodborne Pathog Dis. 2013;10:35–41. doi: 10.1089/fpd.2012.1253. [DOI] [PubMed] [Google Scholar]
- 7.Sithithaworn P, Pipitgool V, Srisawangwong T, Elkins DB, Haswell-Elkins MR. Seasonal variation of Opisthorchis viverrini infection in cyprinoid fish in north-east Thailand: implications for parasite control and food safety. Bull World Health Organ. 1997;75:125–131. [PMC free article] [PubMed] [Google Scholar]
- 8.Vajrasthira S, Harinasuta C, Komiya Y. The morphology of the metacercaria of Opisthorchis viverrini, with special reference to the excretory system. Ann Trop Med Parasitol. 1961;55:413–418. doi: 10.1080/00034983.1961.11686068. [DOI] [PubMed] [Google Scholar]
- 9.Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- 10.Phan VT, Ersboll AK, Nguyen TT, Nguyen KV, Nguyen HT, Murrell D, Dalsgaard A. Freshwater aquaculture nurseries and infection of fish with zoonotic trematodes, Vietnam. Emerg Infect Dis. 2010;16:1905–1909. doi: 10.3201/eid1612.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touch S, Komalamisra C, Radomyos P, Waikagul J. Discovery of Opisthorchis viverrini metacercariae in freshwater fish in southern Cambodia. Acta Trop. 2009;111:108–113. doi: 10.1016/j.actatropica.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Sohn WM, Yong TS, Eom KS, Pyo KH, Lee MY, Lim H, Choe S, Jeong HG, Sinuon M, Socheat D, Chai JY. Prevalence of Opisthorchis viverrini infection in humans and fish in Kratie Province, Cambodia. Acta Trop. 2012;124:215–220. doi: 10.1016/j.actatropica.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisiengmay S. Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. Korean J Parasitol. 2013;51:107–114. doi: 10.3347/kjp.2013.51.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waikagul J. Opisthorchis viverrini metacercaria in Thai freshwater fish. Southeast Asian J Trop Med Public Health. 1998;29:324–326. [PubMed] [Google Scholar]
- 15.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudgeon D. Asian river fishes in the Anthropocene: threats and conservation challenges in an era of rapid environmental change. J Fish Biol. 2011;79:1487–1524. doi: 10.1111/j.1095-8649.2011.03086.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang YC, Feng CC, Sithithaworn P, Feng Y, Petney TN. How do snails meet fish? Landscape perspective needed to study parasite prevalence. EcoHealth. 2011;8:258–260. doi: 10.1007/s10393-011-0701-2. [DOI] [PubMed] [Google Scholar]
- 18.Srisawangwong T, Sithithaworn P, Tesana S. Metacercariae isolated from cyprinoid fishes in Khon Kaen District by digestion technic. Southeast Asian J Trop Med Public Health. 1997;28(suppl 1):224–226. [PubMed] [Google Scholar]
- 19.Sukontason K, Piangjai S, Muangyimpong Y, Methanitikorn R, Chaithong U. Prevalence of trematode metacercariae in cyprinoid fish of Ban Pao district, Chiang Mai Province, northern Thailand. Southeast Asian J Trop Med Public Health. 1999;30:365–370. [PubMed] [Google Scholar]
- 20.Vichasri S, Viyanant V, Upatham ES. Opisthorchis viverrini: intensity and rates of infection in cyprinoid fish from an endemic focus in Northeast Thailand. Southeast Asian J Trop Med Public Health. 1982;13:138–141. [PubMed] [Google Scholar]
- 21.Sithithaworn P, Ziegler AD, Grundy-Warr C, Andrews RH, Petney TN. Changes to the life cycle of liver flukes: dams, roads, and ponds. Lancet Infect Dis. 2012;12:588. doi: 10.1016/S1473-3099(12)70174-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaewpitoon N, Kaewpitoon SJ, Ueng-arporn N, Rujirakul R, Churproong S, Matrakool L, Auiwatanagul S, Sripa B. Carcinogenic human liver fluke: current status of Opisthorchis viverrini metacercariae in Nakhon Ratchasima, Thailand. Asian Pac J Cancer Prev. 2012;13:1235–1240. doi: 10.7314/apjcp.2012.13.4.1235. [DOI] [PubMed] [Google Scholar]
- 23.Nithiuthai S, Suwansaksri J, Wiwanitkit V, Chaengphukeaw P. A survey of metacercariae in cyprinoid fish in Nakhon Ratchasima, northeast Thailand. Southeast Asian J Trop Med Public Health. 2002;33(suppl 3):103–105. [PubMed] [Google Scholar]
- 24.Stumbo AD, James CT, Goater CP, Wisenden BD, Cotter S. Shoaling as an antiparasite defence in minnows (Pimephales promelas) exposed to trematode cercariae. J Anim Ecol. 2012;81:1319–1326. doi: 10.1111/j.1365-2656.2012.02012.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang YC. Examining landscape determinants of Opisthorchis viverrini transmission. EcoHealth. 2012;9:328–341. doi: 10.1007/s10393-012-0789-z. [DOI] [PubMed] [Google Scholar]
- 26.Kiatsopit N, Sithithaworn P, Boonmars T, Tesana S, Chanawong A, Saijuntha W, Petney TN, Andrews RH. Genetic markers for studies on the systematics and population genetics of snails, Bithynia spp., the first intermediate hosts of Opisthorchis viverrini in Thailand. Acta Trop. 2011;118:136–141. doi: 10.1016/j.actatropica.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aunpromma S, Tangkawattana P, Papirom P, Kanjampa P, Tesana S, Sripa B, Tangkawattana S. High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol Int. 2012;61:60–64. doi: 10.1016/j.parint.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Kaewpitoon SJ, Rujirakul R, Kaewpitoon N. Prevalence of Opisthorchis viverrini infection in Nakhon Ratchasima province, Northeast Thailand. Asian Pac J Cancer Prev. 2012;13:5245–5249. doi: 10.7314/apjcp.2012.13.10.5245. [DOI] [PubMed] [Google Scholar]
- 30.Saengsawang P, Promthet S, Bradshaw P. Prevalence of OV infection in Yasothon Province, Northeast Thailand. Asian Pac J Cancer Prev. 2012;13:3399–3402. doi: 10.7314/apjcp.2012.13.7.3399. [DOI] [PubMed] [Google Scholar]
- 31.Forrer A, Sayasone S, Vounatsou P, Vonghachack Y, Bouakhasith D, Vogt S, Glaser R, Utzinger J, Akkhavong K, Odermatt P. Spatial distribution of, and risk factors for, Opisthorchis viverrini infection in southern Lao PDR. PLoS Negl Trop Dis. 2012;6:e1481. doi: 10.1371/journal.pntd.0001481. [DOI] [PMC free article] [PubMed] [Google Scholar]