Abstract
Despite the existence of effective anthelmintics, parasitic infections remain a major public health problem in Southeast Asia, including Thailand. In rural communities, continuing infection is often reinforced by dietary habits that have a strong cultural basis and by poor personal hygiene and sanitation. This study presents a survey of the prevalence of intestinal parasitic infections among the people in rural Thailand. The community-based cross-sectional study was conducted in villages in Khon Kaen Province, northeastern Thailand, from March to August 2013. A total of 253 stool samples from 102 males and 140 females, aged 2-80 years, were prepared using formalin-ethyl acetate concentration methods and examined using light microscopy. Ninety-four individuals (37.2%) were infected with 1 or more parasite species. Presence of parasitic infection was significantly correlated with gender (P=0.001); nearly half of males in this survey (49.0%) were infected. Older people had a higher prevalence than younger members of the population. The most common parasite found was Opisthorchis viverrini (26.9%), followed by Strongyloides stercoralis (9.5%), Taenia spp. (1.6%), echinostomes (0.4%), and hookworms (0.4%). The prevalence of intestinal protozoa was Blastocystis hominis 1.6%, Entamoeba histolytica 0.8%, Entamoeba coli 0.8%, Balantidium coli 0.4%, Iodamoeba bütschlii 0.4%, and Sarcocystis hominis 0.4%. Co-infections of various helminths and protozoa were present in 15.9% of the people. The present results show that the prevalence of parasitic infections in this region is still high. Proactive education about dietary habits, personal hygiene, and sanitation should be provided to the people in this community to reduce the prevalence of intestinal parasite infections. Moreover, development of policies and programs to control parasites is needed.
Keywords: Opisthorchis viverrini, Strongyloides stercoralis, Taenia sp., Blastocystis hominis, echinostome, hookworm, intestinal parasite, parasitosis, prevalence, Thailand
INTRODUCTION
More than a quarter of the world's population is infected with intestinal parasites and 450 million people, especially in developing countries, may host multiple parasite species [1-3]. In Southeast Asia, including Thailand, parasitic infections remain a public health problem. The prevalence is particularly high in northeastern Thailand [4-6]. In rural communities, people still have cultural beliefs concerning diet, poor personal hygiene, and sanitation. Parasites transmitted via fecal-oral route (some trematodes, cestodes, and protozoa) are commonly found in rural communities. The infection may be caused among the people who frequently consume fresh vegetables and raw meat contaminated with food-borne parasites [7,8]. Soil-transmitted parasites (nematodes) occur in people who walk barefoot on contaminated soil or consume soil-contaminated food [9,10]. Lack of access to potable water and hot and humid tropical climate are additional factors associated with intestinal parasitic infections [11,12]. Although effective anthelmintic drugs exist and programs for prevention of parasitic infections have been initiated, some intestinal parasites remain a problem.
This study presents a cross-sectional survey of the prevalence of intestinal parasitic infections among the people in rural communities in northeastern Thailand and explores associations between parasitic infections and sociodemographic data.
MATERIALS AND METHODS
Data collection
A community-based cross-sectional study was conducted in 19 villages (estimated 20,000 people) through which the Shi and Pong Rivers flow in Khon Kaen Province, northeastern Thailand, from March to August 2013. In total, 253 volunteers were recruited in this study. A self-administered questionnaire was used to collect data on sociodemographic characteristics and related data. All participants were informed about the purpose, procedures, risks, and potential benefits of this study. Written informed consent was obtained from each participant. All protocols were approved by the Human Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (ethical clearance no. HE561219).
Stool collection and examination
Plastic containers were distributed, and participants were instructed on the procedure for stool specimen collection. Sample containers were marked with the participant identification number. All samples were stored in an ice-box for less than 6 hr, and then preserved in 10% formalin solution prior to processing using formalin-ether concentration methods [13,14] at the Parasitology Laboratory, Faculty of Medicine, Khon Kaen University. Presence of parasites in the sediments was examined using a light microscope. Each specimen was sampled for 2 slides and observed by 2 parasitologists. A stool sample was considered positive if at least 1 parasite was detected in 1 slide.
Data analysis
The prevalence of intestinal parasites was stratified according to sociodemographic data and was reported by descriptive statistics. Pearson's chi-squared test was used to determine whether there was a statistically significant difference between the parasitic infected and uninfected groups together with variable factors. The P-values of <0.05 were considered statistically significant.
RESULTS
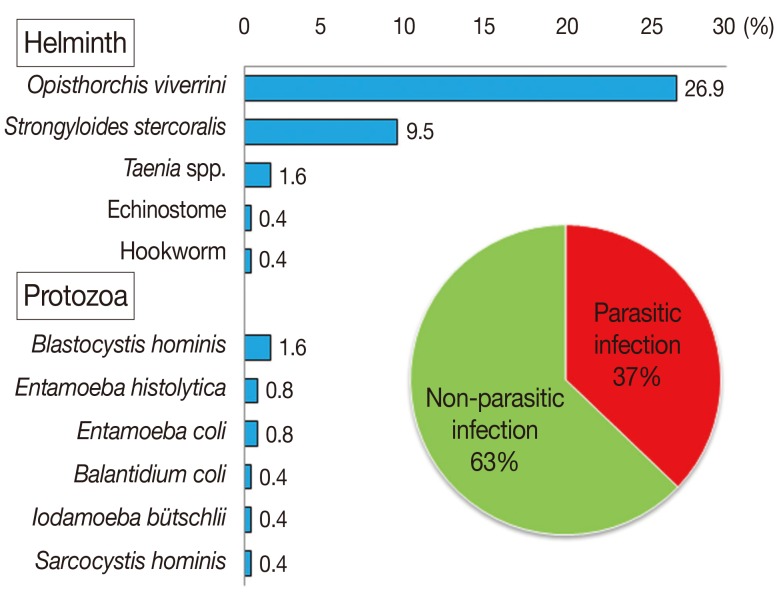
Of the 253 stool samples, 102 were collected from males (42%), 140 from females (58%), and 11 samples without marking the gender, aged 2-80 years with the mean age of 55.0±1.4 years. Most participants (99.2%) were buddhists and native northeastern people. Seventy percent of participants had completed only the primary school. The principal occupations in this community were agriculturist (35.5%), laborer (24.2%), and housewife (18.6%). Ninety-four individuals were infected with 1 or more parasites (Fig. 1). The prevalence rate among males (50 individuals; 20.7%) was slightly higher than that among females (40 individuals; 16.5%). People aged 41-60 years had a higher prevalence (19%) than those in other age groups (Table 1). Those in the agriculturist and labor categories had prevalence of 11.7%. Of those who had completed primary school, 27.8% were infected with parasites (Table 1). The most common intestinal parasite found was Opisthorchis viverrini (68 cases; 26.9%), followed by Strongyloides stercoralis (24 cases; 9.5%), Taenia spp., echinostome, and hookworm with 4 cases (1.6%), 1 case (0.4%), and 1 case (0.4%), respectively (Fig. 1). The prevalence of intestinal protozoa in this community were 4 cases (1.6%) of Blastocystis hominis, 2 cases (0.8%) each of Entamoeba histolytica and E. coli. Balantidium coli, Iodamoeba bütschlii, and Sarcocystis hominis were found in 1 case (0.4%) each. Seventy-nine individuals were infected with a single parasite species (31.2%), and 15 (5.9%) were infected with more than 1 parasite species (Table 2).
Fig. 1.
Prevalence of intestinal parasites found in people among rural communities.
Table 1.
Prevalence of intestinal parasites in rural communities stratified by sociodemographic characteristics
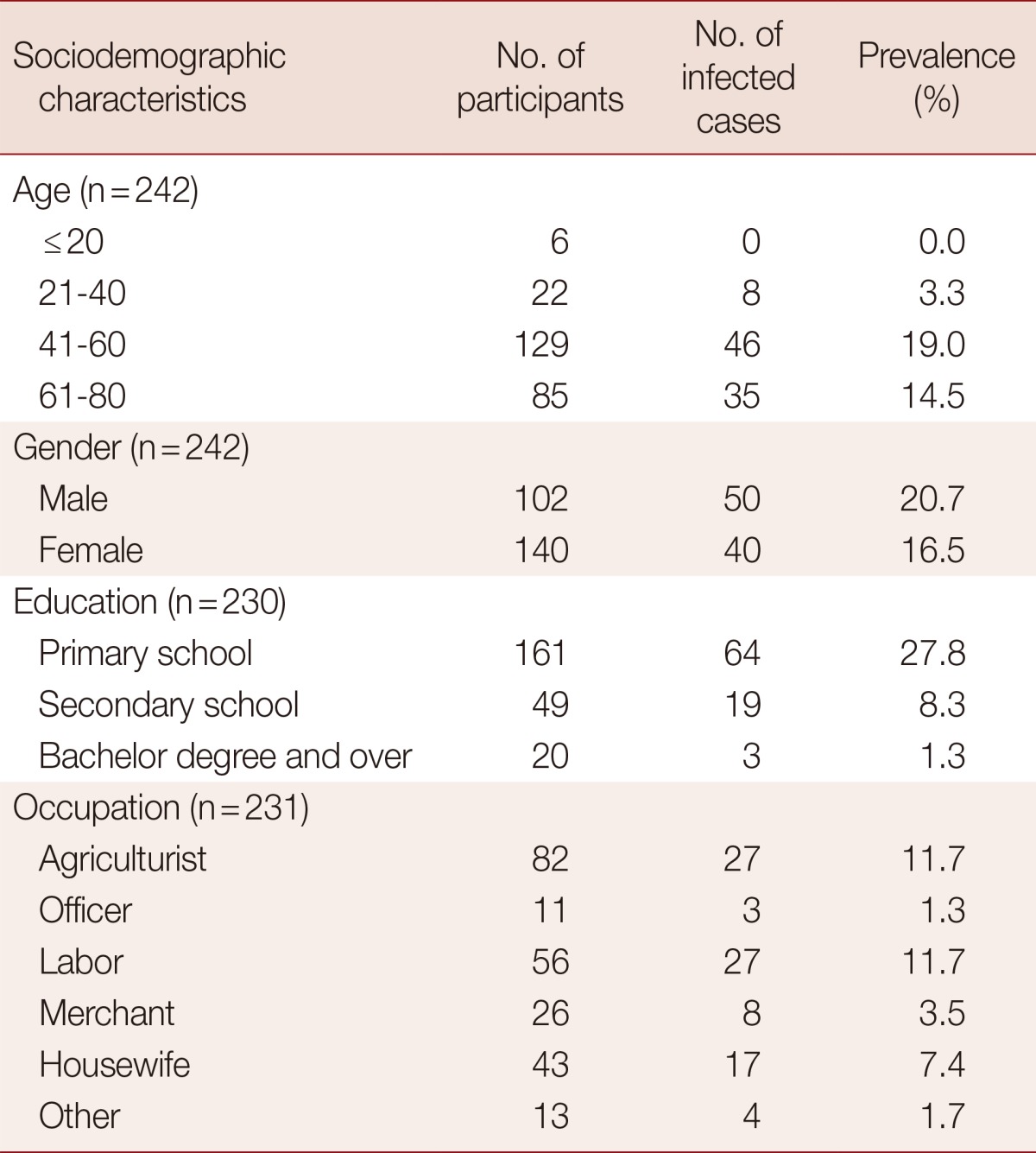
Table 2.
Prevalence of single and double intestinal parasite infections found in people in rural communities
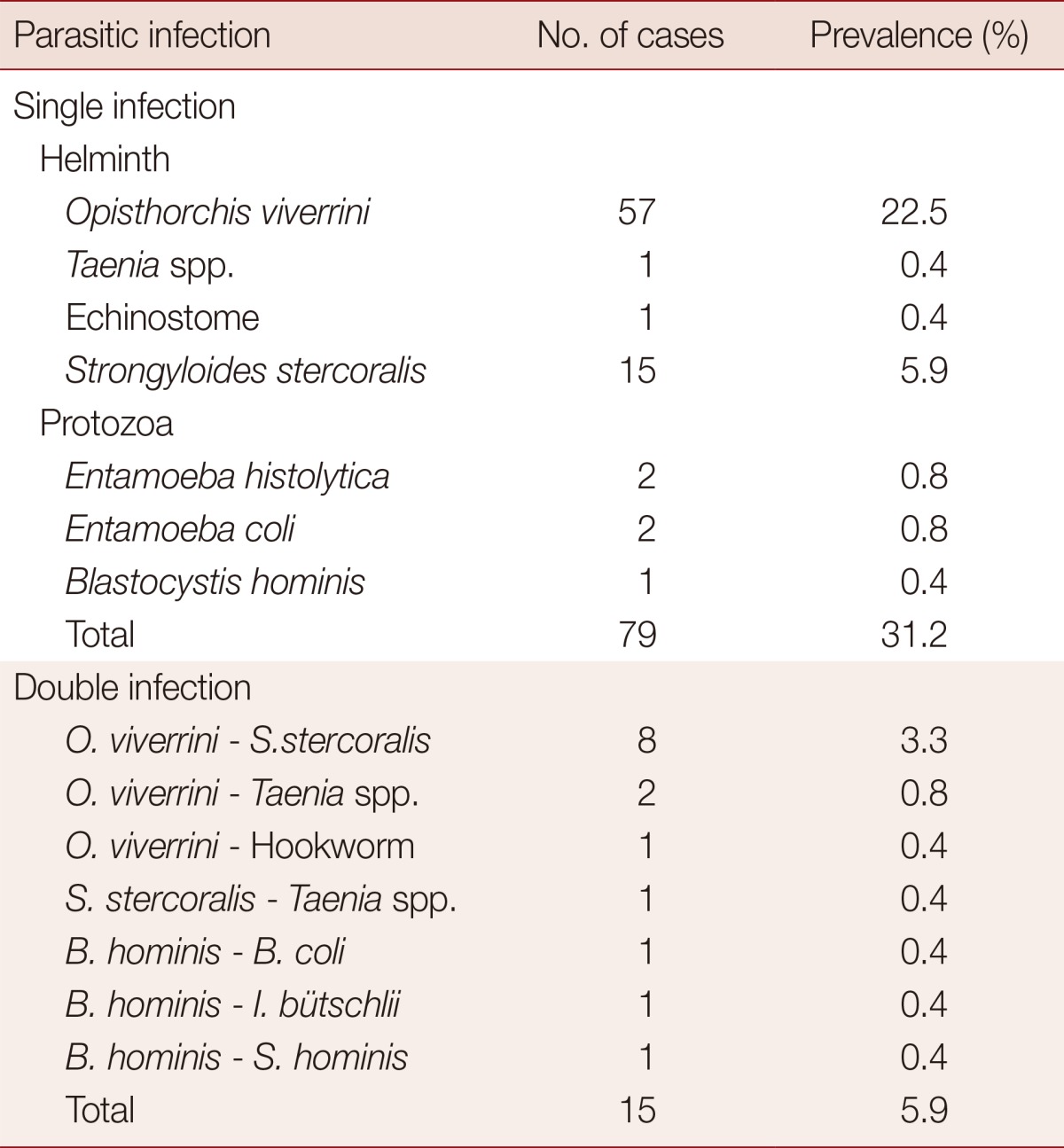
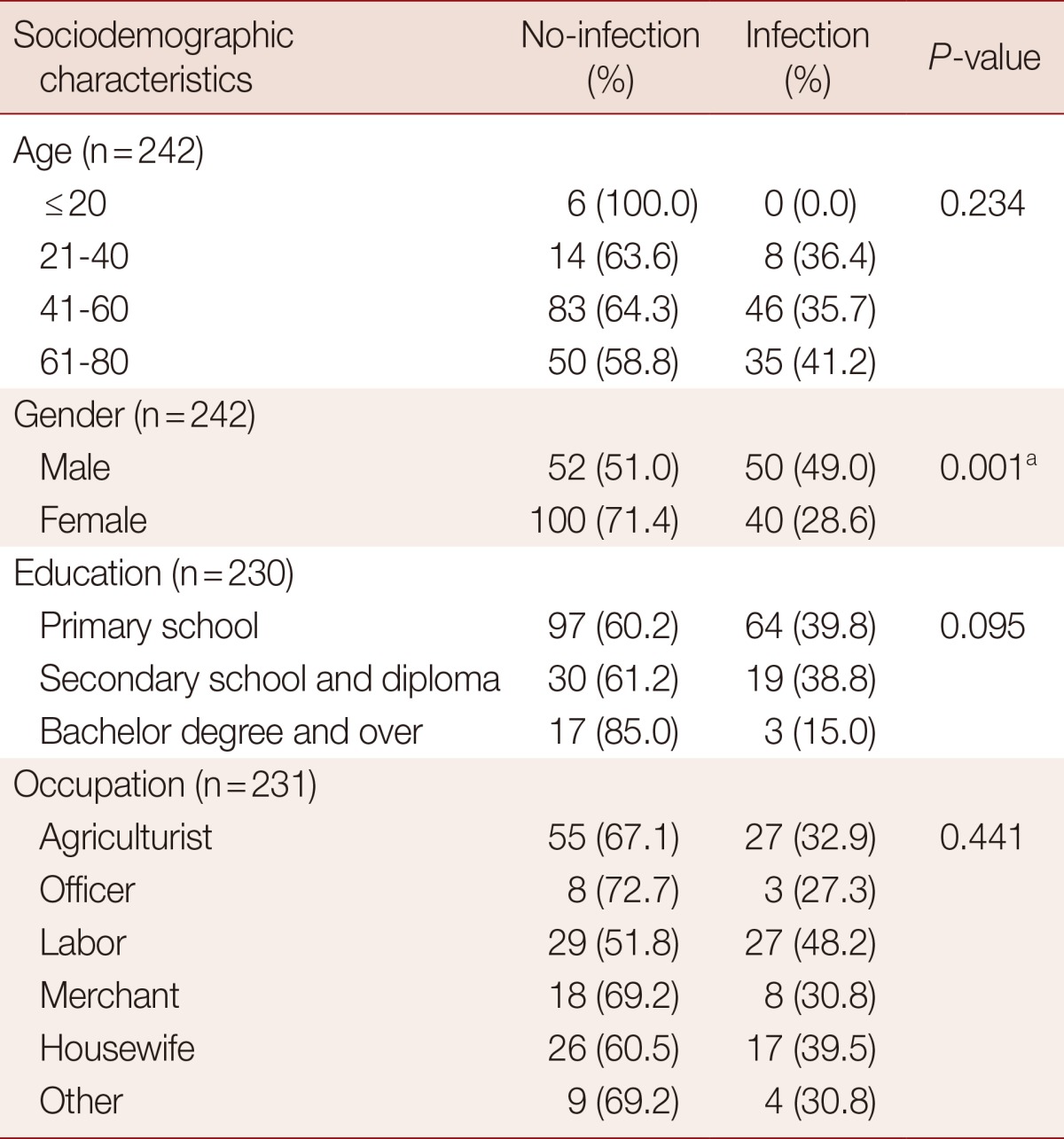
Correlation between parasitic infections and sociodemographic characteristics of the people in rural communities was shown in Table 3. Parasitic infections were significantly correlated with gender (P=0.001). Nearly half of the males (49.0%) were infected. However, there were no correlations of parasitic infection with age, occupation, and education. Certain subgroups exhibited (non-significantly) higher prevalences than other subgroups within their categories. These included 61-80 year age group (relative to younger age subgroups), those who had completed only the primary school (relative to more highly educated subgroups) and laborer relative to other occupation categories (Table 3).
Table 3.
Correlation between parasitic infections and sociodemographic characteristics of people in rural communities
**Statistically significant.
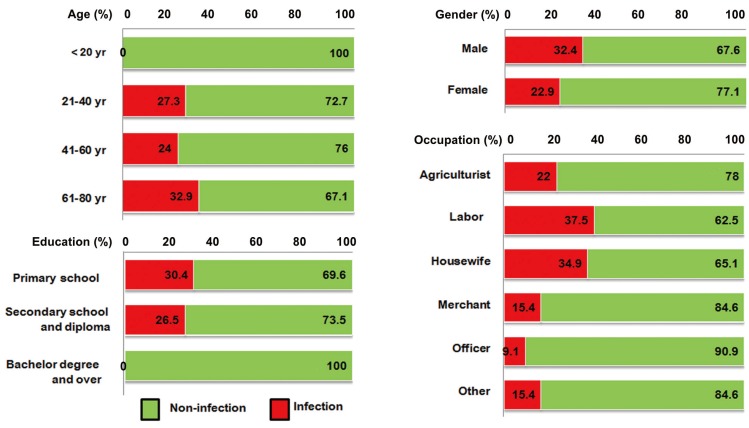
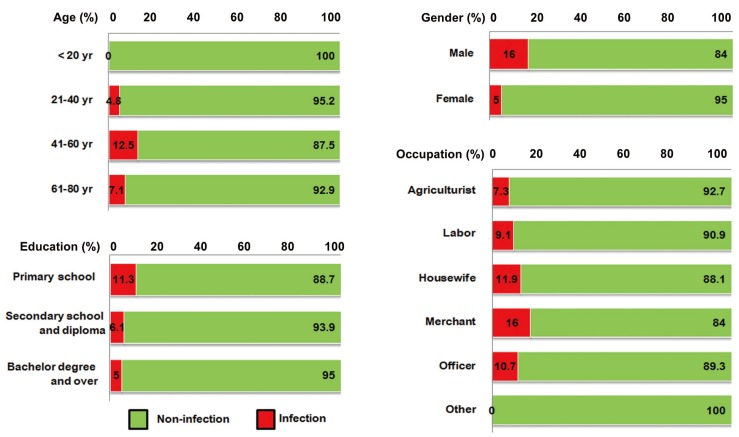
Male individuals, those aged 61-80 years, those who had completed only the primary school, and those in the laborer subcategory exhibited the highest prevalences of O. viverrini (Fig. 2). Fig. 3 shows a rather similar picture for S. stercoralis infection. Again, males and those of lower educational attainment exhibited the highest prevalence in their categories. Merchants and persons aged 41-60 years had the highest prevalence of parasitic infections in the occupation and age categories, respectively.
Fig. 2.
Percentage of Opisthorchis viverrini infection with sociodemographic characteristics
Fig. 3.
Percentage of Strongyloides stercoralis infection with sociodemographic characteristics.
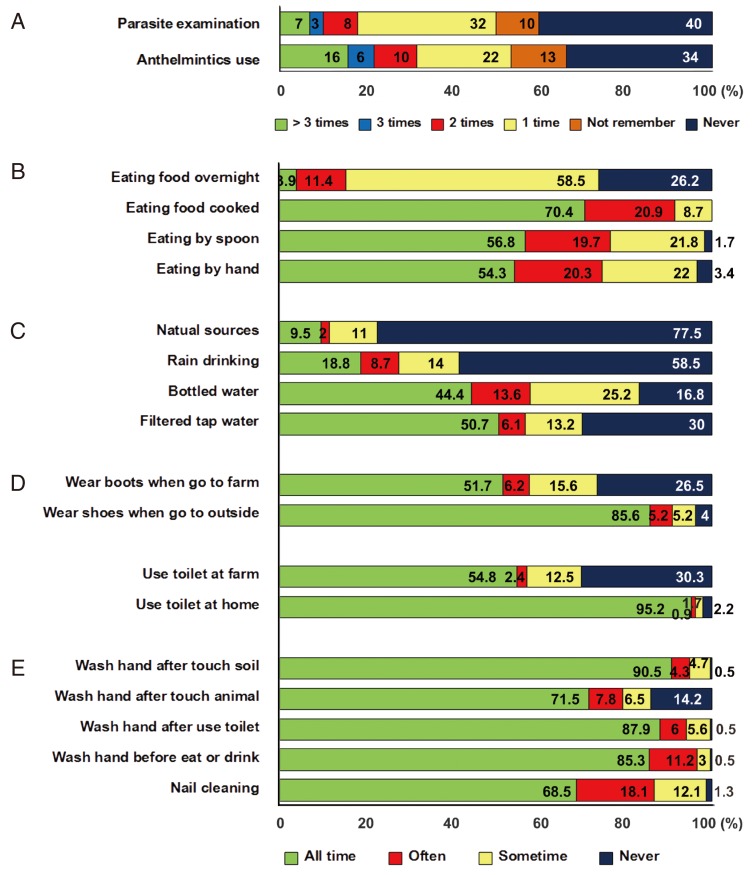
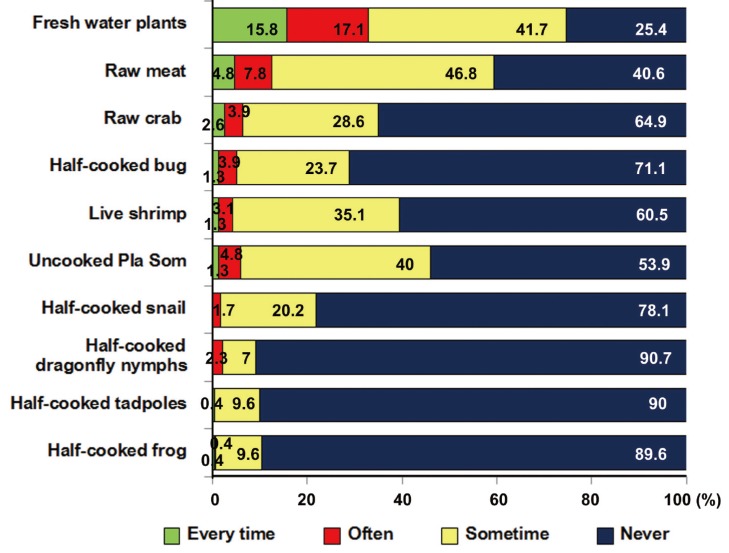
Dietary behavior, personal hygiene, and sanitation were shown in Fig. 4. Forty percent of those surveyed never had stool examinations and 34% never used anthelmintic drugs. People in this community usually ate sticky rice by hand (54.3%) and used a spoon (56.8%) for eating soup. Participants ate food freshly cooked (70.4%) but sometimes left food overnight before eating it (58.5%). People generally drank filtered tap water (50.7%) and bottled water (44.4%), although a few drank natural water or rainwater. Among the subjects, 85.6% wore shoes every time when going outside the house, and 51.7% always wore boots when at their farms, but 26.5% never wore boots. The toilet in their house was always used for urination or defecation (95.2%), but surprisingly 30.3% of people never used the toilet and defecated at the farm. About a half of the people (54.8%) always used toilet at the farm. Most people washed their hands after touching soil, after going to the toilet, before eating, after touching an animal or a pet, and most cleaned their nails regularly (Fig. 4).
Fig. 4.
Percentage of parasite examination and treatment (A), dietary behavior (B), drinking water consumption (C), shoes wearing (D), toilet using (E), and personal hygiene (F).
Likely sources of food-borne parasitic infections in this rural community are presented in Fig. 5. Most people ate freshwater plants but fewer than 10% of villagers always or often ate uncooked or half-cooked meat. Somewhat more popular were uncooked fish salad, half cooked meat, raw crab, half-cooked bug, half-cooked snail, live shrimp, and uncooked pla som. People rarely ate half-cooked dragonfly nymphs, tadpoles, and frogs only 7.0-9.6%.
Fig. 5.
Percentage of foodborne parasitic infectious source consumption.
DISCUSSION
Our study showed that more than 1/3 of 253 participants had infections with 1 or more species of parasites. High prevalences of O. viverrini (26.9%) and S. stercoralis (9.5%) were found. This result was similar to those in previous studies in Thai rural communities that found prevalences of opisthorchiasis to be 21.6/100 person in a year [15] and 24.5% [16]. The prevalence of S. stercoralis in our study was similar to that in the previous study [14] that diagnosed S. stercoralis in 10.5% of the population using a formalin-ether concentration method.
The present study showed a significant correlation between gender and parasitic infections (P=0.001), with males having a higher prevalence for all parasite species. This result was similar to the previous findings [5]. The gender difference may be due to male-specific behavioral factors [17] such as the eating raw meat, alcohol drinking with colleagues, and taking risks with their work in the farm. In our study, the prevalence of parasitic infections in older people (age >40 years) was higher than in young people (age <40 years). Rangsin et al. [15] reported that the prevalence of opisthorchiasis was correlated with age >60 years. Our findings were the same. This may be because 1) older people still have the culturally-embedded habit of eating uncooked food, 2) older people had poorer education and live in conditions of poor sanitation, and 3) survival time of some parasites in the host is one of the important factors for higher prevalence in the old people. Certainly, many people in this community have a low level of education, having completed only the primary school, and these people have a relatively high prevalence of parasites [5]. Health education programs should target this group and teach them about risky eating habits and the benefits of wearing shoes.
This study showed the incidence of parasitic infections was not significantly different between occupations. However, the highest incidence was found in the laborer, agriculturist, and housewife subgroups. Presumably again, social and behavioral factors are responsible for these differences, as suggested in a previous report about Nigerian workers [18] and Thai and Myanmar workers [19]. Among the subjects, 40% never had stool examinations, and many never or rarely used anthelmintic drugs by themselves. These results indicate the lack of public health activities aimed at prevention and control of parasitic infections in this area. Most people still eat food with their fingers, following local tradition. This may contribute to spread of infection by hand contamination.
Household sanitation in this rural community was not poor. Most people drank filtered tap water and bottled water. Only a few people drank rainwater and river/pond water that may cause protozoan infections in this community. In general, the villagers always wore shoes to go outside. When working on their farm, some villagers always wore boots, whereas some others never did. Villagers always used the toilet at home, but many urinated or defecated on the ground when working at the farm. Such behavior promotes spread of soil-transmitted parasites. The personal hygiene in this community was good; almost all villagers cleaned their nails and washed their hands before eating and after using the toilet, touching an animal or a pet and soil. An interesting result was that about 14.2% of people never washed hands after touching an animal or a pet. In daily life, fresh and uncooked vegetables, such as morning glory and mimosa, are the most likely source of food-borne parasitic infections. People still eat uncooked food, such as raw spices, beef salad, half cooked meat (especially infection through beef and pork) more often than they eat raw crab, half-cooked bug, half-cooked snail, live shrimp, and uncooked pla som.
Although the Thai government spends a lot of money on programs for prevention and control of parasitic infections in endemic areas, our study shows that parasites are still prevalent in this community. The main reason for the failure of previous or present programs is likely to be the lack of culturally sensitive and well-formulated health education about raw food consumption attitudes and practices [19]. Therefore, proactive health education to reduce transmission and reinfection by encouraging healthy behaviors in cultural beliefs, personal hygiene, and sanitation should be provided to the people in this community especially in the primary school.
ACKNOWLEDGMENTS
This work was supported by a grant from Khon Kaen University and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS) and TRF Senior Research Scholar Grant no. RTA5580004. We also wish to thank Faculty of Medicine to give research assistance (AS56201), the Department of Parasitology, Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand for their assistance.
References
- 1.Robertson LJ, van der Giessen JW, Batz MB, Kojima M, Cahill S. Have foodborne parasites finally become a global concern? Trends Parasitol. 2013;29:101–103. doi: 10.1016/j.pt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Control of Tropical Diseases. Geneva, Switzwerland: WHO; 1998. [Google Scholar]
- 4.Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick S, Thompson RC. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg. 2012;86:624–634. doi: 10.4269/ajtmh.2012.11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Songserm N, Promthet S, Wiangnon S, Sithithaworn P. Prevalence and co-infection of intestinal parasites among Thai rural residents at high-risk of developing cholangiocarcinoma: a cross-sectional study in a prospective Cohort study. Asian Pac J Cancer Prev. 2012;13:6175–6179. doi: 10.7314/apjcp.2012.13.12.6175. [DOI] [PubMed] [Google Scholar]
- 6.Tun A, Myat SM, Gabrielli AF, Montresor A. Control of soil-transmitted helminthiasis in Myanmar: results of 7 years of deworming. Trop Med Int Health. 2013 doi: 10.1111/tmi.12130. doi: 10.1111/tmi.12130. [DOI] [PubMed] [Google Scholar]
- 7.Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol. 2009;163:196–206. doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson CN, Gottstein B, Geerts S. Parasitic food-borne and water-borne zoonoses. Rev Sci Tech. 2000;19:240–258. doi: 10.20506/rst.19.1.1218. [DOI] [PubMed] [Google Scholar]
- 9.Naish S, McCarthy J, Williams GM. Prevalence, intensity and risk factors for soil-transmitted helminth infection in a South Indian fishing village. Acta Trop. 2004;91:177–187. doi: 10.1016/j.actatropica.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Nyarango RM, Aloo PA, Kabiru EW, Nyanchongi BO. The risk of pathogenic intestinal parasite infections in Kisii Municipality, Kenya. BMC Public Health. 2008;8:237. doi: 10.1186/1471-2458-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez AD, Poole A, Cattadori IM. Climate changes influence free-living stages of soil-transmitted parasites of European rabbits. Glob Chang Biol. 2013;19:1028–1042. doi: 10.1111/gcb.12106. [DOI] [PubMed] [Google Scholar]
- 12.Marcogliese DJ, Cone DK. Parasite communities as indicators of ecosystem stress. Parassitologia. 1997;39:227–232. [PubMed] [Google Scholar]
- 13.Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, Bhudhisawasdi V, Uttaravichien T. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990;84:715–719. doi: 10.1016/0035-9203(90)90159-c. [DOI] [PubMed] [Google Scholar]
- 14.Intapan PM, Maleewong W, Wongsaroj T, Singthong S, Morakote N. Comparison of the quantitative formalin ethyl acetate concentration technique and agar plate culture for diagnosis of human strongyloidiasis. J Clin Microbiol. 2005;43:1932–1933. doi: 10.1128/JCM.43.4.1932-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangsin R, Mungthin M, Taamasri P, Mongklon S, Aimpun P, Naaglor T, Leelayoova S. Incidence and risk factors of Opisthorchis viverrini infections in a rural community in Thailand. Am J Trop Med Hyg. 2009;81:152–155. [PubMed] [Google Scholar]
- 16.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int J Parasitol. 1996;26:1009–1023. [PubMed] [Google Scholar]
- 18.Ejezie GC, Akpan IF. Human ecology and parasitic infections. 1.The effect of occupation on the prevalence of parasitic infections in Calabar, Nigeria. J Hyg Epidemiol Microbiol Immunol. 1992;36:161–167. [PubMed] [Google Scholar]
- 19.Puangsa-art S, Yimsamran S, Buchachart K, Thanyavanich N, Wuthisen P, Rukmanee P, Maneeboonyang W, Prommongkol S, Rukmanee N. Study on the Ecology of Anopheline Larvae in Malaria Endemic Areas of Tanowsri Canton, Suanphung District, Ratchaburi Province. J Trop Med Parasitol. 2006;29:56–64. [Google Scholar]
- 20.Grundy-Warr C, Andrews RH, Sithithaworn P, Petney TN, Sripa B, Laithavewat L, Ziegler AD. Raw attitudes, wetland cultures, life-cycles: socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2012;61:65–70. doi: 10.1016/j.parint.2011.06.015. [DOI] [PubMed] [Google Scholar]