Abstract
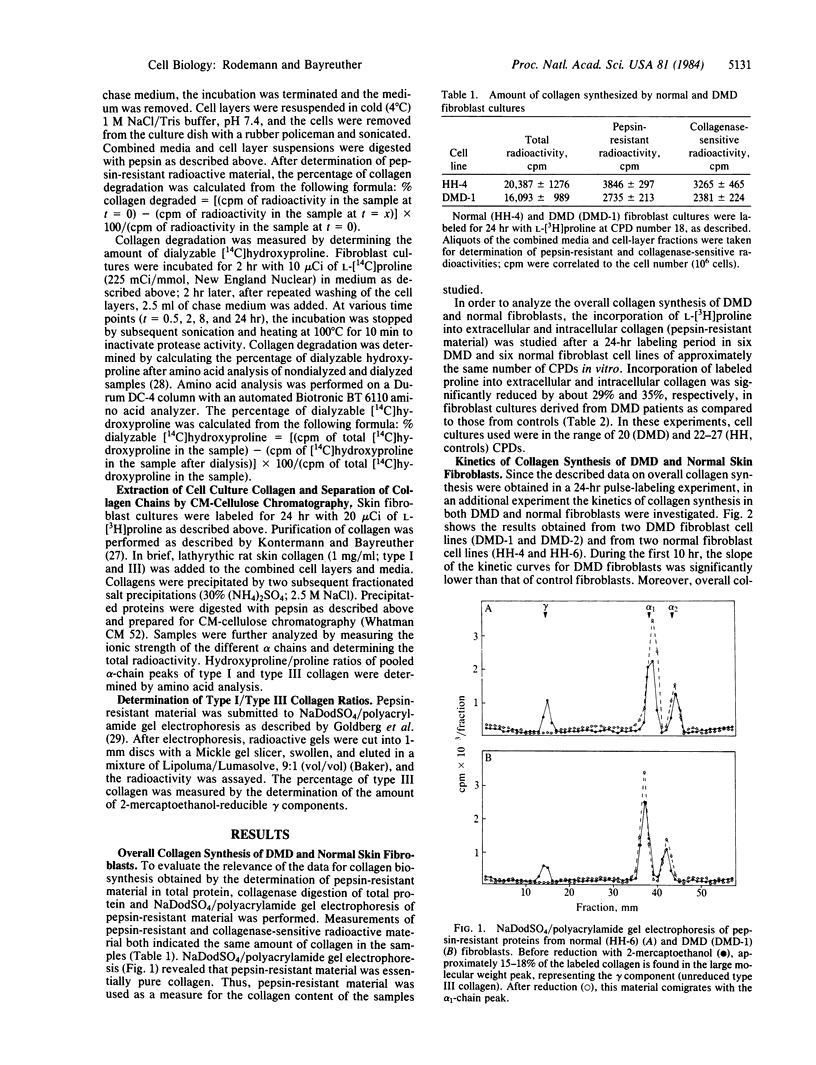
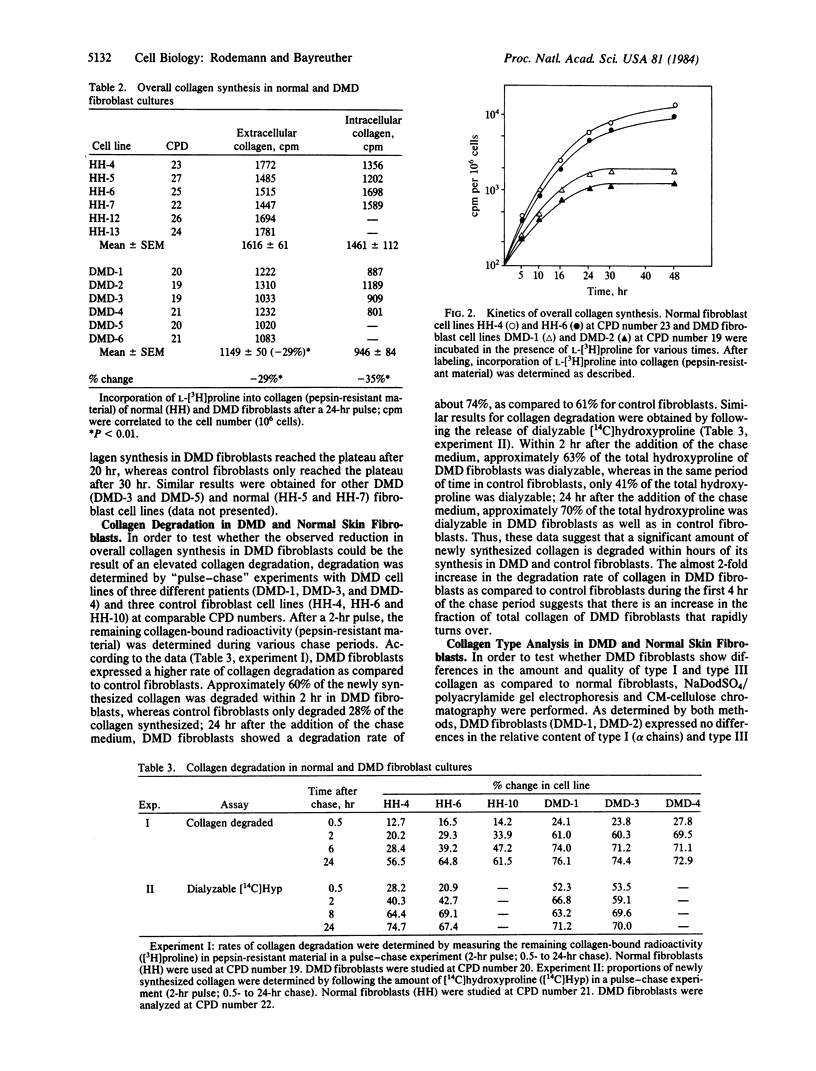
Total collagen synthesis is decreased by about 29% (P less than 0.01) in skin fibroblasts established in vitro from male patients with Duchenne muscular dystrophy (DMD) as compared with that in normal male skin fibroblasts in vitro. The reduction in collagen synthesis is associated with an approximately 2-fold increase in collagen degradation in DMD fibroblasts. Correlated to these alterations in the metabolism of collagen, DMD fibroblasts express a significantly higher hydroxyproline/proline ratio (DMD: 1.36-1.45; P less than 0.01) than do normal fibroblasts (controls: 0.86-0.89). The increased hydroxylation of proline residues of collagen (composed of type I and type III) could be the cause for the enhanced degradation of collagen in DMD fibroblasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbestos and health. Lancet. 1979 Oct 27;2(8148):887–887. [PubMed] [Google Scholar]
- Bader P. I. Lymphocyte capping in carriers of Duchenne muscular dystrophy. N Engl J Med. 1979 Sep 27;301(13):725–725. [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Boulé M., Vanasse M., Brakier-Gingras L. Decrease in the rate of protein synthesis by polysomes from cultured fibroblasts of patients and carriers with Duchenne muscular dystrophy. Can J Neurol Sci. 1979 Aug;6(3):355–358. doi: 10.1017/s0317167100024008. [DOI] [PubMed] [Google Scholar]
- Butterfield D. A. Electron spin resonance investigations of membrane proteins in erythrocytes in muscle diseases. Duchenne and myotonic muscular dystrophy and congenital myotonia. Biochim Biophys Acta. 1977 Oct 3;470(1):1–7. doi: 10.1016/0005-2736(77)90056-6. [DOI] [PubMed] [Google Scholar]
- Duance V. C., Stephens H. R., Dunn M., Bailey A. J., Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980 Apr 3;284(5755):470–472. doi: 10.1038/284470a0. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Gelman B. B., Papa L., Davis M. H., Gruenstein E. Decreased lysosomal dipeptidyl aminopeptidase I activity in cultured human skin fibroblasts in Duchenne's muscular dystrophy. J Clin Invest. 1980 Jun;65(6):1398–1406. doi: 10.1172/JCI109804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Steven F. S., Jackson D. S., Sandberg L. B. Carbohydrate content of insoluble elastins prepared from adult bovine and calf ligamentum nuchae and tropoelastin isolated from copper-deficient porcine aorta. Biochem J. 1971 Jan;121(2):197–202. doi: 10.1042/bj1210197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson A., Pleasure D. Erythrocyte cation-activated adenosine triphosphatases in Duchenne muscular dystrophy. J Neurol Sci. 1977 Jul;32(3):361–369. doi: 10.1016/0022-510x(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Ionasescu V., Lara-Braud C., Zellweger H., Ionasescu R., Burmeister L. Fibroblast cultures in Duchenne muscular dystrophy. Alterations in synthesis and secretion of collagen and noncollagen proteins. Acta Neurol Scand. 1977 May;55(5):407–417. doi: 10.1111/j.1600-0404.1977.tb05659.x. [DOI] [PubMed] [Google Scholar]
- Ionasescu V., Zellweger H., Ionasescu R., Lara-Braud C., Cancilla P. A. Protein synthesis in muscle cultures from patients with Duchenne muscular dystrophy. Calcium and A23187 ionophore dependent changes. Acta Neurol Scand. 1976 Sep;54(3):241–247. doi: 10.1111/j.1600-0404.1976.tb04800.x. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Witkowski J. A. Reduced adhesiveness between skin fibroblasts from patients with Duchenne muscular dystrophy. J Neurol Sci. 1979 Nov;43(3):465–470. doi: 10.1016/0022-510x(79)90025-x. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Activity of some proteolytic enzymes in normal and dystrophic human muscle. Clin Biochem. 1979 Apr;12(2):37–39. doi: 10.1016/s0009-9120(79)80001-6. [DOI] [PubMed] [Google Scholar]
- Kent C. Increased rate of cell-substratum detachment of fibroblasts from patients with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983 May;80(10):3086–3090. doi: 10.1073/pnas.80.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. D., Luthra M. G., Watts R. P., Stern L. Z. Factors influencing osmotic fragility of red blood cells in Duchenne muscular dystrophy. Neurology. 1980 Jul;30(7 Pt 1):726–731. doi: 10.1212/wnl.30.7.726. [DOI] [PubMed] [Google Scholar]
- Kontermann K., Bayreuther K. The cellular aging of rat fibroblasts in vitro is a differentiation process. Gerontology. 1979;25(5):261–274. doi: 10.1159/000212351. [DOI] [PubMed] [Google Scholar]
- Laurent M., Daveloose D., Leterrier F., Fischer S., Schapira G. A spin label study of the erythrocyte membranes in Duchenne muscular dystrophy. Clin Chim Acta. 1980 Aug 4;105(2):183–194. doi: 10.1016/0009-8981(80)90460-x. [DOI] [PubMed] [Google Scholar]
- Liechti-Gallati S., Moser H., Siegrist H. P., Wiesmann U., Herschkowitz N. N. Abnormal growth kinetics and 5'-nucleotidase activities in cultured skin fibroblasts from patients with Duchenne muscular dystrophy. Pediatr Res. 1981 Nov;15(11):1411–1414. doi: 10.1203/00006450-198111000-00004. [DOI] [PubMed] [Google Scholar]
- McKeran R. O., Halliday D., Purkiss P. Increased myofibrillar protein catabolism in Duchenne muscular dystrophy measured by 3-methylhistidine excretion in the urine. J Neurol Neurosurg Psychiatry. 1977 Oct;40(10):979–981. doi: 10.1136/jnnp.40.10.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato C. N., Davis M. H., Doughty M. J., Bryant S. H., Gruenstein E. Increased membrane permeability to chloride in Duchenne muscular dystrophy fibroblasts and its relationship to muscle function. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4732–4736. doi: 10.1073/pnas.80.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S., Bornstein P. In vitro enzymatic hydroxylation of prolyl residues in the alpha 1-CB2 fragment of rat collagen. J Biol Chem. 1971 Jul 10;246(13):4138–4142. [PubMed] [Google Scholar]
- Risteli J., Tryggvason K., Kivirikko K. I. Prolyl 3-hydroxylase: partial characterization of the enzyme from rat kidney cortex. Eur J Biochem. 1977 Mar 1;73(2):485–492. doi: 10.1111/j.1432-1033.1977.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Roses A. D. Erythrocyte membrane autophosphorylation in Duchenne muscular dystrophy: effect of two methods of erythrocyte ghost preparation on results. Clin Chim Acta. 1979 Jul 2;95(1):69–73. doi: 10.1016/0009-8981(79)90337-1. [DOI] [PubMed] [Google Scholar]
- Rourke A. W. Myosin in developing normal and dystrophic chicken pectoralis. I. Synthesis and degradation. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):343–351. doi: 10.1002/jcp.1040860406. [DOI] [PubMed] [Google Scholar]
- Smith G. N., Jr, Linsenmayer T. F., Newsome D. A. Synthesis of type II collagen in vitro by embryonic chick neural retina tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4420–4423. doi: 10.1073/pnas.73.12.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. VII. Studies on the biosynthesis of protein and RNA in various cellular fractions of the muscle of normal and dystrophic mice. Can J Biochem. 1968 Jan;46(1):35–41. doi: 10.1139/o68-006. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Risteli J., Kivirikko K. I. Separation of prolyl 3-hydroxylase and 4-hydroxylase activities and the 4-hydroxyproline requirement for synthesis of 3-hydroxyproline. Biochem Biophys Res Commun. 1976 May 23;76(2):275–281. doi: 10.1016/0006-291x(77)90722-7. [DOI] [PubMed] [Google Scholar]
- Verrill H. L., Pickard N. A., Gruemer H. D. Diminished cap formation in lymphocytes from patients and carriers of Duchenne muscular dystrophy. Clin Chem. 1977 Dec;23(12):2341–2343. [PubMed] [Google Scholar]
- WEINSTOCK I. M., EPSTEIN S., MILHORAT A. T. Enzyme studies in muscular dystrophy. III. In hereditary muscular dystrophy in mice. Proc Soc Exp Biol Med. 1958 Oct;99(1):272–276. doi: 10.3181/00379727-99-24320. [DOI] [PubMed] [Google Scholar]
- Wyatt P. R., Cox D. M. Duchenne's muscular dystrophy: studies in cultured fibroblasts. Lancet. 1977 Jan 22;1(8004):172–174. doi: 10.1016/s0140-6736(77)91768-8. [DOI] [PubMed] [Google Scholar]


