Background: CYP2D6-mediated drug metabolism is enhanced during pregnancy, but the underlying mechanisms remain unknown.
Results: In CYP2D6-humanized mice, CYP2D6 induction during pregnancy was linked to decreased expression of SHP, a repressor of CYP2D6 expression.
Conclusion: Decreased SHP expression may account for CYP2D6 induction during pregnancy.
Significance: This may provide a mechanistic basis in designing optimal dosage regimens in pregnant women.
Keywords: Cytochrome P450, Drug Metabolism, Pregnancy, Retinoid, Transgenic Mice, HNF4α, SHP
Abstract
Substrates of a major drug-metabolizing enzyme CYP2D6 display increased elimination during pregnancy, but the underlying mechanisms are unknown in part due to a lack of experimental models. Here, we introduce CYP2D6-humanized (Tg-CYP2D6) mice as an animal model where hepatic CYP2D6 expression is increased during pregnancy. In the mouse livers, expression of a known positive regulator of CYP2D6, hepatocyte nuclear factor 4α (HNF4α), did not change during pregnancy. However, HNF4α recruitment to CYP2D6 promoter increased at term pregnancy, accompanied by repressed expression of small heterodimer partner (SHP). In HepG2 cells, SHP repressed HNF4α transactivation of CYP2D6 promoter. In transgenic (Tg)-CYP2D6 mice, SHP knockdown led to a significant increase in CYP2D6 expression. Retinoic acid, an endogenous compound that induces SHP, exhibited decreased hepatic levels during pregnancy in Tg-CYP2D6 mice. Administration of all-trans-retinoic acid led to a significant decrease in the expression and activity of hepatic CYP2D6 in Tg-CYP2D6 mice. This study provides key insights into mechanisms underlying altered CYP2D6-mediated drug metabolism during pregnancy, laying a foundation for improved drug therapy in pregnant women.
Introduction
More than 50% of pregnant women take one or more prescription drugs, and the average number of prescriptions per patient during pregnancy ranges from 3 to 5 (1, 2). Clinical evidence indicates that pregnancy alters hepatic drug disposition. However, our current understanding of the underlying mechanisms is limited due to a lack of appropriate study models recapitulating the complex physiological changes accompanying pregnancy (3). This is in turn manifested clinically by an overall paucity of drug safety and dosing guidelines for pregnancy.
Cytochrome P450 (CYP)3 enzymes play a key role in hepatic elimination of xenobiotics as well as in hormone homeostasis. CYP expression is largely regulated by the actions of multiple liver-enriched transcription factors. For example, expression of many CYP isoforms is transcriptionally activated by xenosensor nuclear receptors including pregnane X receptor and constitutive androstane receptor (4). Drugs that bind and activate these transcription factors induce CYP expression, leading to clinically significant drug-drug interactions.
CYP2D6 is a major drug-metabolizing enzyme expressed in the liver and extrahepatic organs including the brain, kidney, and intestine. CYP2D6 mediates the hepatic metabolism of the ∼20% of marketed drugs, second-largest portion only after CYP3A4 (5, 6). Also, CYP2D6 expressed in the brain is involved in the synthesis of neurotransmitters (7) and is implicated in Parkinson disease (8). CYP2D6 has been considered a non-inducible gene based on the finding that none of the previously reported CYP-inducing compounds (including pregnane X receptor-activating rifampin and constitutive androstane receptor-activating phenobarbital) affect CYP2D6 expression. Interestingly, however, pregnancy induces hepatic elimination of CYP2D6 substrates. Elimination of dextromethorphan and metoprolol, the prototypical CYP2D6 substrates, is increased in pregnant women as compared with postpartum controls (9–11). Higher doses of CYP2D6 substrates are required during pregnancy than before pregnancy (12).
Despite the accumulating clinical evidence, the mechanisms underlying CYP2D6 induction during pregnancy remain unknown because appropriate experimental models have been lacking. For example, expression of the endogenous CYP2D6 homologs in rodents (i.e. CYP2D2 in rats and Cyp2d22 in mice) does not increase during pregnancy (13, 14). This likely reflects the large divergence in the regulatory region sequences of genes encoding xenobiotic-metabolizing CYP enzymes between rodents and humans (15).
In this study, using CYP2D6-humanized (Tg-CYP2D6) mice (16) as an animal model, we investigated the molecular mechanisms underlying CYP2D6 induction during pregnancy and demonstrated a potential role for the interplay between retinoic acids and hepatic transcription factors in CYP2D6 induction.
EXPERIMENTAL PROCEDURES
Animals
Tg-CYP2D6 and Hnf4α/AlbCre transgenic mice were previously described (16, 17). Adult female (8 weeks old) mice were mated with male mice of the similar age. Mating between adult mice was confirmed by the presence of vaginal plugs (day 0). Male mice were separated from female mice immediately after a vaginal plug was found. Virgin mice were group-housed so that their estrous cycles were suppressed. All procedures were approved by the Institutional Animal Care and Use Committee in the University of Illinois at Chicago.
Chemicals and Reagents
Debrisoquine and (±)-4-hydroxydebrisoquin were purchased from Biomol (Plymouth Meeting, PA). Formic acid (ACS grade), acetonitrile (ACS grade), and methanol (Optima grade) were purchased from Fisher. All-trans-retinoic acid (atRA) and 13-cis-retinoic acid (13cRA) were purchased from Sigma.
Plasmids
To construct the pGL3-CYP2D6 plasmid, the upstream region of CYP2D6 (−2453 to +90) was PCR-amplified using the genomic DNA of Tg-CYP2D6 mouse as a template and primers listed in supplemental Table S1. The PCR product was digested by KpnI and NcoI restriction enzymes and cloned into promoterless pGL3-basic vectors (Promega, Madison, WI) digested by the same enzymes, yielding pGL3-CYP2D6. pcDNA3-HNF4α was received from Dr. Frances M. Sladek (University of California Riverside Human). pEBG-control and pEBG-SHP expression vector were received from Dr. Hueng-Sik Choi (Chonnam National University, Korea).
Western Blot
CYP2D6, Hepatocyte nuclear factor 4α (HNF4α), and small heterodimer partner (SHP) protein expression levels were determined by using the respective antibodies (CYP2D6, BD GentestTM, catalog #458246; HNF4α, Santa Cruz, sc-6556; Aviva, ARP31946_P050; SHP, Santa Cruz, sc-30169).
CYP2D6 Phenotyping
Liver microsomes or S9 fractions were prepared as described previously (18). Microsomes or S9 fractions were incubated with debrisoquine (at different concentrations ranging from 25 to 1000 μm) for 15 min, and the reaction was stopped by adding two volumes of ice-cold acetonitrile. The concentration of 4-hydroxydebrisoquine was determined by LC-MS/MS (Agilent 1200 HPLC interfaced with Applied Biosystems Qtrap 3200) using an electrospray ion source. Multiple reaction monitoring data acquisition were employed: m/z 192.3/132.2 for 4-hydroxydebrisoquine and 181.1/124.1 for the internal standard paraxanthine.
RNA Isolation and Quantitative Real Time-PCR (qRT-PCR)
Total RNA was isolated from mouse liver tissues using TRIzol (Invitrogen) and converted to cDNA using the High Capacity cDNA Archive kit (Invitrogen). Using the cDNA as template, qRT-PCR was performed using StepOnePlus Real-Time PCR System and primers listed in supplemental Table S1. The results are expressed as -fold changes during pregnancy using the gene expression levels normalized to those of mouse β-actin (2−ΔΔCt method).
Luciferase Reporter Assay
HepG2 or HEK293T cells were seeded in 12-well plates at a density of 4.5 × 105 cells/ml and on the next day transfected with 0.3 μg of luciferase construct, 0.1 μg of expression plasmids (or empty vector as a control), and 0.006 μg of Renilla expression vector (Promega) using FuGENE HD transfection reagent (Promega) according to the manufacturer's protocol. The transfected cells were grown for 48 h and harvested for determination of luciferase activities using a luciferase assay kit (Promega). At least two independent experiments were performed in triplicate.
Gene Knockdown by siRNA
siRNA targeting SHP (Thermo Scientific; siGenome_NR0B2 siRNA, 50 μg in 1 ml of PBS) or control siRNA (siGenome_non-targeting siRNA pool) was injected into Tg-CYP2D6 mice through the tail vein. The injection was repeated 8 h later. Livers were collected 3 days after the last injection for examination of gene expression.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed as previously described with minor modifications (19). Briefly, livers were finely minced and incubated in PBS containing 1% formaldehyde at room temperature for 15 min, and glycine was added to stop the cross-linking reaction. Cell pellets were resuspended in hypotonic buffer (15 mm HEPES, pH 7.9, 60 mm KCl, 2 mm EDTA, 0.5% BSA, 0.15 mm spermine, 0.5 mm spermidine, 0.32 m sucrose) and lysed by homogenization. Nuclei were pelleted and resuspended in nuclei lysis buffer (50 mm Tris-HCl, pH 8.0, 2 mm EDTA, 1% SDS). The samples were sonicated to shear DNA to the length ranging from 100 to 500 bp. After centrifugation, the chromatin sample was immunoprecipitated with 2 μg of antibody (Santa Cruz Biotechnology; HNF4α, sc-6556x; RNA polymerase II, sc-899x; SHP, sc-30169; farnesoid X receptor (FXR; sc-13063x) or immunoglobulin G (IgG) at 4 °C for overnight. The immune complex was collected, the magnetic beads were extensively washed, and the bound chromatin was eluted. Genomic DNA was purified by PCR Clean-up kit (Promega) and used as a template for qPCR. Primer sequences are listed in supplemental Table S1.
Retinoic acid Measurement
The concentrations of atRA and 13cRA were analyzed by LC-MS/MS as previously described (20) with the following modifications; mouse livers (n = 11, liver weight 36–208 mg) were homogenized with a 2-ml glass Dounce homogenizer (Kimble Glass) in a 1:1 volume of 0.9% NaCl, and the sample was transferred into a 15-ml glass culture tube. A 2:1 volume of acetonitrile with 0.1% formic acid was added together with atRA-d5 (internal standard). Retinoic acid was extracted with 10 ml of hexanes, and the organic layer was transferred to a glass tube and dried under nitrogen at 37 °C. The sample was reconstituted in 65 μl of 60:40 acetonitrile/H2O for LC-MS/MS analysis. A standard curve and quality control samples were generated using UV light-exposed mouse liver homogenate spiked with atRA and 13cRA at concentrations of 0, 2, 5, 10, 15, and 20 nm for the standards and 3, 7.5, and 17.5 nm for the quality control samples. Gradient elution with a flow rate of 0.5 ml/min using water +0.1% formic acid (A) and acetonitrile/methanol (60/40) (B) was used. The gradient was from initial 60% (A) for 2 min to 45% (A) over 8 min and then to 10% (A) over 7 min. The column was then washed with 95% (B) for 3 min and returned to initial conditions.
Statistical Analysis
The statistical difference between the two groups was determined by the Student's t test. For statistical testing among different treatment groups, one-way analysis of variance test was performed for multiple comparisons followed by post hoc Dunnett's test.
RESULTS
Tg-CYP2D6 Mice Serve as a Model for CYP2D6 Induction during Pregnancy
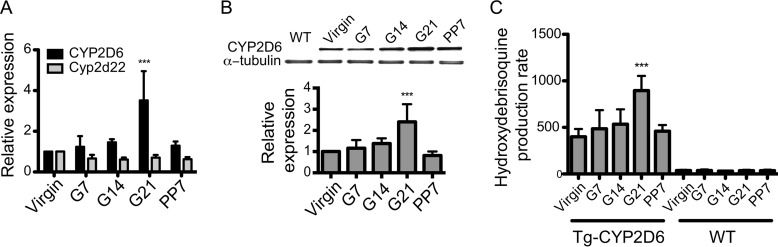
The Tg-CYP2D6 mice carrying the entire human CYP2D6 gene including ∼2.5 kb of the upstream regulatory region was previously used as a model to characterize CYP2D6-mediated drug metabolism in an in vivo system (16). To determine the utility of Tg-CYP2D6 mice as an animal model to study CYP2D6 induction during pregnancy, we first examined the hepatic expression and enzyme activity of CYP2D6 at different gestational time points (virgin, 7/14/21 days of pregnancy (G7/14/21), and 7 days postpartum). The mRNA and protein levels of CYP2D6 were elevated during pregnancy and returned to pre-pregnancy levels by the postpartum time point (Fig. 1, A and B). In contrast, the expression of Cyp2d22 (a mouse homolog of CYP2D6) remained unchanged during pregnancy (Fig. 1A), consistent with recent findings (14). Results from microsomal phenotyping (by using debrisoquine as a probe drug for CYP2D6) revealed a significant increase in CYP2D6 activity at term as compared with pre-pregnancy or postpartum (Fig. 1C), in accordance with the changes observed for CYP2D6 expression. The increased CYP2D6 activity was accompanied by higher Vmax values but no changes in Km (Table 1), indicating that CYP2D6 activity is enhanced by increased protein amounts rather than changes in the catalytic activity of CYP2D6. The magnitude of CYP2D6 induction was similar to the clinically reported increases in elimination of a CYP2D6 substrate metoprolol (9), suggesting that Tg-CYP2D6 mice may serve as a model system for identification and characterization of regulatory mechanisms for CYP2D6 induction during pregnancy.
FIGURE 1.
Hepatic CYP2D6 is induced in Tg-CYP2D6 mice during pregnancy. A, liver tissues of Tg-CYP2D6 mice were collected at different time points during gestation. mRNA expression levels of CYP2D6 and Cyp2d22 were determined by qRT-PCR. B, protein levels of CYP2D6 in the liver tissues were determined by using Western blotting. A representative image of Western blot is shown including a liver tissue sample from wild-type (WT) mice (top), and the band intensities were quantified (bottom). C, microsomes were prepared from the liver tissues of Tg-CYP2D6 or WT mice, and CYP2D6 phenotyping was performed using debrisoquine (200 μm) (n = 4, mean ± S.D.; ***, p < 0.001 one-way ANOVA versus virgin).
TABLE 1.
Kinetic parameters for debrisoquine hydroxylation activity in hepatic microsomes prepared from Tg-CYP2D6 mice (n = 4, mean ± S.D.)
Vmax, maximum velocity; Km, Michaelis-Menten constant.
| Parameter | Virgin | G7 | G14 | G21 | PP7 |
|---|---|---|---|---|---|
| Vmax (pmol/min/mg protein) | 749 ± 125 | 1130 ± 420 | 1240 ± 160a | 2120 ± 60b | 1080 ± 200 |
| Km (μm) | 210 ± 77 | 291 ± 54 | 296 ± 87 | 278 ± 26 | 285 ± 19 |
a p < 0.05.
b p < 0.01 versus virgin.
SHP Is Down-regulated during Pregnancy and Represses HNF4α-mediated Transactivation of CYP2D6
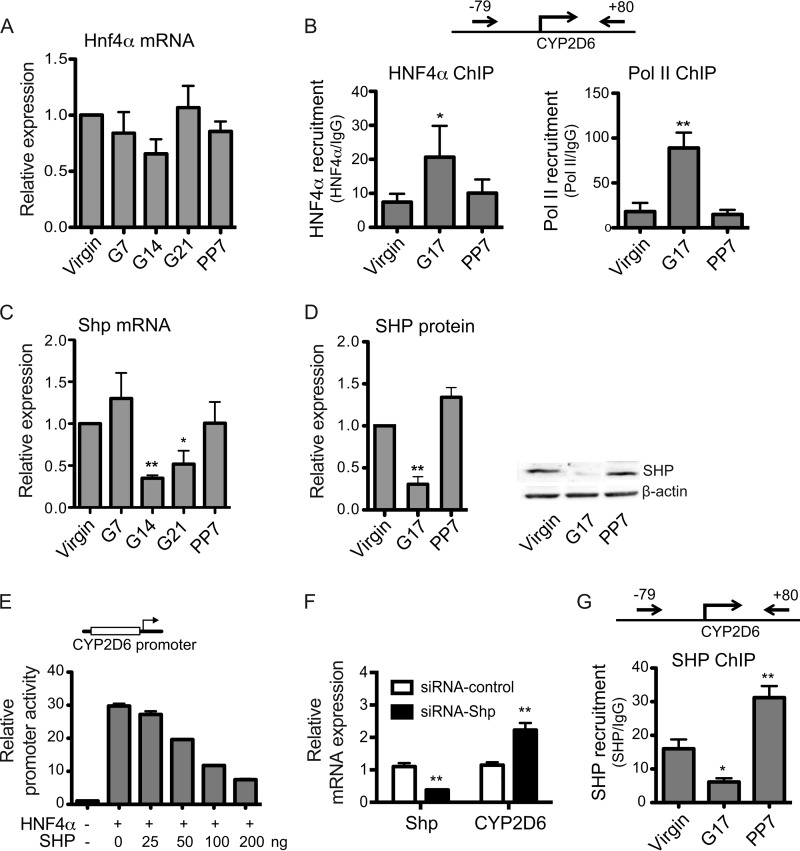
HNF4α is a positive regulator of basal CYP2D6 expression under nonpregnant conditions via direct binding to the CYP2D6 promoter (16, 21, 22). To examine the potential effects of pregnancy on HNF4α expression, mRNA and protein levels of HNF4α were examined in the livers of Tg-CYP2D6 mice at different gestational time points. Pregnancy did not affect hepatic HNF4α expression at the levels of mRNA (Fig. 2A) or protein (data not shown), consistent with results from a previous study (23).
FIGURE 2.
SHP is down-regulated during pregnancy and represses HNF4α transactivation of CYP2D6. A, liver tissues were collected from Tg-CYP2D6 mice at pre-pregnancy (Virgin), 7/14/21 days of pregnancy (G7, G14, and G21, respectively), or 7 days postpartum (PP7), and total RNA and lysates were prepared from the tissues. mRNA levels of Hnf4α were determined by qRT-PCR (n = 4, mean ± S.D.; *, p < 0.05; **, p < 0.01, one-way ANOVA versus virgin). B, ChIP assays were performed using liver tissues of Tg-CYP2D6 mice collected at different gestational time points (n = 4, mean ± S.D.; *, p < 0.05; **, p < 0.01, one-way ANOVA versus virgin). C and D, mRNA and protein levels of SHP in liver tissues were determined by qRT-PCR and Western blot, respectively. A representative image of Western blot (D, right) and the quantified band intensities (D, left; n = 4, mean ± S.D.; **, p < 0.01, one-way ANOVA versus virgin) are shown. E, CYP2D6 promoter activity was examined by dual luciferase assay in HEK293T cells (n = 3, mean ± S.D.). F, Tg-CYP2D6 mice were injected with scrambled siRNA (siRNA-control) or siRNA targeting SHP (siRNA-SHP), and mRNA expression levels were determined by qRT-PCR (n = 3, mean ± S.D.; **, p < 0.01, Student's t test versus siRNA-control). G, ChIP assays were performed using liver tissues of Tg-CYP2D6 mice collected at different gestational time points (n = 4, mean ± S.D.; *, p < 0.05; **, p < 0.01, one-way ANOVA versus virgin).
HNF4α activity can be functionally modulated by interaction with other transcription factors (24). To determine whether HNF4α activity on CYP2D6 is enhanced in mouse livers during pregnancy, in vivo ChIP assays were performed. To this end, liver tissues were collected from Tg-CYP2D6 mice at different gestational time points and subjected to ChIP using antibodies against HNF4α or RNA polymerase II (a positive control for CYP2D6 transcription). The protein-bound DNA was measured using a primer set that can detect the HNF4α response element at −55/−43 of CYP2D6 (22). The results showed that HNF4α and polymerase II recruitment to the CYP2D6 promoter significantly increased at term as compared with the pre-pregnancy level (Fig. 2B), indicating increased HNF4α activity during pregnancy.
SHP is known to repress HNF4α transactivation of a HNF4α target gene (i.e. CYP8B1) (25). To examine whether SHP expression is altered during pregnancy, mRNA and protein levels of SHP were examined in liver tissues collected at different gestational time points. The results showed significantly decreased SHP expression at term as compared with pre-pregnancy or postpartum period (Fig. 2, C and D).
To determine the effects of SHP on HNF4α transactivation of CYP2D6 promoter, luciferase assays were performed in HEK293T cells where basal expression of HNF4α is minimal. Transient transfection of HNF4α led to a significant increase in CYP2D6 promoter activity as expected (Fig. 2E). SHP repressed HNF4α transactivation of the promoter in a SHP concentration-dependent manner (Fig. 2E), indicating that SHP is a repressor of CYP2D6 promoter potentially by repressing HNF4α action.
To mimic the state of pregnancy, where SHP expression is decreased, we knocked down SHP expression by using siRNA and examined its effects on CYP2D6 expression. Tg-CYP2D6 mice injected with SHP-targeting siRNA exhibited substantial knockdown of SHP and at the same time a marked increase in CYP2D6 expression when compared with those injected with scrambled siRNA (Fig. 2F). These results suggest that repressed SHP expression plays a role in the up-regulation of CYP2D6 promoter activity during pregnancy.
To determine whether the enhanced HNF4α activity on CYP2D6 promoter during pregnancy (Fig. 2B) is accompanied by any changes in SHP recruitment to the promoter, in vivo ChIP assays were performed. Liver tissues collected at different gestational time points were subjected to ChIP using antibodies against SHP. The results showed that SHP recruitment to the CYP2D6 promoter significantly decreased at term (Fig. 2G). Together, these results suggest that down-regulated SHP expression during pregnancy de-represses HNF4α activity on CYP2D6 promoter.
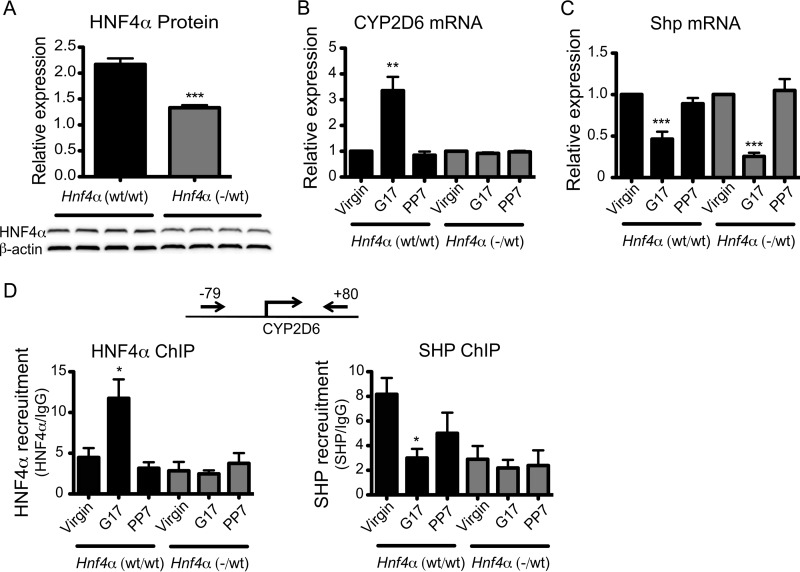
CYP2D6 Induction during Pregnancy Is Abrogated in Hnf4α(−/wt) Mice
To verify the importance of HNF4α for CYP2D6 induction during pregnancy, we examined whether reduced hepatic HNF4α expression influences the extent of CYP2D6 induction during pregnancy. Complete deletion of Hnf4α even when localized to the liver is fatal due to disruption of hepatic lipid homeostasis (17). Thus, Tg-CYP2D6 mice were crossed with heterozygous AlbCre;Hnf4α fl/wt mice, generating pups carrying CYP2D6 as well as either one or two copies of the Hnf4α allele in the liver (called Hnf4α(−/wt) and Hnf4α(wt/wt), respectively), and the extent of CYP2D6 induction during pregnancy was compared. None of these mice exhibited any prominent phenotypes, and all grew normally. The hepatic protein level of HNF4α in Hnf4α(−/wt) mice was about half that of the littermate control, as expected (Fig. 3A). In Hnf4α(−/wt) mice, CYP2D6 induction during pregnancy was completely abrogated (Fig. 3B), and the enhanced HNF4α recruitment to CYP2D6 promoter disappeared (Fig. 3D, left). On the other hand, pregnancy-mediated repression of Shp was maintained in Hnf4α(−/wt) mice (Fig. 3C), while decreased recruitment of SHP to the CYP2D6 promoter was abrogated in the mice (Fig. 3D, right). Together, these results indicate that CYP2D6 induction during pregnancy is highly dependent on HNF4α expression levels and suggest an essential role of HNF4α in mediating CYP2D6 induction by SHP during pregnancy.
FIGURE 3.
Pregnancy-mediated CYP2D6 induction was abrogated in Hnf4α(−/wt) mice. A, liver tissues were collected from Hnf4α(wt/wt) and Hnf4α(−/wt) mice at pre-pregnancy. HNF4α protein expression levels were determined by a Western blot (bottom). The signal intensity was measured by densitometry and expressed relative to that of β-actin (top) (n = 4, mean ± S.D.; ***, p < 0.001, Student's t test versus Hnf4α(wt/wt) mice). B and C, mRNA levels of CYP2D6 (B) and Shp (C) in the liver tissues were determined by qRT-PCR (n = 4, mean ± S.D.; **, p < 0.01; ***, p < 0.001, one-way ANOVA versus virgin). D, ChIP assays were performed in liver tissues collected at different gestational time points (n = 4, mean ± S.D.; *, p < 0.05, one-way ANOVA versus virgin).
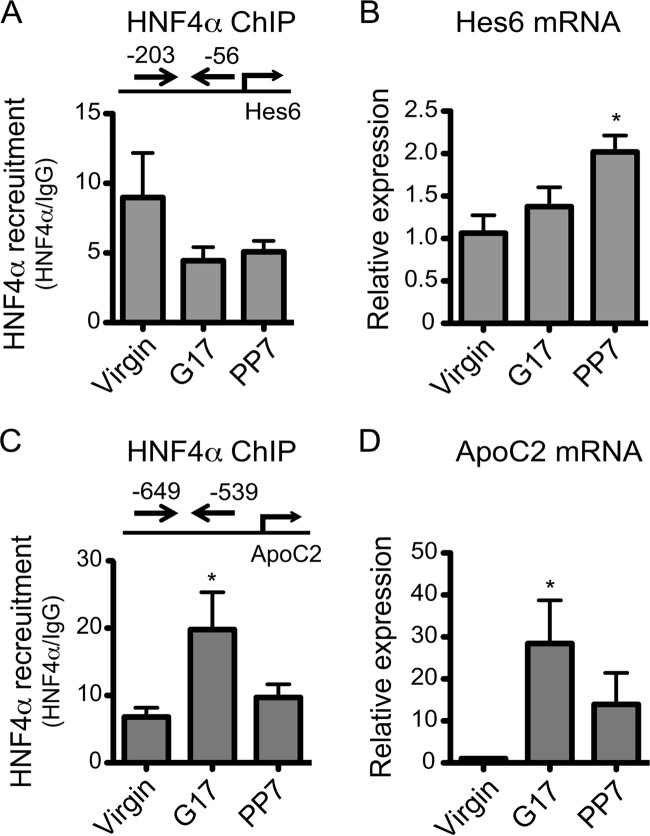
To determine whether pregnancy enhances HNF4α activity on other target genes of HNF4α, HNF4α recruitment to promoters of two other known target genes (Hes6 (26) and ApoC2 (27, 28)) was examined in the mouse livers at different gestational time points. HNF4α recruitment to the Hes6 promoter was not affected by pregnancy (Fig. 4A), in agreement with insignificant changes in the mRNA level of Hes6 at term as compared with pre-pregnancy levels (Fig. 4B). At postpartum, however, Hes6 mRNA level was significantly increased, potentially due to altered expression and/or activities of transcription factors known to regulate Hes6 expression, such as Ascl1, Myod, and Clock/Bmal1 (29–31). On the other hand, HNF4α recruitment to the ApoC2 promoter was increased at term (Fig. 4C), but the extent of the increase (∼2-fold) was much less than that observed for ApoC2 mRNA (>20-fold) (Fig. 4D), suggesting a relatively minor role of HNF4α in enhanced ApoC2 expression during pregnancy. Together, these results suggest that the regulatory effects of pregnancy on the expression of HNF4α target genes are likely gene-specific.
FIGURE 4.
Effects of pregnancy on HNF4α activity are target gene-specific. A and C, HNF4α recruitment to Hes6 (A) and Apoc2 (C) promoter was analyzed by ChIP assay (n = 4, mean ± S.D.; *, p < 0.05, one-way ANOVA versus virgin). B and D, mRNA expression of Hes6 (B) and Apoc2 (D) was determined by qRT-PCR (n = 4, mean ± S.D.; *, p < 0.05, one-way ANOVA versus virgin).
Hepatic FXR Activity Is Enhanced at Postpartum
To identify potential the upstream regulator(s) responsible for the changes in SHP expression during pregnancy, the effects of pregnancy on factors previously known to modulate SHP expression were examined.
SHP is a representative target gene of FXR, a nuclear receptor activated upon binding to bile acids (32). To determine whether altered expression and/or activity of FXR is responsible for SHP repression during pregnancy, the expression levels of FXR as well as FXR recruitment to the Shp promoter were examined in the livers of Tg-CYP2D6 mice collected at different gestational time points. FXR expression showed insignificant changes during pregnancy (Fig. 5A), in agreement with a previous report (33). Also, ChIP results showed that FXR recruitment to the Shp promoter did not decrease at term as compared with pre-pregnancy (Fig. 5B), indicating an insignificant role for FXR in reduced SHP expression during pregnancy. Of note, FXR recruitment to Shp promoter increased at postpartum (Fig. 5B), suggesting a potential role for FXR in the return of decreased SHP expression to pre-pregnancy levels. Expression levels of other known regulators of SHP expression, liver X receptor (LXR) (34) and liver receptor homolog-1 (LRH-1) (35), were not affected by pregnancy (data not shown).
FIGURE 5.
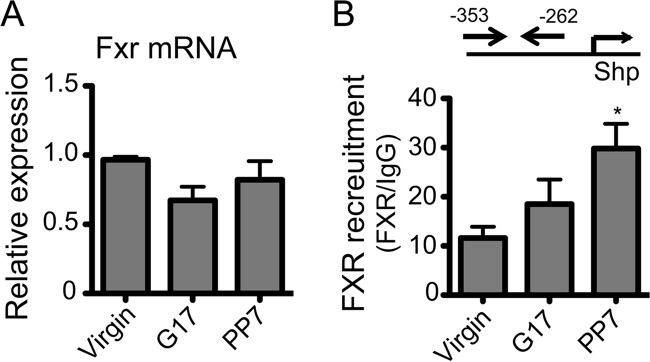
FXR expression and activity do not decrease during pregnancy. Liver tissues of Tg-CYP2D6 mice were collected at pre-pregnancy (Virgin), 17 days of pregnancy (G17), and 7 days postpartum (PP7). A, mRNA expression was determined by qRT-PCR (n = 4, mean ± S.D.). B, FXR recruitment to Shp promoter was analyzed by a ChIP assay (n = 4, mean ± S.D.; *, p < 0.05, one-way ANOVA versus virgin).
Retinoic Acid Modulates CYP2D6 Expression
A previous study reported that atRA (the bioactive form of vitamin A) induces SHP expression in HepG2 cells (36). To examine whether changes in RA levels are potentially responsible for CYP2D6 induction during pregnancy, hepatic levels of the two predominant RA isomers atRA and 13cRA were measured by using liquid chromatography-mass spectrometry. The results revealed significantly decreased hepatic levels of atRA at term as compared with those at pre-pregnancy (Fig. 6A, left). A similar finding was observed for 13cRA (Fig. 6A, right). This suggests that the decreased hepatic RA content may be in part responsible for the reduced SHP expression observed during pregnancy.
FIGURE 6.
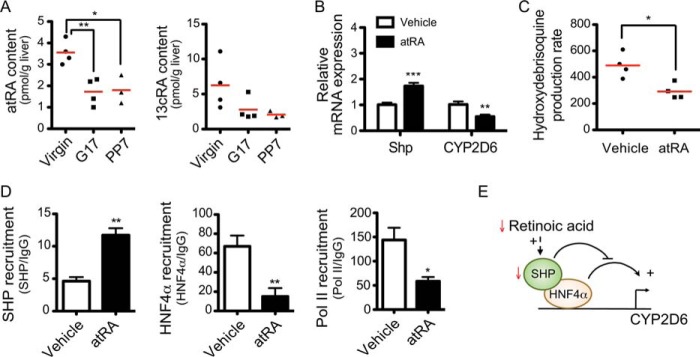
atRA represses CYP2D6 expression. A, hepatic levels of atRA and 13cRA in mouse liver tissues (n = 3–4; *, p < 0.05; **, p < 0.01, one-way ANOVA versus virgin). B, Tg-CYP2D6 mice were injected with atRA (5 mg/kg/day intraperitoneally for 5 days). CYP2D6 and Shp mRNA expression was determined by qRT-PCR. C, liver S9 fractions were prepared from the liver tissues of Tg-CYP2D6 mice treated with vehicle or atRA, and CYP2D6 phenotyping was performed using debrisoquine (200 μm) (n = 4, mean ± S.D.; *, p < 0.05, Student's t test versus vehicle). Data shown are the metabolite production rate in pmol/min/mg of protein. D, recruitment of SHP, HNF4α, and RNA polymerase II to CYP2D6 promoter was analyzed by ChIP assay (n = 4, mean ± S.D.; *, p < 0.05; **, p < 0.01, Student's t test versus vehicle). E, proposed model for CYP2D6 induction during pregnancy. The red arrows indicate pregnancy-related changes in the hepatic contents or expression levels.
To determine whether RA alters CYP2D6 expression and activity in vivo, atRA was intraperitoneally administered to Tg-CYP2D6 mice, and livers were collected to measure expression and activity of hepatic CYP2D6. atRA significantly decreased CYP2D6 in mRNA levels, and this was accompanied by enhanced SHP expression (Fig. 6B). Results from CYP2D6 phenotyping (by using debrisoquine as a probe drug for CYP2D6) revealed a significant decrease in CYP2D6 activity upon atRA treatment as compared with vehicle treatment (Fig. 6C). To determine whether the repressive effect of atRA on CYP2D6 is mediated by changes in HNF4α activity on CYP2D6 promoter, a ChIP assay was performed using liver tissues of Tg-CYP2D6 mice administered with atRA. The results showed that atRA decreased the recruitment of HNF4α as well as polymerase II to the CYP2D6 promoter as compared with the vehicle-treated control while increasing SHP recruitment to the promoter (Fig. 6D). A model depicting the proposed mechanisms underlying CYP2D6 induction during pregnancy is shown in Fig. 6E.
DISCUSSION
CYP2D6-mediated drug metabolism is increased during pregnancy, but the underlying mechanisms remained unknown. Results from a previous study in human hepatocytes indicate that estrogen and progesterone (whose concentrations increase >100-fold during human pregnancy) do not affect CYP2D6 expression (37), suggesting that female hormones play minor roles (if any) in CYP2D6 induction during pregnancy. In this study, using Tg-CYP2D6 mice as an in vivo model, we provide evidence that CYP2D6 induction during pregnancy is coordinated by liver-enriched transcription factors SHP and HNF4α and that this is in part triggered by the altered hepatic RA contents. This study represents the first report on the mechanisms underlying CYP2D6 induction during pregnancy.
The identification of mechanisms underlying altered drug metabolism during pregnancy has been challenging due to the lack of appropriate experimental models. In this study we demonstrate that Tg-CYP2D6 mice can serve as an in vivo model for CYP2D6 induction during pregnancy, achieving a major breakthrough for the mechanistic studies of pregnancy-mediated changes in drug metabolism. An improved understanding of these mechanisms may provide a basis to develop physiologically based pharmacokinetic models (38) that can be used to predict dosage adjustments required for pregnant women. This underscores the necessity and utility of appropriate in vivo mouse models for CYP2D6 regulation during pregnancy.
Our mechanistic studies reveal that repressed SHP expression during pregnancy leads to enhanced HNF4α activity on CYP2D6 promoter. SHP is a nuclear receptor lacking the DNA binding domain and represses the activity of multiple transcription factors (39). A previous study has shown that SHP binds to N-terminal region of HNF4α, thereby blocking HNF4α binding to the promoter of a target gene (40). Consistent with the reported mechanism, our ChIP results from Tg-CYP2D6 mice showed increased HNF4α recruitment to CYP2D6 promoter at term pregnancy accompanied by decreased the recruitment of SHP to the promoter. The reciprocal relationship between HNF4α and SHP recruitment to CYP2D6 promoter is likely due to the rapid and dynamic interaction between DNA and transcription factors (41). Of note, in the repression of HNF4α transactivation of CYP2D6 by SHP, an appropriate expression level of HNF4α appears to be critical; the pregnancy-mediated changes in CYP2D6 expression or in SHP recruitment to CYP2D6 promoter were abrogated in Hnf4α(wt/−) mice. HNF4α expression is known to decrease during hyperinsulinemia accompanying diabetes (42). Whether diabetes during pregnancy (affecting ∼18% of pregnancies) impacts the extent of CYP2D6 induction remains to be determined.
The pregnancy-mediated changes in HNF4α activity apparently affect only a subset of HNF4α target genes (including CYP2D6) because mRNA levels of other target genes (ApoC2 and Hes6) as well as HNF4α recruitment to their promoters exhibited patterns different from those of CYP2D6. These findings are in part consistent with the previous ChIP results that SHP associates with only a subset of the promoters occupied by HNF4α (43). The promoter context may provide additional specificity of SHP-mediated repression of HNF4α action.
FXR is a key mediator in maintenance of cholesterol/bile acid homeostasis; during cholestasis, bile acids bind to FXR, which transactivates SHP promoter. SHP in turn represses the expression of genes involved in bile acid synthesis (43). Hepatic Shp mRNA levels in Fxr-null mice was only ∼80% of those in wild type (32), suggesting that FXR plays a key role in modulating SHP expression. Our data indicate, however, that pregnancy affects neither FXR expression nor FXR recruitment to Shp promoter. Furthermore, pregnancy did not affect expression of protein arginine methyltransferase type I (PRMT-1), a chromatin modifier that is known to modulate FXR transactivation without altering FXR recruitment to its target gene promoters (44) (data not shown). Together, these results indicate that FXR plays a minimal role, if any, in the repressed SHP expression during pregnancy.
Repressed SHP expression at term pregnancy was associated with decreased hepatic levels of RA. The decrease in hepatic contents of RA may be attributable to enhanced hepatic expression of CYP26A1 during pregnancy (45). CYP26A1 expressed in livers is a major RA-metabolizing enzyme and a key contributor to endogenous RA clearance (46). How pregnancy increases Cyp26a1 expression remains unknown. atRA was shown to induce SHP expression in HepG2 cells (36), and our results further showed that exogenously administered atRA decreases CYP2D6 expression. Together, these results suggest that decreased hepatic RA contents and subsequent decreases in SHP expression may be in part responsible for enhanced CYP2D6 expression during pregnancy.
The repressed SHP expression levels at term returned to pre-pregnancy levels after delivery, whereas hepatic levels of RA did not. This suggests the presence of other regulators of SHP expression whose expression and/or activity may be altered during the postpartum period. Our ChIP study demonstrated a significant increase in FXR recruitment to the Shp promoter at postpartum, suggesting that activation of FXR may be responsible for the rebound in SHP expression after delivery. Of note, expression levels of other target genes of FXR (including Bsep) did not differ between the term pregnancy and postpartum period (data not shown), indicating that enhanced FXR activity after delivery may be specific for Shp promoter. Shp promoter harbors an ERα-binding site overlapping with the FXR-binding site (47) such that ligand-activated ERα can repress FXR transactivation (33). After delivery, plasma concentrations of both estrogens and bile acids change in the direction of promoting FXR transactivation; concentrations of estrogen decrease, whereas those of bile acid increase (48). These changes may lead to increased FXR transactivation of Shp promoter in an additive or synergistic manner. Together, SHP expression level during postpartum period may be coordinated by multiple endogenous substances, including estrogens and bile acids.
CYP2D6 shows the largest phenotypic variability among drug-metabolizing CYPs. This is in part due to genetic polymorphisms of CYP2D6; >100 different variants have been reported to date, and some of the gene products exhibit reduced or no enzyme activity. On the other hand, CYP2D6 activity in human liver tissues correlates well with CYP2D6 mRNA levels (the correlation coefficient ranging from 0.71 to 0.91) (49–51), suggesting that transcriptional regulation of CYP2D6 may play a major role in governing the extent of CYP2D6-mediated drug metabolism. The finding of interplay among retinoic acid and hepatic transcription factors HNF4α and SHP during pregnancy may provide a basis to better understand the factors contributing to large phenotypic variability in CYP2D6 activity. For example, one can speculate that vitamin A deficiency or overload may impact CYP2D6-mediated drug metabolism through modulating SHP expression.
In conclusion, we present the first case of utilizing CYP-humanized mice for the in vivo functional and mechanistic investigation of altered drug metabolism during pregnancy. Our results will likely provide a basis to improve drug therapy during pregnancy as well as to better understand factors governing variability in CYP2D6-mediated drug metabolism.
Acknowledgments
We thank Drs. Timothy Tracy, Wooin Lee, Hyunwoo Lee, and Jennifer Chang for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HD065532 and Fellowship K12HK055892 (NICHD; to H. J.).

This article contains supplemental Table S1.
- CYP
- cytochrome P450
- Tg
- transgenic
- RA
- retinoic acid
- atRA
- all-trans-retinoic acid
- 13cRA
- 13-cis-retinoic acid
- HNF4α
- hepatocyte nuclear factor 4α
- SHP
- small heterodimer partner
- FXR
- farnesoid X receptor
- qRT
- quantitative real-time
- ANOVA
- analysis of variance.
REFERENCES
- 1. Andrade S. E., Gurwitz J. H., Davis R. L., Chan K. A., Finkelstein J. A., Fortman K., McPhillips H., Raebel M. A., Roblin D., Smith D. H., Yood M. U., Morse A. N., Platt R. (2004) Prescription drug use in pregnancy. Am. J. Obstet. Gynecol. 191, 398–407 [DOI] [PubMed] [Google Scholar]
- 2. Glover D. D., Amonkar M., Rybeck B. F., Tracy T. S. (2003) Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am. J. Obstet. Gynecol. 188, 1039–1045 [DOI] [PubMed] [Google Scholar]
- 3. Isoherranen N., Thummel K. E. (2013) Drug metabolism and transport during pregnancy. How does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab. Dispos. 41, 256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jover R., Moya M., Gómez-Lechón M. J. (2009) Transcriptional regulation of cytochrome p450 genes by the nuclear receptor hepatocyte nuclear factor 4-α. Curr. Drug. Metab. 10, 508–519 [DOI] [PubMed] [Google Scholar]
- 5. Yu A. M., Idle J. R., Gonzalez F. J. (2004) Polymorphic cytochrome P450 2D6. Humanized mouse model and endogenous substrates. Drug Metab. Rev. 36, 243–277 [DOI] [PubMed] [Google Scholar]
- 6. Zanger U. M., Raimundo S., Eichelbaum M. (2004) Cytochrome P450 2D6. Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch. Pharmacol. 369, 23–37 [DOI] [PubMed] [Google Scholar]
- 7. Yu A. M., Idle J. R., Byrd L. G., Krausz K. W., Küpfer A., Gonzalez F. J. (2003) Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics 13, 173–181 [DOI] [PubMed] [Google Scholar]
- 8. Kurth M. C., Kurth J. H. (1993) Variant cytochrome P450 CYP2D6 allelic frequencies in Parkinson's disease. Am. J. Med. Genet. 48, 166–168 [DOI] [PubMed] [Google Scholar]
- 9. Högstedt S., Lindberg B., Peng D. R., Regårdh C. G., Rane A. (1985) Pregnancy-induced increase in metoprolol metabolism. Clin. Pharmacol. Ther. 37, 688–692 [DOI] [PubMed] [Google Scholar]
- 10. Wadelius M., Darj E., Frenne G., Rane A. (1997) Induction of CYP2D6 in pregnancy. Clin. Pharmacol. Ther. 62, 400–407 [DOI] [PubMed] [Google Scholar]
- 11. Tracy T. S., Venkataramanan R., Glover D. D., Caritis S. N., and National Institute for Child Health and Human Development Network of Maternal-Fetal-Medicine Units (2005) Temporal changes in drug metabolism (CYP1A2, CYP2D6, and CYP3A activity) during pregnancy. Am. J. Obstet. Gynecol. 192, 633–639 [DOI] [PubMed] [Google Scholar]
- 12. Sit D. K., Perel J. M., Helsel J. C., Wisner K. L. (2008) Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J. Clin. Psychiatry 69, 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickmann L. J., Tay S., Senn T. D., Zhang H., Visone A., Unadkat J. D., Hebert M. F., Isoherranen N. (2008) Changes in maternal liver Cyp2c and Cyp2d expression and activity during rat pregnancy. Biochem. Pharmacol. 75, 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koh K. H., Xie H., Yu A. M., Jeong H. (2011) Altered cytochrome P450 expression in mice during pregnancy. Drug Metab. Dispos. 39, 165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson M. D., Barbosa-Morais N. L., Schmidt D., Conboy C. M., Vanes L., Tybulewicz V. L., Fisher E. M., Tavaré S., Odom D. T. (2008) Species-specific transcription in mice carrying human chromosome 21. Science 322, 434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corchero J., Granvil C. P., Akiyama T. E., Hayhurst G. P., Pimprale S., Feigenbaum L., Idle J. R., Gonzalez F. J. (2001) The CYP2D6 humanized mouse. Effect of the human CYP2D6 transgene and HNF4α on the disposition of debrisoquine in the mouse. Mol. Pharmacol. 60, 1260–1267 [DOI] [PubMed] [Google Scholar]
- 17. Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. (2001) Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Felmlee M. A., Lon H. K., Gonzalez F. J., Yu A. M. (2008) Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab. Dispos. 36, 435–441 [DOI] [PubMed] [Google Scholar]
- 19. Fang S., Miao J., Xiang L., Ponugoti B., Treuter E., Kemper J. K. (2007) Coordinated recruitment of histone methyltransferase G9a and other chromatin-modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol. Cell. Biol. 27, 1407–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnold S. L., Amory J. K., Walsh T. J., Isoherranen N. (2012) A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J. Lipid Res. 53, 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S. S., Cha E. Y., Jung H. J., Shon J. H., Kim E. Y., Yeo C. W., Shin J. G. (2008) Genetic polymorphism of hepatocyte nuclear factor-4α influences human cytochrome P450 2D6 activity. Hepatology 48, 635–645 [DOI] [PubMed] [Google Scholar]
- 22. Cairns W., Smith C. A., McLaren A. W., Wolf C. R. (1996) Characterization of the human cytochrome P4502D6 promoter. A potential role for antagonistic interactions between members of the nuclear receptor family. J. Biol. Chem. 271, 25269–25276 [DOI] [PubMed] [Google Scholar]
- 23. Sweeney T. R., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. (2006) Decreased nuclear hormone receptor expression in the livers of mice in late pregnancy. Am. J. Physiol. Endocrinol. Metab. 290, E1313–E1320 [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez F. J. (2008) Regulation of hepatocyte nuclear factor 4 α-mediated transcription. Drug Metab. Pharmacokinet. 23, 2–7 [DOI] [PubMed] [Google Scholar]
- 25. Zhang M., Chiang J. Y. (2001) Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1). Roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J. Biol. Chem. 276, 41690–41699 [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Jimenez C. P., Kyrmizi I., Cardot P., Gonzalez F. J., Talianidis I. (2010) Hepatocyte nuclear factor 4α coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol. Cell. Biol. 30, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garrison W. D., Battle M. A., Yang C., Kaestner K. H., Sladek F. M., Duncan S. A. (2006) Hepatocyte nuclear factor 4α is essential for embryonic development of the mouse colon. Gastroenterology 130, 1207–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nikolaidou-Neokosmidou V., Zannis V. I., Kardassis D. (2006) Inhibition of hepatocyte nuclear factor 4 transcriptional activity by the nuclear factor κB pathway. Biochem. J. 398, 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson B. R., Hartman B. H., Ray C. A., Hayashi T., Bermingham-McDonogh O., Reh T. A. (2009) Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev. Dyn. 238, 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee Y. J., Han D. H., Pak Y. K., Cho S. H. (2012) Circadian regulation of low density lipoprotein receptor promoter activity by CLOCK/BMAL1, Hes1, and Hes6. Exp. Mol. Med. 44, 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malone C. M., Domaschenz R., Amagase Y., Dunham I., Murai K., Jones P. H. (2011) Hes6 is required for actin cytoskeletal organization in differentiating C2C12 myoblasts. Exp. Cell Res. 317, 1590–1602 [DOI] [PubMed] [Google Scholar]
- 32. Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 [DOI] [PubMed] [Google Scholar]
- 33. Milona A., Owen B. M., Cobbold J. F., Willemsen E. C., Cox I. J., Boudjelal M., Cairns W., Schoonjans K., Taylor-Robinson S. D., Klomp L. W., Parker M. G., White R., van Mil S. W., Williamson C. (2010) Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology 52, 1341–1349 [DOI] [PubMed] [Google Scholar]
- 34. Goodwin B., Watson M. A., Kim H., Miao J., Kemper J. K., Kliewer S. A. (2003) Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-α. Mol. Endocrinol. 17, 386–394 [DOI] [PubMed] [Google Scholar]
- 35. Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6, 507–515 [DOI] [PubMed] [Google Scholar]
- 36. Cai S. Y., He H., Nguyen T., Mennone A., Boyer J. L. (2010) Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J. Lipid Res. 51, 2265–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi S. Y., Koh K. H., Jeong H. (2013) Isoform-specific Regulation of Cytochromes P450 Expression by Estradiol and Progesterone. Drug Metab. Dispos. 41, 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dickmann L. J., Isoherranen N. (2013) Quantitative prediction of CYP2B6 induction by estradiol during pregnancy. Potential explanation for increased methadone clearance during pregnancy. Drug Metab. Dispos. 41, 270–274 [DOI] [PubMed] [Google Scholar]
- 39. Chanda D., Park J. H., Choi H. S. (2008) Molecular basis of endocrine regulation by orphan nuclear receptor small heterodimer partner. Endocr. J. 55, 253–268 [DOI] [PubMed] [Google Scholar]
- 40. Shimamoto Y., Ishida J., Yamagata K., Saito T., Kato H., Matsuoka T., Hirota K., Daitoku H., Nangaku M., Yamagata K., Fujii H., Takeda J., Fukamizu A. (2004) Inhibitory effect of the small heterodimer partner on hepatocyte nuclear factor-4 mediates bile acid-induced repression of the human angiotensinogen gene. J. Biol. Chem. 279, 7770–7776 [DOI] [PubMed] [Google Scholar]
- 41. Bagamasbad P., Ziera T., Borden S. A., Bonett R. M., Rozeboom A. M., Seasholtz A., Denver R. J. (2012) Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology 153, 5334–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie X., Liao H., Dang H., Pang W., Guan Y., Wang X., Shyy J. Y., Zhu Y., Sladek F. M. (2009) Down-regulation of hepatic HNF4α gene expression during hyperinsulinemia via SREBPs. Mol. Endocrinol. 23, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boulias K., Katrakili N., Bamberg K., Underhill P., Greenfield A., Talianidis I. (2005) Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 24, 2624–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rizzo G., Renga B., Antonelli E., Passeri D., Pellicciari R., Fiorucci S. (2005) The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol. Pharmacol. 68, 551–558 [DOI] [PubMed] [Google Scholar]
- 45. Topletz A. R., Le H. N., Lee N., Chapman J. D., Kelly E. J., Wang J., Isoherranen N. (2013) Hepatic Cyp2d and Cyp26a1 mRNAs and activities are increased during mouse pregnancy. Drug Metab. Dispos. 41, 312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thatcher J. E., Isoherranen N. (2009) The role of CYP26 enzymes in retinoic acid clearance. Expert Opin. Drug Metab. Toxicol. 5, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lai K., Harnish D. C., Evans M. J. (2003) Estrogen receptor α regulates expression of the orphan receptor small heterodimer partner. J. Biol. Chem. 278, 36418–36429 [DOI] [PubMed] [Google Scholar]
- 48. Heikkinen J., Mäentausta O., Ylöstalo P., Jänne O. (1981) Changes in serum bile acid concentrations during normal pregnancy, in patients with intrahepatic cholestasis of pregnancy, and in pregnant women with itching. Br. J. Obstet. Gynaecol. 88, 240–245 [PubMed] [Google Scholar]
- 49. Temesvári M., Kóbori L., Paulik J., Sárváry E., Belic A., Monostory K. (2012) Estimation of drug-metabolizing capacity by cytochrome P450 genotyping and expression. J Pharmacol. Exp. Ther. 341, 294–305 [DOI] [PubMed] [Google Scholar]
- 50. Carcillo J. A., Adedoyin A., Burckart G. J., Frye R. F., Venkataramanan R., Knoll C., Thummel K., Roskos L., Wilson J. W., Sereika S., Romkes M., Bebia Z., Branch R. A. (2003) Coordinated intrahepatic and extrahepatic regulation of cytochrome p4502D6 in healthy subjects and in patients after liver transplantation. Clin. Pharmacol. Ther. 73, 456–467 [DOI] [PubMed] [Google Scholar]
- 51. Ohtsuki S., Schaefer O., Kawakami H., Inoue T., Liehner S., Saito A., Ishiguro N., Kishimoto W., Ludwig-Schwellinger E., Ebner T., Terasaki T. (2012) Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver. Comparison with mRNA levels and activities. Drug Metab. Dispos. 40, 83–92 [DOI] [PubMed] [Google Scholar]