Background: TGF-β is a mediator of lung diseases treated with glucocorticoids, but TGF-β/glucocorticoid interactions in the lung have not been studied.
Results: Glucocorticoids drive Smad1 and inhibit Smad3 TGF-β signaling in lung cells.
Conclusion: Glucocorticoids modulate pulmonary TGF-β signaling in vitro and in vivo.
Significance: Glucocorticoid effects on TGF-β signaling are relevant drug/cell interactions and may be relevant drug/disease interactions.
Keywords: Fibroblast, Glucocorticoids, Lung, SMAD Transcription Factor, Transforming Growth Factor Beta (TGFbeta)
Abstract
Glucocorticoids represent the mainstay therapy for many lung diseases, providing outstanding management of asthma but performing surprisingly poorly in patients with acute respiratory distress syndrome, chronic obstructive pulmonary disease, lung fibrosis, and blunted lung development associated with bronchopulmonary dysplasia in preterm infants. TGF-β is a pathogenic mediator of all four of these diseases, prompting us to explore glucocorticoid/TGF-β signaling cross-talk. Glucocorticoids, including dexamethasone, methylprednisolone, budesonide, and fluticasone, potentiated TGF-β signaling by the Acvrl1/Smad1/5/8 signaling axis and blunted signaling by the Tgfbr1/Smad2/3 axis in NIH/3T3 cells, as well as primary lung fibroblasts, smooth muscle cells, and endothelial cells. Dexamethasone drove expression of the accessory type III TGF-β receptor Tgfbr3, also called betaglycan. Tgfbr3 was demonstrated to be a “switch” that blunted Tgfbr1/Smad2/3 and potentiated Acvrl1/Smad1 signaling in lung fibroblasts. The Acvrl1/Smad1 axis, which was stimulated by dexamethasone, was active in lung fibroblasts and antagonized Tgfbr1/Smad2/3 signaling. Dexamethasone acted synergistically with TGF-β to drive differentiation of primary lung fibroblasts to myofibroblasts, revealed by acquisition of smooth muscle actin and smooth muscle myosin, which are exclusively Smad1-dependent processes in fibroblasts. Administration of dexamethasone to live mice recapitulated these observations and revealed a lung-specific impact of dexamethasone on lung Tgfbr3 expression and phospho-Smad1 levels in vivo. These data point to an interesting and hitherto unknown impact of glucocorticoids on TGF-β signaling in lung fibroblasts and other constituent cell types of the lung that may be relevant to lung physiology, as well as lung pathophysiology, in terms of drug/disease interactions.
Introduction
Glucocorticoids are endogenously produced steroid hormones such as cortisol that bind to the ubiquitously expressed glucocorticoid receptor and thereby regulate the expression of glucocorticoid-responsive genes. In this way, glucocorticoids influence a broad spectrum of physiological processes, including fat, protein and carbohydrate metabolism, and inflammation (1, 2). Synthetic glucocorticoids, including the earlier generation drugs dexamethasone and methylprednisolone and later generation budesonide and fluticasone, by virtue of their anti-inflammatory and other properties, have found widespread clinical application (1).
Although widely and successfully used in respiratory medicine, for example in the management of obstructive airway diseases such as asthma (3, 4) and antenatal use in pregnant women at risk for preterm birth (5), glucocorticoids have performed surprisingly poorly in the management of other respiratory diseases, including stable chronic obstructive pulmonary disease (6, 7), the acute respiratory distress syndrome (8, 9), and lung fibrosis (10), as well as in the postnatal management of bronchopulmonary dysplasia (11–14), where the use of glucocorticoid therapy cannot be currently recommended and may even be deleterious and dangerous. The failure of glucocorticoids in the context of stable chronic obstructive pulmonary disease, lung fibrosis, acute respiratory distress syndrome, and bronchopulmonary dysplasia therapy may well reflect (i) our somewhat limited understanding of the disease mechanisms at play, (ii) our limited understanding of alternative as yet undiscovered activities of these powerful steroids, and (iii) a lack of consideration of the interaction between glucocorticoids and disease mechanisms.
Although the anti-inflammatory properties of glucocorticoids have been well characterized, less attention has been paid to the impact of glucocorticoids on other pathological signaling pathways (2). Among these pathways, signaling by the TGF-β family of polypeptide growth factors has been ascribed a key role, not only in the regulation of inflammation (15), but also in the pathophysiological mechanisms at play in several lung diseases. In lung fibrosis, the pro-fibrotic activities of TGF-β drive the production of the fibrotic mediator connective tissue growth factor and plasminogen activator inhibitor-1 (encoded by the SERPINE1 gene in humans) by lung fibroblasts and promote fibroblast to pathological myofibroblast differentiation (16). TGF-β also drives aberrant production of extracellular matrix molecules such pro-collagen, which limit alveolar repair and proper alveolar development in diseases such as chronic obstructive pulmonary disease (17–19), lung fibrosis (17, 20), acute respiratory distress syndrome (21), and bronchopulmonary dysplasia (22, 23). In general, matrix production relies on TGF-β signaling by the type I TGF-β receptor Tgfbr1 (also called Alk-5), in complex with the type II receptor (Tgfbr2), which together recruit the downstream signaling molecules Smad2 and Smad3 to transduce signals to the nucleus, regulating the expression of TGF-β-responsive genes in the so-called “Tgfbr1/Smad2/3 axis” (24–26).
In the pulmonary vasculature and systemic circulation, an alternative type 1 TGF-β receptor Acvrl1 (also called Alk-1) similarly recruits Smad1 (and perhaps Smad5 and Smad8) to drive expression of a different subset of TGF-β-responsive genes via the “Acvrl1/Smad1 axis” (27–29). This Acvrl1/Smad1 axis is thought to play a role in pulmonary vascular diseases such as such as pulmonary hypertension and the hereditary hemorrhagic telangiectasias (27). The Acvrl1/Smad1 axis, which can promote the acquisition of smooth muscle actin and smooth muscle myosin in several cell types, including lung fibroblasts, is emerging as a regulator of fibroblast to myofibroblast differentiation (30–36). Several accessory molecules, including the inhibitory Smad proteins Smad6 and Smad7, as well as the accessory type III TGF-β receptors endoglin (also called CD105) and Tgfbr3 (also called betaglycan) (37), play poorly defined regulatory roles, although Tgfbr3 has emerged as a mediator of cancer progression (38) and epithelial to mesenchymal differentiation (39). Although both the glucocorticoid and TGF-β signaling pathways have been well characterized individually, few studies to date have addressed the intersection of the TGF-β and glucocorticoid signaling pathways in pulmonary physiology. Such information may prove useful, both to further our understanding of how the TGF-β system may be regulated by endogenous glucocorticoids such as cortisol, as well as possible (deleterious or desired) drug-disease interactions, given the widespread use of glucocorticoids in the management of lung and other diseases.
In this study, it was hypothesized that the TGF-β signaling pathway, which plays a key pathological role in a broad spectrum of restrictive and obstructive lung diseases, is impacted by glucocorticoids, which are a class of drugs that are widely but generally unsuccessfully (with the exception of asthma) used to treat these same diseases. To address this idea, the influence of glucocorticoids on TGF-β signaling was assessed in vitro in a mouse fibroblast cell line and primary lung fibroblasts and in vivo in C57BL/6J mice. These investigations revealed that four widely used synthetic glucocorticoids dramatically impact TGF-β signaling in lung fibroblasts, shifting the balance of TGF-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis. Mechanistically, glucocorticoids impacted the expression of components of the TGF-β signaling machinery. In particular, glucocorticoids recruited Tgfbr3, which acted as a switch between the two TGF-β signaling axes, to inhibit Tgfbr1/Smad2/3-driven processes and promote Acvrl1/Smad1-driven signaling. By redirecting TGF-β signaling, glucocorticoids were demonstrated to potentiate fibroblast to myofibroblast differentiation. Taken together, our data indicate that glucocorticoids have a powerful effect on TGF-β signaling, and as such, glucocorticoids may drive or inhibit TGF-β signaling pathways that are relevant to disease pathogenesis.
EXPERIMENTAL PROCEDURES
Cells and Cell Lines
Primary human lung pulmonary microvascular endothelial cells (C0085C) and pulmonary artery smooth muscle cells (C0095C) were obtained from Invitrogen. Primary human lung fibroblasts were obtained from Lonza. Primary mouse lung fibroblasts were isolated as described previously (22). The NIH/3T3 mouse fibroblast-like cell line (CRL-1658TM) and H441 human Clara cell-like airway epithelial cell line (HTB-174TM) were obtained from the American Type Culture Collection. H441 cells formed polarized monolayers on membranes of Transwell inserts and were maintained on an air/liquid interface, as described previously (40). H441 cells were exposed to dexamethasone from the basolateral side, with TGF-β stimulation made from the basolateral side. The other three cell types were plated on plastic and maintained in liquid culture as recommended by the manufacturers.
Glucocorticoid and TGF-β Stimulation
Cells were stimulated with dexamethasone (20 nm), methylprednisolone (20 nm), budesonide (2 nm), or fluticasone (2 nm) (all from Sigma) for 18 h, where indicated. These concentrations represent the mean, circulating, clinically relevant doses when these agents are employed therapeutically (41, 42). When cells were intended for the analysis of Smad protein phosphorylation, cells were subsequently stimulated with TGF-β1 (2 ng/ml; R&D Systems) for 30 min, after the 18-h incubation with glucocorticoids. When cells were intended for analysis of gene expression by real time RT-PCR after TGF-β1 stimulation, cells were stimulated with TGF-β1 (2 ng/ml) for 12 h, after the 18-h incubation with glucocorticoids (total, 30 h).
siRNA Knockdown of Gene Expression
Expression of components of the TGF-β signaling machinery was abrogated by siRNA-mediated knockdown. The siRNA were from Santa Cruz, and the optimal working concentration was assessed for each siRNA, as: mouse Tgfbr3 (sc-40225; 200 nm) and mouse Smad1 (sc-36507; 200 nm). In all cases, cells were treated with scrambled siRNA (Ambion; AM-4611; at the equivalent concentration) to serve as a negative control. The knockdown efficiency was assessed at the protein level by immunoblot for Tgfbr3 and Smad1. The siRNA transfections were performed with LipofectamineTM 2000 (Invitrogen), followed by a 6-h transfection period in serum-free Opti-MEM® (Invitrogen), after which the medium was exchanged for DMEM supplemented with 10% FCS, and the cells were then stimulated with glucocorticoids and/or TGF-β1 (or vehicle alone, where indicated).
Tgfbr3 Overexpression
Tgfbr3 was overexpressed in NIH/3T3 cells using an expression construct containing the mouse tgfbr3 (43) gene that was obtained from Dr. Fernando Lopéz-Casillas (Universidad Nacional Autónoma de México). The human TGFBR3 gene was cloned from human lung cDNA using forward (5′-AA GAT ATC ATG ACT TCC CAT TAT GTG AT-3′; containing an EcoRV site, in bold type) and reverse (5′-A AGC GGC CGC CTA GGC CGT GCT GCT GCT GG-3′; containing a NotI site, in bold type) primers, which generated a 2556-bp amplicon containing the complete TGFBR3 coding sequence that was cloned into the EcoRV and NotI sites of pIRES hrGFPII (Agilent). Plasmids were transfected into NIH/3T3 cells using LipofectamineTM 2000 (Invitrogen) as described above for siRNA. Overexpression was validated by immunoblot.
Immunoblotting
Proteins were prepared from cultured cells by scraping in lysis buffer: 20 mm Tris-Cl, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% (v/v) Nonidet P-40, 1 mm sodium vanadate, and 1× CompleteTM protease inhibitor mixture (Roche Applied Science). Proteins from mouse lung tissue were homogenized in lysis buffer (1 ml/0.1 g of wet tissue) by disruption in a Precellys® 24-Dual Homogenisator (PeqLab) using 1.4-mm ceramic beads (PeqLab) to disrupt tissue. Protein concentration was determined by Bradford assay. Proteins (25 μg/lane) were resolved by SDS-PAGE and transferred to nitrocellulose membranes, and immunoblots were probed as described previously (22), using the following antibodies: mouse anti-rabbit Tgfbr3 (Cell Signaling Technology; 2519; 1:1000), mouse anti-rabbit β-actin (Cell Signaling Technology; 4967; 1:1000), mouse anti-rabbit phospho-Smad1/5/8 (Cell Signaling Technology; 9511; 1:800), mouse anti-rabbit Smad1 (Cell Signaling Technology; 9743; 1:1000), mouse anti-rabbit phospho-Smad2 (Cell Signaling Technology; 3101; 1:1000), mouse anti-rabbit phospho-Smad3 (Cell Signaling Technology; 9520; 1:1000), mouse anti-mouse Smad2 (Cell Signaling Technology; 3103; 1:1000), mouse anti-rabbit Smad2/3 (Cell Signaling Technology; 3102; 1:1000), rabbit anti-bovine MYH11 (smooth muscle myosin heavy chain 11; Abcam, ab53219; 1:1000), and monoclonal mouse anti-rabbit ACTA2 (α-smooth muscle actin; Sigma, A-2547; 1:1000). Immune complexes were detected using peroxidase-conjugated secondary antibodies: anti-rabbit (ThermoFisher Scientific; rb:13460; 1:3000) and anti-mouse (ThermoFisher Scientific; ms:31450; 1:3000). Densitometric analysis of immunoblot bands was performed using the Multi Gauge MFC application version 3.0.0.0.
Real Time RT-PCR Analysis
Total RNA was harvested from cell cultures or mouse lung tissue, after homogenization as described for immunoblotting, using the PeqGold total RNA kit (Peqlab; 12–6834-01) and screened by quantitative real time RT-PCR with the primers listed in Table 1, as described previously (22, 44, 45). Changes in mRNA expression were assessed using the gapdh gene as a reference, as described previously (22, 44, 45). Changes in mRNA expression were reflected as fold change, using the formula: fold change = 2ΔΔCT values (22, 44, 45).
TABLE 1.
Primers employed for real time RT-PCR
| Gene | Forward primer | Reverse primer | Amplicon size | Number of cycles | Annealing temperature |
|---|---|---|---|---|---|
| bp | °C | ||||
| tgfbr3 | 5′-ATGGCAGTGACATCCCACCACAt-3′ | 5′-agaacggtgaagctctccatca-3′ | 152 | 45 | 60.0 |
| acvrl1 | 5′-CACCTACATGTGGAGATCT-3′ | 5′-CGATATCCAGGTAATCGCTG-3′ | 160 | 45 | 60.0 |
| smad1 | 5′-GCCTCTGGAATGCTGTGAGTTCCCA-3′ | 5′-GAGCCAGAAGGCTGTGCTGAGCA-3′ | 152 | 45 | 60.0 |
| gapdh | 5′-ATGGTGAAGGTCGGTGTGAA-3′ | 5′-TCATACTGGAACATGTAGACC-3′ | 143 | 45 | 60.0 |
Dual Luciferase Assay
The Dual-Luciferase assay, using both the firefly luciferase and Renilla luciferase reporters was employed to assess TGF-β signaling. The (CAGA)9-firefly luciferase (p(CAGA)9-luc) (46) and the BRE-firefly luciferase (pBRE-luc) (47) constructs were obtained from Dr. Daizo Koinuma (University of Tokyo). NIH/3T3 cells or primary lung fibroblasts were co-transfected with both the appropriate firefly luciferase-expressing construct and pRL-SV40 (Promega; which constitutively expresses Renilla luciferase) using LipofectamineTM 2000 (Invitrogen), followed by a 6-h transfection period in serum-free Opti-MEM® (Invitrogen). If cells were intended for subsequent siRNA transfection, the siRNA was also transfected with LipofectamineTM 2000 (Invitrogen), and cells were incubated with siRNA in serum-free Opti-MEM® (Invitrogen) medium, after which medium was exchanged for DMEM supplemented with 10% FCS, and cells were then stimulated with glucocorticoids and/or TGF-β1 (or vehicle alone, where indicated). The Dual-Luciferase ratio (DLR),4 was calculated from luminescence units generated by firefly luciferase normalized for luminescence units generated by Renilla luciferase, as described previously (23).
Animal Studies
Animal experiments performed in Germany were approved by the Regierungspräsidium Darmstadt (housing the Institutional Animal Care and Use Committee equivalent in Germany) under approval number B2/331. To assess the impact of glucocorticoid administration on TGF-β signaling in vivo in the mouse lung, six female C57Bl/6J mice received an intraperitoneal injection (100 μl) of dexamethasone (10 mg/kg of body mass; from a dexamethasone sodium phosphate 4 mg/ml injection solution (JENAPHARM®, mibe GmbH), diluted in PBS], whereas six control mice received an intraperitoneal injection (100 μl) of vehicle (PBS) alone. Twenty-four hours later, the mice were sacrificed, and the lung, liver, heart, and kidneys were harvested for protein and RNA isolation. Organ homogenates were screened for changes in Tgfbr3, Smad1, phospho-Smad1/5/8, Smad2/3, and phospho-Smad2 expression by immunoblot and for changes in mRNA expression of acvrl1, tgfbr3, and smad1 by real time RT-PCR.
Statistical Analyses
Data are indicated as means ± S.D. Statistical comparisons were made between two samples with an unpaired Student's t test and by one-way ANOVA followed by a Bonferroni post hoc test (for more than two samples), to evaluate changes between mean values.
RESULTS
Glucocorticoids Inhibit Classical TGF-β Signaling
The effect of glucocorticoids on TGF-β signaling was assessed by the activation of the (CAGA)9 Smad3-binding element that is common to promoters of Tgfbr1/Smad2/3-regulated genes (46), which was assessed in a luminescence-based Dual-Luciferase assay. TGF-β activated the (CAGA)9 element of the p(CAGA)9-luc construct, which was evident by an increase in DLR from 0.014 ± 0.00007 (Fig. 1A, first bar) in the unstimulated condition to 0.168 ± 0.01 (Fig. 1A, third bar) after TGF-β1 (2 ng/ml; 12 h) stimulation. The presence of dexamethasone (20 nm) did not impact the DLR, at 0.014 ± 0.0003 (Fig. 1A, second bar), relative to the unstimulated condition, where a DLR of 0.014 ± 0.00007 was attained (Fig. 1A, first bar). However, pretreatment of NIH/3T3 cells with dexamethasone (20 nm) for 18 h prior to stimulation with TGF-β1 caused a decrease in DLR, from 0.168 ± 0.01 (Fig. 1A, third bar) in the absence of dexamethasone to 0.054 ± 0.005 (Fig. 1A, fourth bar) in the presence of dexamethasone. These data indicate that the activation of the (CAGA)9 element by TGF-β1 was inhibited by dexamethasone. Identical trends were also seen with another older generation synthetic glucocorticoid, methylprednisolone (20 nm; Fig. 1B), as well as with two newer generation synthetic glucocorticoids: budesonide (2 nm; Fig. 1C) and fluticasone (2 nm; Fig. 1D).
FIGURE 1.
Glucocorticoids inhibit canonical Tgfbr1/Smad2/3 TGF-β signaling in NIH/3T3 cells. A–D, activation of the TGF-β-responsive (CAGA)9 promoter element by TGF-β1 (2 ng/ml), assessed by Dual-Luciferase reporter assay. NIH/3T3 cells were transfected with p(CAGA)9-luc and pRL-SV40 constructs and, 6 h later, treated with dexamethasone (A, dex, 20 nm), methylprednisolone (B, met, 20 nm), budesonide (C, bud, 2 nm), or fluticasone (D, flu, 2 nm), or vehicle alone for 18 h, followed by TGF-β1 (2 ng/ml) for an additional 12 h. The data indicate means ± S.D. (n = 6). The p values were assessed by one-way ANOVA followed by a Bonferroni post hoc test.
Glucocorticoids Alter Expression of Tgfbr3
Glucocorticoids blocked TGF-β signaling via the Tgfbr1/Smad2/3 axis, as assessed by activation of the (CAGA)9 element (Fig. 1). One possible explanation for this was that glucocorticoids might alter the expression of components of the TGF-β signaling machinery. To address this possibility, the expression of key components of the Tgfbr1/Smad2/3 axis signaling machinery was assessed in NIH/3T3 cells by real time RT-PCR 18 h after dexamethasone (20 nm) treatment and also after 18 h dexamethasone (20 nm) treatment followed by 12 h of TGF-β1 (2 ng/ml) stimulation (30 h total). Interestingly, the mRNA abundance of the gene accessory type III TGF-β receptor, Tgfbr3 (also called betaglycan (37–39)) was increased by dexamethasone 1.88 ± 0.35-fold (p < 0.001, relative to the unstimulated condition), hinting at a mechanism by which glucocorticoids might inhibit TGF-β signaling in fibroblasts. For this reason, a possible role for Tgfbr3 in regulating TGF-β signaling in NIH/3T3 cells was explored in detail.
Glucocorticoids Recruit Tgfbr3 to Shift TGF-β Signaling from Smad2/3 to Smad1
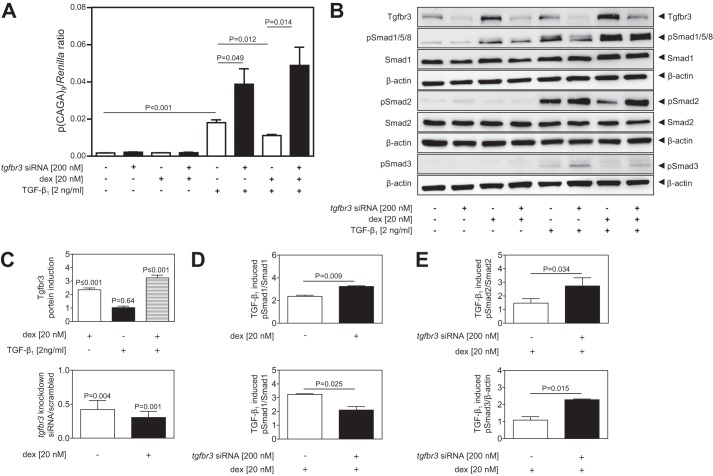
To examine the functional contribution of Tgfbr3 to the effects of dexamethasone on TGF-β signaling, the expression of tgfbr3 was ablated by transfection of NIH/3T3 cells with siRNA directed against tgfbr3, with scrambled siRNA serving as a negative control. Using a luciferase-based DLR assay, TGF-β1 induced activation of the TGF-β-responsive (CAGA)9 element (Fig. 2A), and this effect was potentiated (i.e., (CAGA)9-driven luciferase production was increased) when tgfbr3 expression was ablated by siRNA, confirming that Tgfbr3 was antagonistic to the Tgfbr1/Smad2/3 axis. Consistent with the data presented in Fig. 1A, dexamethasone blocked TGF-β induction of luciferase expression by the (CAGA)9 element (Fig. 2A; compare the seventh bar versus the fifth bar). When tgfbr3 expression was ablated, dexamethasone lost the ability to dampen (CAGA)9 responsiveness to TGF-β (Fig. 2A, compare the eighth bar versus the seventh bar). These DLR data confirm that Tgfbr3 impacts both the responsiveness of the Tgfbr1/Smad2/3 (CAGA)9 element to TGF-β1 and that Tgfbr3 mediates the effect of glucocorticoids on TGF-β1 induction of the (CAGA)9 element.
FIGURE 2.
Glucocorticoids recruit Tgfbr3 to shift TGF-β signaling from the Tgfbr1/Smad2/3 to the Acvrl1/Smad1 axis in NIH/3T3 cells. A, Tgfbr3 expression was knocked down by siRNA transfection, and the effects of dexamethasone (dex; 20 nm) and TGF-β1 (2 ng/ml), alone or in combination, were assessed in a luminescence-based Dual-Luciferase assay employing p(CAGA)9-luc and pRL-SV40. The data represent means ± S.D. (n = 6), and p values were assessed by one-way ANOVA followed by a Bonferroni post hoc test (groups of four). B, the impact of reduced Tgfbr3 expression on the phosphorylation of Smad1, Smad2, and Smad3 induced by dexamethasone and TGF-β1 stimulation (alone, or in combination), was assessed by immunoblot. C, densitometric analysis was employed to assess the impact of dexamethasone and/or TGF-β1 on Tgfbr3 levels in NIH/3T3 cells treated with scrambled siRNA alone (upper panel) or with siRNA directed against tgfbr3 (lower panel). D, densitometric analysis was employed to assess the impact of dexamethasone on TGF-β1-induced Smad1 phosphorylation, in the presence of Tgfbr3 (upper panel) and after ablation of Tgfbr3 expression (lower panel). For D, p values compare mean values in the stimulated versus unstimulated condition. E, densitometric analysis was employed to assess the impact of ablation of Tgfbr3 expression on TGF-β1-induced Smad2 (upper panel) and Smad3 (lower panel) phosphorylation. The data represent means ± S.D. (n = 3), and p values were assessed by unpaired Student's t test.
To examine the mechanistic role of Tgfbr3 further, the more proximal aspects of the Tgfbr1/Smad2/3 and Acvrl1/Smad1 axes were examined, by assessing Smad2/3 phosphorylation, and Smad1 phosphorylation, respectively (Fig. 2B; quantified from three independent experiments in Fig. 2, D and E). In support of the increased tgfbr3 mRNA levels observed in response to dexamethasone stimulation (reported above), increased Tgfbr3 protein expression in response to dexamethasone stimulation was also observed by immunoblot (Fig. 2B, compare the third lane versus the first lane; quantified in Fig. 2C, upper panel). In the case of Smad2/3, TGF-β stimulation drove phosphorylation of both Smad2 and Smad3 (Fig. 2B, fifth lane), and this effect was potentiated (i.e., more Smad phosphorylation was seen) when tgfbr3 expression was knocked down (Fig. 2B, sixth lane), with the knockdown of Tgfbr3 validated by immunoblot (Fig. 2B, compare even versus odd lanes; quantified in Fig. 2C, lower panel). In the presence of Tgfbr3, dexamethasone reduced Smad2/3 phosphorylation levels (Fig. 2B, compare the seventh lane versus the fifth lane; quantified in Fig. 2E), whereas after siRNA-mediated ablation of tgfbr3 expression, the inhibitory effect of dexamethasone on TGF-β1-induced phosphorylation of Smad2/3 was lost (Fig. 2B, compare the eighth lane versus the sixth lane; quantified in Fig. 2E). These data clearly indicate that (i) Tgfbr3 is antagonistic to TGF-β-driven Smad2/3 phosphorylation in NIH/3T3 cells and (ii) dexamethasone requires Tgfbr3 to block phosphorylation of Smad2/3 induced by TGF-β.
Interestingly, an analysis of Smad1 phosphorylation, which lies in the alternative Acvrl1/Smad1 TGF-β signaling axis, was dramatically—and oppositely—impacted by dexamethasone (Fig. 2B). Indeed, dexamethasone alone was able to drive Smad1 phosphorylation (Fig. 2B, compare the third lane versus the first lane; quantified in Fig. 2D). Furthermore, TGF-β1 stimulated Smad1 phosphorylation, and this effect was stronger in the presence of Tgfbr3 (Fig. 2B, compare the sixth lane versus the fifth lane), which is the opposite of what is seen for Smad2 phosphorylation in the presence of Tgfbr3. Most notably, Tgfbr3 had a pivotal effect on the ability of dexamethasone to impact the ability of TGF-β1 to phosphorylate Smad1 (Fig. 2B, sixth lane versus fifth lane, compared with eighth lane versus seventh lane). This effect is highlighted in the densitometric analysis presented in Fig. 2D. Together, these data demonstrate two important facts: (i) that dexamethasone redirects TGF-β signaling in NIH/3T3 cells, blocking the Smad2/3 axis and favoring the Smad1 axis, and (ii) that Tgfbr3 is central to the ability of dexamethasone to redirect TGF-β signaling in this way.
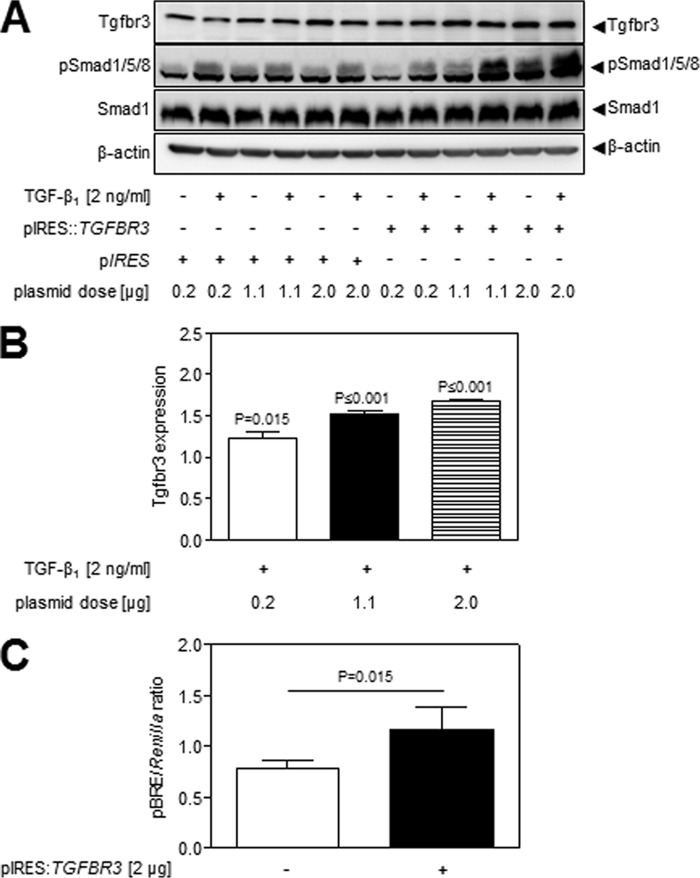
The ability of Tgfbr3 to impact TGF-β signaling via the Acvrl1/Smad1 axis was validated by the overexpression of human TGFBR3 (which mimics the effect of dexamethasone on Tgfbr3 expression in NIH/3T3 cells) from plasmid pIRES::TGFBR3. Overexpression of TGFBR3 dose-dependently increased both base-line and TGF-β-stimulated Smad1/5/8 phosphorylation (Fig. 3A; quantified in Fig. 3B). Employing a DLR-based luciferase reporter system, where the Smad1-responsive BMP response element (BRE) drives luciferase production in plasmid pBRE-luc, co-transfection of pBRE-luc with p pIRES::TGFBR3 increased base-line activity of the Smad1-responsive BRE (Fig. 3C). The impact of dexamethasone on TGF-β-driven BRE activity was not assessed, because dexamethasone alone drove pBRE-luc luciferase expression, suggesting the presence of a glucocorticoid response element in the pBRE-luc plasmid (data not shown). These data demonstrate that overexpression of Tgfbr3, as would be induced by dexamethasone, would drive signaling via the Acvrl1/Smad1 axis. It is important to note that the level of increased Tgfbr3 expression required to drive Smad1/5/8 phosphorylation (Fig. 3B) was below or equal to the levels of Tgfbr3 expression increased by dexamethasone (Fig. 2C, upper panel). As such, the levels of Tgfbr3 increased by dexamethasone are sufficient to drive increased Smad1/5/8 phosphorylation. These data also support the idea that dexamethasone-driven Tgfbr3 expression would shift the balance of TGF-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in NIH/3T3 cells.
FIGURE 3.
Overexpression of TGFBR3 drove the Acvrl1/Smad1 axis. A, the impact of the overexpression of human TGFBR3 in NIH/3T3 cells in Smad1/5/8 phosphorylation was assessed by immunoblot, employing pIRES::TGFBR3 for TGFBR3 overexpression or pIRES as empty vector. Identical data were obtained with the mouse Tgfbr3-expressing construct; however, the increased expression of human TGFBR3 over the background, endogenous mouse Tgfbr3 was more evident, and hence, these data are presented here. B, expression changes in Tgfbr3 in the TGF-β-stimulated groups were assessed by densitometry, where p values compare mean values in the pIRES-transfected versus pIRES::TGFBR3-transfected cells. C, to validate that the expression of TGFBR3 can (in the absence of TGF-β stimulation) drive Acvrl1/Smad1 signaling, the expression of the Smad1-responsive “BMP-responsive element” in pBRE-luc was assessed by Dual-Luciferase assay, in the presence of either pIRES::TGFBR3 or pIRES as empty vector. The data represent means ± S.D. (n = 6), and p values were assessed by unpaired Student's t test.
Smad1 Functionally Contributes to the Effects of Dexamethasone on TGF-β Signaling
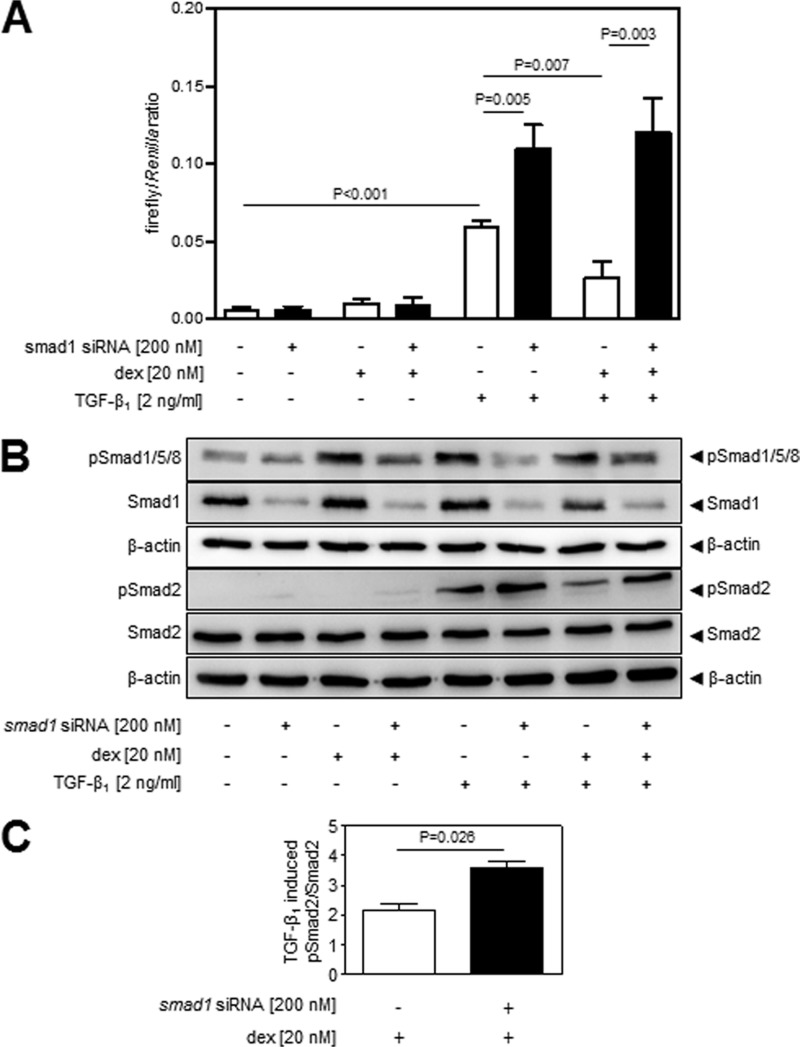
Both dexamethasone and Tgfbr3 impacted Smad1 phosphorylation in NIH/3T3 cells (Fig. 2B), suggesting a role for Smad1, which is the key mediator of the Acvrl1/Smad1 axis, in the effects of dexamethasone on TGF-β signaling. The Acvrl1/Smad1 axis, which is traditionally considered to be active primarily in the endothelium (29, 48–50), was demonstrated to be active in NIH/3T3 cells (results not shown) and in fibroblasts (51, 52). To examine the functional contribution of Smad1 to the effects of dexamethasone on TGF-β signaling, the expression of smad1 was ablated by transfection of NIH/3T3 cells with siRNA directed against smad1, with scrambled siRNA serving as a negative control. Using a luciferase-based promoter reporter assay, TGF-β1 could induce expression of the TGF-β-responsive (CAGA)9 element (Fig. 4A), and this effect was potentiated when smad1 expression was ablated by siRNA (Fig. 4A, compare the sixth bar versus the fifth bar). Consistent with the data presented in Fig. 1A, dexamethasone blocked TGF-β1 induction of luciferase expression by the (CAGA)9 element (Fig. 4A). These DLR data confirm that Smad1 impacts the responsiveness of the Tgfbr1/Smad2/3 (CAGA)9 element to TGF-β, which is consistent with the Acvrl1/Smad1 axis being antagonistic to the Tgfbr1/Smad2/3 axis. Additionally, these data reveal that Smad1 is a central mediator of the effects of dexamethasone on Tgfbr1/Smad2/3-driven (CAGA)9 activation, because when smad1 expression was ablated, the inhibitory effects of dexamethasone on the responsiveness of the Tgfbr1/Smad2/3 (CAGA)9 element to TGF-β were lost (Fig. 4A, compare the eighth bar versus the sixth bar). These data support the idea that increased Tgfbr3 expression would redirect TGF-β signaling by two separate but related mechanisms: increased Tgfbr3 expression would (i) drive the Acvrl1/Smad1 pathway directly by enhancing Smad1/5/8 phosphorylation and (ii) inhibit the Tgfbr1/Smad2/3 pathway through the antagonistic impact of increased Smad1 activity on Tgfbr1/Smad2/3 signaling.
FIGURE 4.
Smad1 functionally contributes to the effects of glucocorticoids on Tgfbr1/Smad2 signaling in NIH/3T3 cells. Smad2 was preferentially employed as a readout of Tgfbr1/Smad2/3 axis activation, because Smad2 is more abundant than Smad3, which made quantification and visualization easier. A, Smad1 expression was knocked down by siRNA transfection, and the effects of dexamethasone (dex) and TGF-β1 (2 ng/ml), alone or in combination, were assessed in a luminescence-based Dual-Luciferase assay employing p(CAGA)9-luc and pRL-SV40. The data represent means ± S.D. (n = 6), and p values were assessed by one-way ANOVA followed by a Bonferroni post hoc test. B, the impact of reduced Smad1 expression on the phosphorylation of Smad2 induced by dexamethasone and TGF-β1 stimulation (alone, or in combination) was assessed by immunoblot. C, densitometric analysis was employed to assess the impact of smad1 ablation on TGF-β1-induced Smad2 phosphorylation in the presence or absence of dexamethasone. The data represent means ± S.D. (n = 3), and p values were assessed by unpaired Student's t test.
TGF-β1 stimulation drove Smad2 phosphorylation (Fig. 4B, compare the fifth lane versus the first lane), and this effect was potentiated (i.e., more Smad2 phosphorylation was seen) when smad1 expression was knocked down (Fig. 4B, compare the sixth lane versus the fifth lane). In the presence of Smad1, dexamethasone dramatically reduced Smad2 phosphorylation levels (Fig. 4B, compare the seventh lane versus the fifth lane), whereas after siRNA-mediated ablation of smad1 expression, pretreatment with dexamethasone did not appreciably impact the ability of TGF-β to drive phosphorylation of Smad2 (Fig. 4B, compare the eighth lane versus the sixth lane). The Smad2 phosphorylation data are quantified in Fig. 4C, where Smad2 phosphorylation has been preferentially used as a proximal readout for TGF-β/Tgfbr1/Smad2/3 signaling, because the low abundance of Smad3 makes total Smad3 detection troublesome, and the phospho-Smad3 antibody yields high background. Together, these data confirm (i) that Smad1 is antagonistic to the Tgfbr1/Smad2/3 axis in NIH/3T3 cells and (ii) that dexamethasone requires Smad1 to dampen the activity of the Tgfbr1/Smad2/3 axis.
Glucocorticoids Have Comparable Effects in Primary Lung Fibroblasts
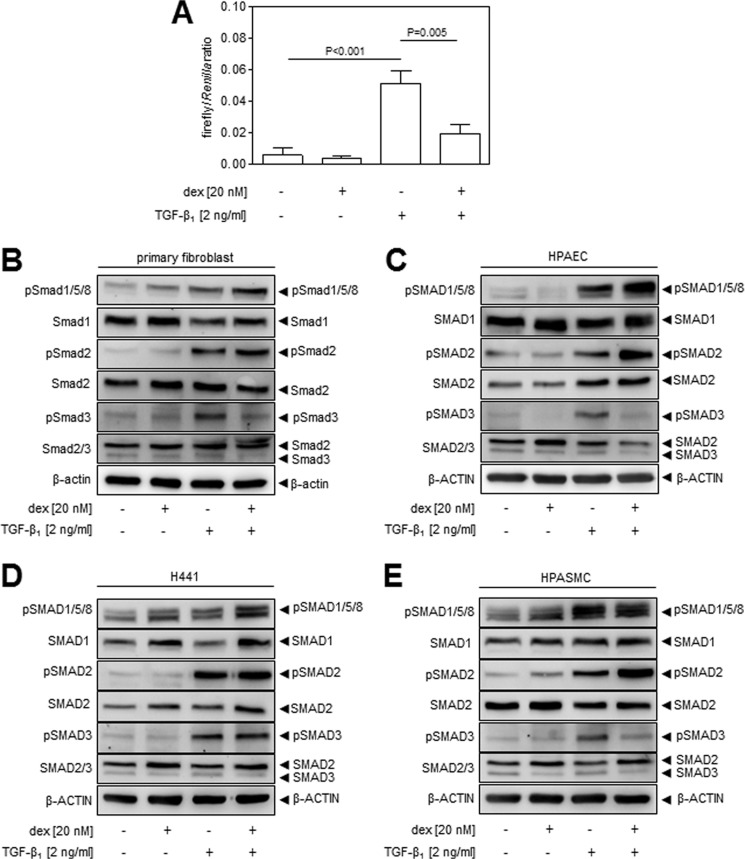
Because the NIH/3T3 cell line essentially serves as a model for fibroblasts, the effects of glucocorticoids on TGF-β signaling were also assessed in primary lung fibroblasts. Indeed, responses were seen in primary lung fibroblasts comparable to those observed in the preceding data obtained with NIH/3T3 cells, where exposure to dexamethasone dampened activation of the Tgfbr1/Smad2/3-responsive (CAGA)9 element in p(CAGA)9-luc after TGF-β stimulation (Fig. 5A). Furthermore, exposure of primary fibroblasts potentiated Smad1 phosphorylation and dampened Smad3 phosphorylation in primary lung fibroblasts in response to TGF-β stimulation (Fig. 5B), which is comparable to the effects of dexamethasone on NIH/3T3 cells. However, dexamethasone did not impact Smad2 phosphorylation in primary lung fibroblasts (Fig. 5B), which contrasts with observations made in NIH/3T3 cells (Fig. 2B).
FIGURE 5.
The impact of glucocorticoids on the Acvrl1/Smad1 and Tgfbr1/Smad2/3 axes are paralleled in primary human lung fibroblasts and are evident in other constituent cell types of the lung. A, activation of the TGF-β-responsive (CAGA)9 promoter element by TGF-β1 (2 ng/ml), assessed by Dual-Luciferase reporter assay. Primary adult human lung fibroblasts were transfected with p(CAGA)9-luc and pRL-SV40 constructs and, 6 h later, treated with dexamethasone (dex; 20 nm) or vehicle alone for 18 h, followed by TGF-β1 (2 ng/ml) for an additional 12 h. The data indicate means ± S.D. (n = 6), where p values were assessed by one-way ANOVA followed by a Bonferroni post hoc test. The impact of dexamethasone on Smad1, Smad2, and Smad3 phosphorylation was also assessed by immunoblot in primary adult human lung fibroblasts (B), primary adult human pulmonary artery endothelial cells (C), the human lung H441 cell line (D), and primary adult human pulmonary artery smooth muscle cells (E). In each case, cells were treated with dexamethasone (20 nm) for 18 h, followed by TGF-β1 (2 ng/ml) for 30 min, prior to assessing Smad expression and phosphorylation by immunoblot.
The impact of dexamethasone on Smad phosphorylation was also assessed in other cells that represent the constituent cell types of the lung, including H441 cells, which are a human airway epithelial cell line that polarizes in culture and are similar to Clara cells (Fig. 5D); primary human lung microvascular endothelial cells (Fig. 5C); and primary human pulmonary artery smooth muscle cells (Fig. 5). In all of these cell types, TGF-β-driven Smad1 phosphorylation and, thus, activation of the Acvrl1/Smad1 axis, were potentiated by dexamethasone. Thus, the impact of dexamethasone on the Acvr1/Smad1 axis appears to be a mechanism common to many lung (and perhaps other) cell types. As with lung fibroblasts and NIH/3T3 cells, dexamethasone dampened the phosphorylation of Smad3 in response to TGF-β1, in both primary pulmonary artery endothelial cells (Fig. 5C) and primary lung pulmonary artery smooth muscle cells (Fig. 5E). Thus, dexamethasone was also antagonistic to Tgfbr1/Smad2/3 signaling in these cells, although no impact on Smad2 phosphorylation was noted for either cell type. Furthermore, dexamethasone had no appreciable impact on Smad2 or Smad3 phosphorylation in airway epithelial cells (Fig. 5D). Noteworthy among the observations made here are (i) that dexamethasone has largely the same impact on primary lung fibroblasts as seen with NIH/3T3 cells and (ii) that dexamethasone has different effects on different lung cell types, although the ability of dexamethasone to potentiate TGF-β-driven Smad1 phosphorylation appears to be common to all lung cell types explored.
Glucocorticoids Functionally Impact TGF-β-regulated Physiological Processes
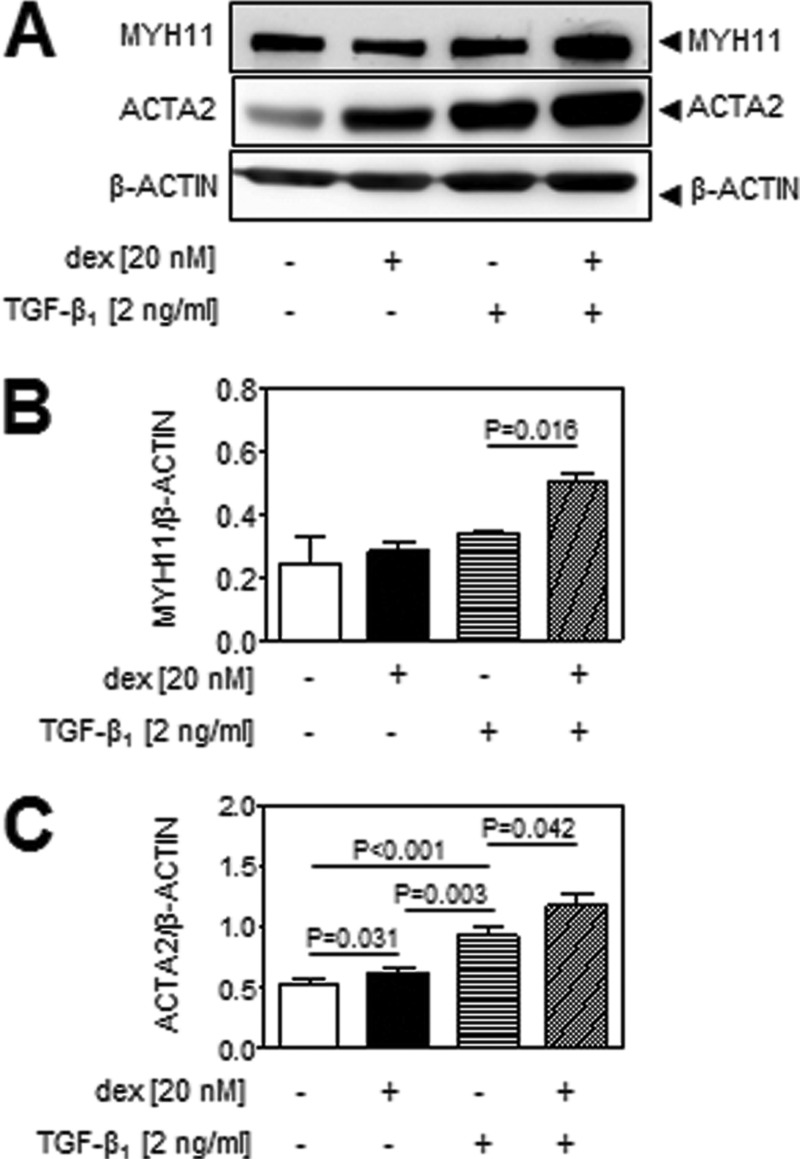
TGF-β regulates a broad spectrum of processes in lung fibroblasts. To assess the impact of glucocorticoids on some of these processes, the TGF-β-driven differentiation of primary human lung fibroblasts into myofibroblasts was selected to demonstrate proof of principle. This process is described to be driven by the Acvrl1/Smad1 axis (30–36) and thus should be enhanced in fibroblasts after exposure to dexamethasone. The acquisition of smooth muscle myosin (MYH11) and α-smooth muscle actin (ACTA2) markers are the hallmark characteristics of myofibroblast differentiation. No effect of TGF-β1 alone or dexamethasone alone was evident on MYH11 expression (Fig. 6, A and B) but applied consecutively (18 h dexamethasone followed by 12 h TGF-β1) increased MYH11 abundance. In contrast, both TGF-β1 and dexamethasone applied alone drove ACTA2 expression, and this effect was potentiated by consecutive application (Fig. 6, A and C). This observation is consistent with the increased activation of the Acvrl1/Smad1 axis by dexamethasone that was observed in NIH/3T3 cells (Fig. 2B) and primary lung fibroblasts (Fig. 5B) and supports the idea that the impact of glucocorticoids on TGF-β signaling may be of physiological and pathophysiological importance.
FIGURE 6.
Glucocorticoids act synergistically with TGF-β1 to drive the differentiation of primary adult human lung fibroblasts to myofibroblasts. A, primary adult human lung fibroblasts were stimulated with TGF-β1 (2 ng/ml; 30 min) after prestimulation with dexamethasone (dex; 20 nm; 18 h) or PBS (as vehicle), prior to the assessment of acquisition of markers of myofibroblast differentiation: smooth muscle myosin (MYH11) and smooth muscle actin (ACTA2) by immunoblot. B and C, densitometric analysis was employed to assess changes in MYH11 (B) and ACTA2 (C) abundance. The data represent means ± S.D. (n = 3), and p values were assessed by one-way ANOVA followed by a Bonferroni post hoc test.
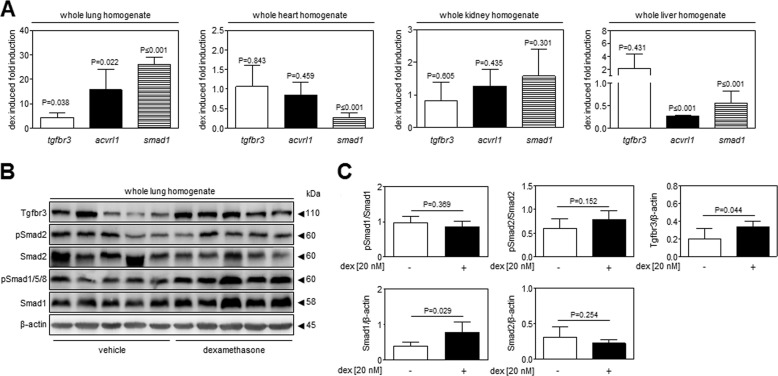
Glucocorticoids Modulate Tgfbr3, Acvrl1, and Smad1 Expression in Vivo
Intraperitoneal administration of dexamethasone (10 mg/kg) for 24 h to living mice resulted in increased mRNA abundance of tgfbr3, smad1, and acvrl1 in the lungs, but not generally in the extrapulmonary organs, of live mice (Fig. 7A). Interestingly, these changes were confined largely to the lung, with down-regulation of smad1 expression in the heart and liver and down-regulation of acvrl1 expression in the liver being the only changes observed in the three extrapulmonary organs examined. The basis for the lung-selective effect of dexamethasone is not known. In support of the gene expression data, the protein expression of Tgfbr3 and Smad1 was increased in the lungs of dexamethasone-treated live mice (Fig. 7B). These data are consistent with the trends observed in NIH/3T3 cells (Fig. 2B) and in primary lung fibroblasts (Fig. 5B). Additionally, an increased abundance of Smad1 was observed in the lungs from dexamethasone-treated versus vehicle-treated mice (Fig. 7B). In contrast, the abundance of phospho-Smad2 was unchanged comparing lungs from dexamethasone-treated mice versus lungs from vehicle-treated mice (Fig. 7B), which is also consistent with the data from primary lung fibroblasts (Fig. 5B). Unfortunately, neither phospho-Smad3 nor total Smad3 could be reliably detected in mouse lung homogenates (data not shown). Together, these data suggest that dexamethasone can drive TGF-β/Acvrl1/Smad1 signaling in the lungs of live mice, lending credence to our suggestion that this phenomenon may be physiologically relevant.
FIGURE 7.
Glucocorticoids modulate expression of the TGF-β signaling machinery in the lungs of living mice. To assess whether the effects observed in NIH/3T3 cells and primary human lung fibroblasts were applicable in vivo in living mice, six adult female C57Bl/6J mice received an intraperitoneal injection of dexamethasone (10 mg/kg body mass), whereas six control adult female C57Bl/6J mice received an intraperitoneal injection of vehicle alone (PBS, 200 μl). 24 h later, mouse tissues were harvested for analysis. A, assessment of tgfbr3, acvrl1, and smad1 expression by real time RT-PCR in the lungs, heart, kidney, and liver of dexamethasone (dex)- and vehicle-treated mice. The data represent means ± S.D. (n = 6), and p values were assessed by unpaired Student's t test and compare gene expression in dexamethasone-treated versus vehicle-treated mice. B, the expression of Tgfbr3 and total and phosphorylated Smad1 and Smad2 were assessed by immunoblot in mouse lung tissues. C, data were quantified by densitometric analysis, where data represent mean ± S.D. (n = 5/group), and p values were assessed by unpaired Student's t test.
DISCUSSION
The data presented here demonstrate that glucocorticosteroids impact TGF-β signaling in lung fibroblasts, as well as in other constituent cell types of the lung. This was demonstrated for four synthetic glucocorticoids used in clinical practice: dexamethasone, methylprednisolone, budesonide, and fluticasone. These data are important because (i) TGF-β is recognized as a key mediator of both normal physiological processes that take place in the lung and pathological processes that underlie a broad spectrum of lung diseases; (ii) lung fibroblasts are a key disease-mediating cell type in several lung diseases and are an important regulator of organogenesis and tissue repair; and (iii) glucocorticoids are a mainstay therapy for several lung diseases. The possible interaction between glucocorticoids and the TGF-β signaling system should be considered when glucocorticoids are used to treat lung disease.
The primary impact of glucocorticoids on TGF-β signaling in fibroblasts was to shift TGF-β signaling away from the Tgfbr1/Smad2/Smad3 axis and in favor of the Acvrl1/Smad1/Smad5/Smad8 axis. This was achieved primarily by glucocorticoid-driven expression of Tgfbr3, where Tgfbr3 acted as a redirecting “switch.” In this study, the effects of glucocorticoids on Tgfbr3 expression have been demonstrated to be a functionally relevant mechanism by which glucocorticoids dampen Tgfbr1/Smad2/3 signaling and, at the same time, enhance Acvrl1/Smad1 signaling in fibroblasts.
In the endothelium, the presence of functional Tgfbr1/Smad2/3 and Acvrl1/Smad1 TGF-β signaling axes has been described (28, 29), although no role for Tgfbr3 has ever been implicated in the balance of activity of these two axes. In the endothelium, the balance between these two signaling axes has been credited with profound effects on vascular homeostasis, where the Tgfbr1/Smad2/3 pathway leads to inhibition of endothelial cell migration and proliferation, and the Acvrl1/Smad1 pathway induces endothelial cell migration and proliferation (28, 29). Specifically in the pulmonary arteries, reduced Acvrl1/Smad1 signaling in the endothelium plays a role in the aberrant pulmonary vascular remodeling seen in pulmonary arterial hypertension and hereditary hemorrhagic telangiectasia, where Smad1 signaling is blocked, because of dysfunctional Acvrl1 caused by ACVRL1 mutations (53). Diminished Smad1 phosphorylation is also seen in the monocrotaline-based rat model of pulmonary hypertension (44). In other (nonhereditary) forms of telangiectasia, such as radiation-induced telangiectasia, a similar pattern emerges, where ionizing radiation shifts the balance from Tgfbr1/Smad2/3 to Acvrl1/Smad1 signaling in human dermal and lung microvascular endothelial cells, driving pathological activation of Notch signaling (54). In addition, blunted Acvrl1/Smad1 signaling is associated with the development of pulmonary arterial hypertension (44, 55–57), and Smad1-driven endothelial cell migration and proliferation are associated with pulmonary vascular development (58).
This highlights the importance of proper Acvrl1/Smad1 signaling in normal vascular homeostasis, as well as in pathology. It is tempting to speculate, based on the data presented here, that glucocorticoids may be employed to correct this defect by driving Smad1 signaling in endothelial cells, particularly considering that both Tgfbr3 and Smad1 mRNA expression is also down-regulated in patients with idiopathic pulmonary arterial hypertension (59). To date, the value of glucocorticoids in patients with pulmonary arterial hypertension has not been evaluated in a randomized controlled clinical trial; however, dexamethasone has been reported to reverse monocrotaline-induced pulmonary hypertension in rats (60), but Smad1 signaling was not assessed in that study. Pathological consequences caused by disturbances to the balance between the Tgfbr1/Smad2/3 and the Acvrl1/Smad1 axes are not limited to the vascular endothelium, where a shift in favor of the Acvrl1/Smad1 axis has been reported in chondrocytes in osteoarthritis. This pro-Acvrl1/Smad1 shift is regarded as pathogenic, because Acvrl1/Smad1 signaling promotes the pathological terminal differentiation of chondrocytes, driving cartilage destruction and osteoarthritis (61), which is interesting, given the widespread use of intra-articular injections of glucocorticoids to manage inflammatory flares associated with osteoarthritis (62).
Our observations that glucocorticoids modulate TGF-β signaling by promoting Acvrl1/Smad1-driven processes and suppressing Tgfbr1/Smad2/3-driven processes in lung fibroblasts are interesting when seen in the background of glucocorticoid use in lung disease. Indeed, these data may explain some observations recently reported in the literature. In a chorioamnionitis preterm lamb model, administration of the glucocorticoid betamethasone to pregnant sheep, in which intrauterine inflammation had been induced by intra-amniotic injection of Escherichia coli lipopolysaccharide, caused a decrease in Tgfbr1-dependent Smad2 phosphorylation in fetal lungs (63). This is consistent with the data we present here, where we propose that glucocorticoids dampen the activity of the Tgfbr1/Smad2/3 axis. In further support of this idea, in another study, antenatal betamethasone dampened lung elastin and collagen deposition (64). Because the deposition of elastin and collagen is Tgfbr1/Smad2/Smad3-dependent, the reduced elastin and collagen deposition would be expected, given the impact of glucocorticoids on Tgfbr1/Smad2/Smad3 signaling reported here.
Glucocorticoids exhibited different effects on TGF-β signaling in the primary constituent cell types of the lung. Consistent across all four primary lung cell types was the ability of dexamethasone to promote increased base-line Smad1/5/8 phosphorylation. Additionally, dexamethasone acted synergistically with TGF-β to drive Smad1/5/8 phosphorylation in H441, fibroblast, and endothelial cells, although not in vascular smooth muscle cells. Thus, dexamethasone generally promoted Smad1/5/8 activation in multiple lung cell types. The opposite was seen with the Tgfbr1/Smad2/3 axis, where dexamethasone blunted TGF-β-induced Smad3 phosphorylation in lung fibroblasts, endothelial, and smooth muscle cells, but not H441 cells. In primary fibroblasts and H441 cells, dexamethasone did not impact TGF-β-induced Smad2 phosphorylation and acted synergistically with TGF-β to increase TGF-β-driven Smad2 phosphorylation in endothelial and smooth muscle cells. In general, the Tgfbr1/Smad2/Smad3 axis in H441 cells appeared relatively resistant to the effects of dexamethasone, although it has been suggested that in human fetal lung epithelial cells, dexamethasone, and TGF-β antagonize one another (65). Thus, in sum, although dexamethasone generally drove the Acvrl1/Smad1 pathway, the impact of dexamethasone on the Tgfbr1/Smad2/3 (and Smad2) pathway was variable and cell type-dependent.
The impact of glucocorticoids on TGF-β signaling in primary adult human lung fibroblasts was physiologically relevant, because dexamethasone and TGF-β acted synergistically to drive fibroblast to myofibroblast differentiation, as assessed by the acquisition of MYH11 (smooth muscle myosin) and ACTA2 (α-smooth muscle actin) markers. These contentions are supported by the observations of others that dexamethasone and TGF-β act synergistically to drive the differentiation of primary fetal human lung fibroblasts to myofibroblasts, as monitored by the acquisition of ACTA2 (66). The myofibroblast is a key pathogenic mediator of asthma, chronic obstructive pulmonary disease, bronchopulmonary dysplasia, and acute respiratory distress syndrome (67), and the data presented here indicate that glucocorticoids may drive myofibroblast differentiation in the background of glucocorticoid use in these pathologies. Although not addressed in this study, our data suggest a mechanism by which TGF-β signaling may also be modulated by endogenous glucocorticoids, such as cortisol, which are active in lung and airway diseases such as asthma (68, 69). Indeed, Smad1 signaling is reported to be activated (and proposed to drive pathology) in airway epithelial cells during experimental allergic airway inflammation (70), and it would be interesting to assess whether increased Smad1 activation might be due to or exacerbated by glucocorticoids, either endogenous or applied exogenously, because budesonide and fluticasone are the mainstay of asthma therapy today (3, 4). When dexamethasone and TGF-β were applied in the reverse sequence (first TGF-β, then dexamethasone), no impact on TGF-β signaling was observed (results not shown); however, no impact was anticipated, because the effects of dexamethasone on TGF-β signaling are attributed here to dexamethasone-induced changes in the expression of Tgfbr3, a component of the TGF-β signaling machinery. The idea we present here is likely to be of disease relevance, because in a patient that is chronically treated with glucocorticoids, the expression of Tgfbr3 is likely to be increased. At the same time, TGF-β is generated continuously over the course of disease, and lung fibroblasts (and other lung cells) are likely to become more (through Acvrl1/Smad1) and less (through Tgfbr1/Smad2/3) responsive to TGF-β over time.
Used in vivo in mice, glucocorticoids influenced TGF-β signaling, and most notably, dexamethasone drove Tgfbr3 expression, as well as Smad1 expression (leading to an overall increase of phospho-Smad1 levels) in the lung. Unfortunately, neither Smad3 nor pSmad3 could be reliably detected in whole lung homogenates from mice. However, these data document that the two key effects of dexamethasone: increased Tgfbr3 and Smad1 expression, also occur in vivo. Although examined in the context of the respiratory system, the impact of glucocorticoids on TGF-β signaling almost certainly occurs in other cell types (and organs) as well, and as such, the data presented here are of broad general interest, in systems other than the respiratory system. Although, we also report here that when administered via the intraperitoneal route, the impact of dexamethasone on the expression of the TGF-β signaling machinery was seen predominantly in the lung, with little or no changes observed in the heart, kidney, or liver. The reasons for this are currently not apparent and should form the basis of future work exploring systemic glucocorticoid use to treat lung disease.
Acknowledgments
We thank Peter Rauschkolb (Max Planck Institute for Heart and Lung Research) for assistance with the isolation of primary fibroblasts from mouse lungs, Dr. Ardeshir Ghofrani (University Hospital Giessen) for helpful discussions regarding glucocorticoid use in patients with pulmonary arterial hypertension, Dr. Fernando Lopéz-Casillas (Universidad Nacional Autónoma de México) for providing the Tgfbr3 expression construct, and Dr. Daizo Koinuma (University of Tokyo) for providing the p(CAGA)9-luc and pBRE-luc reporter constructs.
This work was supported by the Max Planck Society; the German Research Foundation through Individual Grant Mo1789/1-1 (to R. E. M.) and Excellence Cluster 147 “Cardio-Pulmonary System” (to K. M., S. H., I. V., W. S., and R. E. M.); the Federal Ministry of Higher Education, Research and the Arts of the State of Hessen LOEWE-Programme (to K. M., S. H., I. V., W. S., and R. E. M.); and the German Center for Lung Research (Deutsches Zentrum für Lungenforschung).
- DLR
- Dual-Luciferase ratio
- ANOVA
- analysis of variance
- BRE
- BMP response element.
REFERENCES
- 1. Rhen T., Cidlowski J. A. (2005) Antiinflammatory action of glucocorticoids. New mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 2. Barnes P. J. (2006) Corticosteroid effects on cell signalling. Eur. Respir. J. 27, 413–426 [DOI] [PubMed] [Google Scholar]
- 3. Barnes P. J. (2011) Biochemical basis of asthma therapy. J. Biol. Chem. 286, 32899–32905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes P. J. (2011) Pathophysiology of allergic inflammation. Immunol. Rev. 242, 31–50 [DOI] [PubMed] [Google Scholar]
- 5. Wapner R., Jobe A. H. (2011) Controversy. Antenatal steroids. Clin. Perinatol. 38, 529–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes P. J. (2011) Glucocorticosteroids. Current and future directions. Br J. Pharmacol. 163, 29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes P. J. (2010) Inhaled corticosteroids in COPD. A controversy. Respiration 80, 89–95 [DOI] [PubMed] [Google Scholar]
- 8. Steinberg K. P., Hudson L. D., Goodman R. B., Hough C. L., Lanken P. N., Hyzy R., Thompson B. T., Ancukiewicz M. (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 354, 1671–1684 [DOI] [PubMed] [Google Scholar]
- 9. Thompson B. T. (2010) Corticosteroids for ARDS. Minerva Anestesiol. 76, 441–447 [PubMed] [Google Scholar]
- 10. Walter N., Collard H. R., King T. E., Jr. (2006) Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 3, 330–338 [DOI] [PubMed] [Google Scholar]
- 11. Doyle L. W., Ehrenkranz R. A., Halliday H. L. (2010) Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants. A systematic review. Neonatology 98, 217–224 [DOI] [PubMed] [Google Scholar]
- 12. Watterberg K. (2012) Evidence-based neonatal pharmacotherapy. Postnatal corticosteroids. Clin. Perinatol. 39, 47–59 [DOI] [PubMed] [Google Scholar]
- 13. de Benedictis F. M., Bush A. (2012) Corticosteroids in respiratory diseases in children. Am. J. Respir. Crit. Care. Med. 185, 12–23 [DOI] [PubMed] [Google Scholar]
- 14. Madurga A., Mizíková I., Ruiz-Camp J., Morty R. E. (2013) Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L893–L905 [DOI] [PubMed] [Google Scholar]
- 15. Sanjabi S., Zenewicz L. A., Kamanaka M., Flavell R. A. (2009) Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 9, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wynn T. A., Ramalingam T. R. (2012) Mechanisms of fibrosis. Therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warburton D., Shi W., Xu B. (2013) TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis. An epigenetic aberration of normal development? Am. J. Physiol. Lung. Cell Mol. Physiol. 304, L83–L85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farkas L., Farkas D., Warburton D., Gauldie J., Shi W., Stampfli M. R., Voelkel N. F., Kolb M. (2011) Cigarette smoke exposure aggravates air space enlargement and alveolar cell apoptosis in Smad3 knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L391–L401 [DOI] [PubMed] [Google Scholar]
- 19. Xu B., Chen H., Xu W., Zhang W., Buckley S., Zheng S. G., Warburton D., Kolb M., Gauldie J., Shi W. (2012) Molecular mechanisms of MMP9 overexpression and its role in emphysema pathogenesis of Smad3-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L89–L96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarantal A. F., Chen H., Shi T. T., Lu C. H., Fang A. B., Buckley S., Kolb M., Gauldie J., Warburton D., Shi W. (2010) Overexpression of transforming growth factor-β1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur. Respir. J. 36, 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jain M., Budinger G. R., Lo A., Urich D., Rivera S. E., Ghosh A. K., Gonzalez A., Chiarella S. E., Marks K., Donnelly H. K., Soberanes S., Varga J., Radigan K. A., Chandel N. S., Mutlu G. M. (2011) Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am. J. Respir. Crit. Care Med. 183, 1490–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alejandre-Alcázar M. A., Kwapiszewska G., Reiss I., Amarie O. V., Marsh L. M., Sevilla-Pérez J., Wygrecka M., Eul B., Köbrich S., Hesse M., Schermuly R. T., Seeger W., Eickelberg O., Morty R. E. (2007) Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L537–L549 [DOI] [PubMed] [Google Scholar]
- 23. Kumarasamy A., Schmitt I., Nave A. H., Reiss I., van der Horst I., Dony E., Roberts J. D., Jr., de Krijger R. R., Tibboel D., Seeger W., Schermuly R. T., Eickelberg O., Morty R. E. (2009) Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am. J. Respir Crit Care Med. 180, 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazawa K., Shinozaki M., Hara T., Furuya T., Miyazono K. (2002) Two major Smad pathways in TGF-β superfamily signalling. Genes Cells 7, 1191–1204 [DOI] [PubMed] [Google Scholar]
- 25. Massagué J. (2012) TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feng X. H., Derynck R. (2005) Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 27. Eickelberg O., Morty R. E. (2007) Transforming growth factor β/bone morphogenic protein signaling in pulmonary arterial hypertension. Remodeling revisited. Trends Cardiovasc. Med. 17, 263–269 [DOI] [PubMed] [Google Scholar]
- 28. Goumans M. J., Lebrin F., Valdimarsdottir G. (2003) Controlling the angiogenic switch. A balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc. Med. 13, 301–307 [DOI] [PubMed] [Google Scholar]
- 29. Goumans M. J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P., ten Dijke P. (2002) Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 21, 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Araoka T., Abe H., Tominaga T., Mima A., Matsubara T., Murakami T., Kishi S., Nagai K., Doi T. (2010) Transcription factor 7-like 2 (TCF7L2) regulates activin receptor-like kinase 1 (ALK1)/Smad1 pathway for development of diabetic nephropathy. Mol. Cells 30, 209–218 [DOI] [PubMed] [Google Scholar]
- 31. Chen W., Guo Y., Walker E. J., Shen F., Jun K., Oh S. P., Degos V., Lawton M. T., Tihan T., Davalos D., Akassoglou K., Nelson J., Pile-Spellman J., Su H., Young W. L. (2013) Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 33, 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Islam S. S., Mokhtari R. B., Kumar S., Maalouf J., Arab S., Yeger H., Farhat W. A. (2013) Spatio-temporal distribution of Smads and role of Smads/TGF-β/BMP-4 in the regulation of mouse bladder organogenesis. PLoS One 8, e61340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abe H., Tominaga T., Matsubara T., Abe N., Kishi S., Nagai K., Murakami T., Araoka T., Doi T. (2012) Scleraxis modulates bone morphogenetic protein 4 (BMP4)-Smad1 protein-smooth muscle α-actin (SMA) signal transduction in diabetic nephropathy. J. Biol. Chem. 287, 20430–20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mima A., Abe H., Nagai K., Arai H., Matsubara T., Araki M., Torikoshi K., Tominaga T., Iehara N., Fukatsu A., Kita T., Doi T. (2011) Activation of Src mediates PDGF-induced Smad1 phosphorylation and contributes to the progression of glomerulosclerosis in glomerulonephritis. PLoS One 6, e17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeffery T. K., Upton P. D., Trembath R. C., Morrell N. W. (2005) BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L370–L378 [DOI] [PubMed] [Google Scholar]
- 36. Matsubara T., Abe H., Arai H., Nagai K., Mima A., Kanamori H., Sumi E., Takahashi T., Matsuura M., Iehara N., Fukatsu A., Kita T., Doi T. (2006) Expression of Smad1 is directly associated with mesangial matrix expansion in rat diabetic nephropathy. Lab. Invest. 86, 357–368 [DOI] [PubMed] [Google Scholar]
- 37. López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. (1991) Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor system. Cell 67, 785–795 [DOI] [PubMed] [Google Scholar]
- 38. Dong M., How T., Kirkbride K. C., Gordon K. J., Lee J. D., Hempel N., Kelly P., Moeller B. J., Marks J. R., Blobe G. C. (2007) The type III TGF-β receptor suppresses breast cancer progression. J. Clin. Invest. 117, 206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gordon K. J., Dong M., Chislock E. M., Fields T. A., Blobe G. C. (2008) Loss of type III transforming growth factor β receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis 29, 252–262 [DOI] [PubMed] [Google Scholar]
- 40. Althaus M., Pichl A., Clauss W. G., Seeger W., Fronius M., Morty R. E. (2011) Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells. Am. J. Respir. Cell Mol. Biol. 44, 53–65 [DOI] [PubMed] [Google Scholar]
- 41. Czock D., Keller F., Rasche F. M., Häussler U. (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44, 61–98 [DOI] [PubMed] [Google Scholar]
- 42. Hübner M., Hochhaus G., Derendorf H. (2005) Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin. North Am. 25, 469–488 [DOI] [PubMed] [Google Scholar]
- 43. Ponce-Castañeda M. V., Esparza-López J., Vilchis-Landeros M. M., Mendoza V., López-Casillas F. (1998) Murine betaglycan primary structure, expression and glycosaminoglycan attachment sites. Biochim. Biophys. Acta 1384, 189–196 [DOI] [PubMed] [Google Scholar]
- 44. Morty R. E., Nejman B., Kwapiszewska G., Hecker M., Zakrzewicz A., Kouri F. M., Peters D. M., Dumitrascu R., Seeger W., Knaus P., Schermuly R. T., Eickelberg O. (2007) Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 27, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 45. Zakrzewicz A., Kouri F. M., Nejman B., Kwapiszewska G., Hecker M., Sandu R., Dony E., Seeger W., Schermuly R. T., Eickelberg O., Morty R. E. (2007) The transforming growth factor-β/Smad2,3 signalling axis is impaired in experimental pulmonary hypertension. Eur. Respir J. 29, 1094–1104 [DOI] [PubMed] [Google Scholar]
- 46. Dennler S., Itoh S., Vivien D., ten Dijke P., Huet S., Gauthier J. M. (1998) Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17, 3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Korchynskyi O., ten Dijke P. (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883–4891 [DOI] [PubMed] [Google Scholar]
- 48. Goumans M. J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C., Karlsson S., ten Dijke P. (2003) Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol. Cell 12, 817–828 [DOI] [PubMed] [Google Scholar]
- 49. Lebrin F., Goumans M. J., Jonker L., Carvalho R. L., Valdimarsdottir G., Thorikay M., Mummery C., Arthur H. M., ten Dijke P. (2004) Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J. 23, 4018–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oh S. P., Seki T., Goss K. A., Imamura T., Yi Y., Donahoe P. K., Li L., Miyazono K., ten Dijke P., Kim S., Li E. (2000) Activin receptor-like kinase 1 modulates transforming growth factor-β 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 97, 2626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alejandre-Alcázar M. A., Michiels-Corsten M., Vicencio A. G., Reiss I., Ryu J., de Krijger R. R., Haddad G. G., Tibboel D., Seeger W., Eickelberg O., Morty R. E. (2008) TGF-β signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev. Dyn. 237, 259–269 [DOI] [PubMed] [Google Scholar]
- 52. Morris E., Chrobak I., Bujor A., Hant F., Mummery C., Ten Dijke P., Trojanowska M. (2011) Endoglin promotes TGF-β/Smad1 signaling in scleroderma fibroblasts. J. Cell. Physiol. 226, 3340–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Upton P. D., Davies R. J., Trembath R. C., Morrell N. W. (2009) Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem. 284, 15794–15804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scharpfenecker M., Kruse J. J., Sprong D., Russell N. S., Ten Dijke P., Stewart F. A. (2009) Ionizing radiation shifts the PAI-1/ID-1 balance and activates notch signaling in endothelial cells. Int. J. Radiat Oncol. Biol. Phys. 73, 506–513 [DOI] [PubMed] [Google Scholar]
- 55. Girerd B., Montani D., Coulet F., Sztrymf B., Yaici A., Jaïs X., Tregouet D., Reis A., Drouin-Garraud V., Fraisse A., Sitbon O., O'Callaghan D. S., Simonneau G., Soubrier F., Humbert M. (2010) Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am. J. Respir Crit. Care Med. 181, 851–861 [DOI] [PubMed] [Google Scholar]
- 56. Long L., Crosby A., Yang X., Southwood M., Upton P. D., Kim D. K., Morrell N. W. (2009) Altered bone morphogenetic protein and transforming growth factor-β signaling in rat models of pulmonary hypertension. Potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 119, 566–576 [DOI] [PubMed] [Google Scholar]
- 57. Yang X., Long L., Southwood M., Rudarakanchana N., Upton P. D., Jeffery T. K., Atkinson C., Chen H., Trembath R. C., Morrell N. W. (2005) Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ. Res. 96, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 58. Southwood M., Jeffery T. K., Yang X., Upton P. D., Hall S. M., Atkinson C., Haworth S. G., Stewart S., Reynolds P. N., Long L., Trembath R. C., Morrell N. W. (2008) Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J. Pathol. 214, 85–95 [DOI] [PubMed] [Google Scholar]
- 59. Geraci M. W., Moore M., Gesell T., Yeager M. E., Alger L., Golpon H., Gao B., Loyd J. E., Tuder R. M., Voelkel N. F. (2001) Gene expression patterns in the lungs of patients with primary pulmonary hypertension. A gene microarray analysis. Circ. Res. 88, 555–562 [DOI] [PubMed] [Google Scholar]
- 60. Price L. C., Montani D., Tcherakian C., Dorfmüller P., Souza R., Gambaryan N., Chaumais M. C., Shao D. M., Simonneau G., Howard L. S., Adcock I. M., Wort S. J., Humbert M., Perros F. (2011) Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur. Respir. J. 37, 813–822 [DOI] [PubMed] [Google Scholar]
- 61. Blaney Davidson E. N., Remst D. F., Vitters E. L., van Beuningen H. M., Blom A. B., Goumans M. J., van den Berg W. B., van der Kraan P. M. (2009) Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 182, 7937–7945 [DOI] [PubMed] [Google Scholar]
- 62. Bijlsma J. W., Berenbaum F., Lafeber F. P. (2011) Osteoarthritis. An update with relevance for clinical practice. Lancet 377, 2115–2126 [DOI] [PubMed] [Google Scholar]
- 63. Collins J. J., Kunzmann S., Kuypers E., Kemp M. W., Speer C. P., Newnham J. P., Kallapur S. G., Jobe A. H., Kramer B. W. (2013) Antenatal glucocorticoids counteract LPS changes in TGF-β pathway and caveolin-1 in ovine fetal lung. Am. J. Physiol. Lung. Cell Mol. Physiol. 304, L438–L444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Collins J. J., Kuypers E., Nitsos I., Jane Pillow J., Polglase G. R., Kemp M. W., Newnham J. P., Cleutjens J. P., Frints S. G., Kallapur S. G., Jobe A. H., Kramer B. W. (2012) LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L778–L787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McDevitt T. M., Gonzales L. W., Savani R. C., Ballard P. L. (2007) Role of endogenous TGF-β in glucocorticoid-induced lung type II cell differentiation. Am. J. Physiol. Lung. Cell Mol. Physiol. 292, L249–L257 [DOI] [PubMed] [Google Scholar]
- 66. Gu L., Zhu Y. J., Guo Z. J., Xu X. X., Xu W. B. (2004) Effect of IFN-γ and dexamethasone on TGF-β1-induced human fetal lung fibroblast-myofibroblast differentiation. Acta Pharmacol. Sin. 25, 1479–1488 [PubMed] [Google Scholar]
- 67. Phan S. H. (2012) Genesis of the myofibroblast in lung injury and fibrosis. Proc. Am. Thorac. Soc. 9, 148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Josephson M. B., Jiao J., Xu S., Hu A., Paranjape C., Grunstein J. S., Grumbach Y., Nino G., Kreiger P. A., McDonough J., Grunstein M. M. (2012) IL-13-induced changes in endogenous glucocorticoid metabolism in the lung regulate the proasthmatic response. Am. J. Physiol. Lung. Cell Mol. Physiol. 303, L382–L390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yao H., Rahman I. (2012) Role of histone deacetylase 2 in epigenetics and cellular senescence. Implications in lung inflammaging and COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L557–L566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosendahl A., Pardali E., Speletas M., Ten Dijke P., Heldin C. H., Sideras P. (2002) Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am. J. Respir. Cell Mol. Biol. 27, 160–169 [DOI] [PubMed] [Google Scholar]