Background: There is renewed interest in the possibility of using Vc as an anticancer agent.
Results: Activation of HIF triggers a Warburg effect that renders cancer cells more sensitive to Vc-induced toxicity.
Conclusion: These results provide a link between the metabolic state and the susceptibility to Vc.
Significance: Our work helps to understand the preferential toxicity of Vc toward cancer cells.
Keywords: Cancer, DNA Damage, Hypoxia-inducible Factor (HIF), Oxidative Stress, Vitamin C, Warburg Effect, Renal Cancer
Abstract
Megadose vitamin C (Vc) is one of the most enduring alternative treatments for diverse human diseases and is deeply engrafted in popular culture. Preliminary studies in the 1970s described potent effects of Vc on prolonging the survival of patients with terminal cancer, but these claims were later criticized. An improved knowledge of the pharmacokinetics of Vc and recent reports using cancer cell lines have renewed the interest in this subject. Despite these findings, using Vc as an adjuvant for anticancer therapy remains questionable, among other things because there is no proper mechanistic understanding. Here, we show that a Warburg effect triggered by activation of the hypoxia-inducible factor (HIF) pathway greatly enhances Vc-induced toxicity in multiple cancer cell lines, including von Hippel-Lindau (VHL)-defective renal cancer cells. HIF increases the intracellular uptake of oxidized Vc through its transcriptional target glucose transporter 1 (GLUT1), synergizing with the uptake of its reduced form through sodium-dependent Vc transporters. The resulting high levels of intracellular Vc induce oxidative stress and massive DNA damage, which then causes metabolic exhaustion by depleting cellular ATP reserves. HIF-positive cells are particularly sensitive to Vc-induced ATP reduction because they mostly rely on the rather inefficient glycolytic pathway for energy production. Thus, our experiments link Vc-induced toxicity and cancer metabolism, providing a new explanation for the preferential effect of Vc on cancer cells.
Introduction
Vc,2 also termed ascorbic acid or ascorbate (the anion of ascorbic acid), is a natural compound whose severe deficiency causes a disease called scurvy, which is characterized by abnormalities in connective tissues and other manifestations (1). In contrast to many mammalian species, humans cannot produce Vc, so it is required in the diet (1). Human cells take up Vc through SVCT1 and 2. The former is expressed in epithelial tissues, whereas the latter is ubiquitous (2). SVCT1 has a high capacity for Vc transport but a low affinity, whereas SVCT2 is the opposite (2). Notably, Vc is unstable in aqueous solution and spontaneously oxidizes to its biologically inactive form, dehydroascorbate (DHA) (1), also producing ascorbate radical as an intermediate product. Cells can take up DHA using a different type of low-affinity transporters: the glucose transporters GLUT1, GLUT2, GLUT3, GLUT4, GLUT8, and GLUT10 (3, 4). Among these GLUT isoforms, only GLUT1 has a ubiquitous distribution (5, 6). Yet, GLUT1 is only expressed at modest levels by normal cells, except in the hematopoietic system and brain (5, 6), indicating that DHA uptake is not a general mechanism for accumulating intracellular Vc throughout the body. Notably, the levels of GLUT1 are increased potently under specific circumstances, including oncogenic signaling (7) and lowered oxygenation (hypoxia) (8). The latter is achieved by stabilization of the transcription factor HIF, whose α subunits (HIF1α or HIF2α) undergo quick proteasomal-mediated degradation in the presence of oxygen (8).
The role of Vc in human health has been traditionally poorly defined, except for its effect on extracellular matrix assembly through the collagen prolyl hydroxylases, which helps explain why its deficiency causes scurvy (1). In addition, Vc is most commonly known for its antioxidant effects, which are due to its ability to donate one or two electrons (1). Nowadays, Vc is implicated in an expanding list of seemingly unrelated functions that range from protection from Alzheimer disease (9) to the generation of induced pluripotent stem cells (10, 11). Some of these functions are explained by the role of Vc as a cofactor in enzymatic reactions involving 2-oxoglutarate-dependent dioxygenases, to which collagen prolyl hydroxylases belong (12). Diverse families of epigenetic regulators, for example the Jumonji histone demethylases (13) and ten-eleven-translocation (Tet) DNA hydroxylases (14, 15), also belong to the abovementioned dioxygenase superfamily. Together, these findings highlight the huge potential of Vc to influence human health, as postulated by the double Nobel Prize laureate Linus Pauling back in the 1970s. Pauling asserted that megadose Vc could combat a wide range of human diseases, in particular cancer (16). He and Cameron performed clinical trials showing a significant efficacy of intravenous Vc in delaying the death of patients with terminal cancer (17, 18). However, these results were heavily criticized after subsequent studies in the Mayo Clinic failed to reproduce this outcome with oral administration (19, 20). It was shown later that a possible reason for the discrepancy is that intravenous Vc produces much higher plasma concentrations than those achieved by oral consumption (21, 22). More recently, Chen et al. (23, 24) demonstrated that megadose Vc has potent cytotoxic effects on a variety of cancer cell lines in vitro and when they were grown as xenografts, findings that have been reproduced by others (25–27). Chen et al. (23, 24) also showed that megadose Vc has little or no effect on normal cells in vitro. These observations have reactivated interest in the subject and prompted further investigations, including ongoing clinical trials (28). Yet, the mechanisms underlying the preferential toxicity of Vc in cancer cells are not well understood.
One possibility proposed by Chen et al. (23) involves the formation of reactive oxygen species in the extracellular space because of the interaction of ascorbate radical (the intermediate product of DHA conversion) with iron and other transition metals. Supporting this idea, the levels of hydrogen peroxide in the extracellular fluid of tumor cell xenografts are high following intravenous injection of megadose Vc (29). High levels of ROS would preferentially destroy cancer cells because, compared with normal tissue, they frequently have impaired antioxidant defense mechanisms (30). However, this link has not been formally demonstrated. In contrast, Hong et al. (27) demonstrated that increased intracellular Vc uptake through SVCT2 contributes to the toxicity of Vc. SVCT2 is highly expressed in some breast cancers, suggesting that this type of cancer is more susceptible to megadose Vc than others. Interestingly, another group described that p53 inactivation diminishes the sensitivity of different cancer cell lines to Vc (26). The latter is important because P53 is the most frequently mutated tumor suppressor gene in human cancers (31). This implies that, for improved clinical efficacy of megadose Vc, it may be appropriate to select cancers with a lower probability of P53 mutations. In this regard, it is interesting to note that breast cancer has a lower frequency of mutations in P53 than other cancers (32). Meanwhile, others have shown that unrelated p53-deficient cancer cell lines are sensitive to megadose Vc (33).
In this report, we demonstrate that the activation of the HIF transcriptional pathway, a widespread phenomenon in cancer cells in vivo (8), produces a metabolic shift that enhances their susceptibility to the toxicity of Vc.
EXPERIMENTAL PROCEDURES
Cell Culture
VHL-defective RCC10 and RCC4 cell lines have been reported previously (34). Human primary renal proximal tubule epithelial cells (RPTECs) were purchased from the ATCC. The Bel-7402, HeLa, HCT116, MDA-MB-435S, SK-OV-3, SW480, and U251 cell lines were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences in Shanghai, China. All cell lines were maintained at 37 °C in 5% CO2. The RCC4, RCC10, Bel-7402, SK-OV-3, and SW480 cell lines were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (PAA). RPTECs were maintained in REBM (renal epithelial cell basal medium) (renal epithelial cell basal medium; Lonza) supplemented with REGM (renal epithelial cell growth medium) SingleQuot kit supplements and growth factors (Lonza). HeLa, HCT116, MDA-MB-435S, and U251 cells were maintained in high-glucose DMEM (Hyclone) supplemented with 10% fetal bovine serum.
Reagents
Sodium L-ascorbate (reduced Vc), dimethyloxaloylglycine (DMOG), meta-phosphoric acid, EDTA, 2-deoxy-d-glucose, DHA, sodium L-ascorbyl-2-phosphate (As-2P), ascorbic acid 6-palmitate (AA6P), N-acetyl-L-cysteine (NAC), tris(2-carboxyethyl) phosphine hydrochloride (TCEP), 1,10-phenanthroline, 2,2′-bipyridine, diethylenetriaminepentaacetic acid, Hoechst 33342, propidium iodide (PI), 3-aminobenzamide, nicotinamide, 3-aminobenzoic acid, nicotinic acid, sodium dichloroacetate (DCA), and 3-methyladenine were purchased from Sigma; d-glucose was purchased from Amersco. L-[1-14C]ascorbic acid was purchased from PerkinElmer Life Sciences. MTT was purchased from MP Biomedicals. Oligomycin was purchased from Aladdin.
Cytotoxicity Assessment
For cell counting experiments, cells were plated at 60–90% confluence in 24-well plates the day before the experiment. A stock solution (250 mm) of Vc was made with deionized water (pH 7.0) and stored at −20 °C. The stock solution was diluted in RPMI 1640 medium to make different concentrations of Vc. Before treatment with Vc, cells were washed with RPMI 1640 medium once to remove serum. Vc was added for 1 h, and then cells were washed and cultured for an another 24 h in growth medium. Dead cells were washed off with Dulbecco's phosphate-buffered saline (Dulbecco's phosphate-buffered saline; Invitrogen), and the attached cells were trypsinized and counted twice. Each item was analyzed in duplicate. Other forms of Vc were prepared and administered following a similar procedure. For the MTT assay, cells were seeded at 1.5 × 104 in a 96-well plate the day before the experiment. Vc, prepared as above, was added for 1 h, and then cells were washed and cultured for another 20 h in growth medium. MTT (0.5 mg/ml) was added and incubated at 37 °C for an additional 4 h. For detection, 150 μl of DMSO was added to each well, and the plate was read at 570 nm using a Synergy HT multimode microplate reader (Bio-Tek). Each item was analyzed as five replicates.
Intracellular Vc Measurements
HPLC
Cells were washed twice with cold DPBS after Vc incubation, trypsinized, and collected by centrifugation (302 g, 3 min). Then they were resuspended in a mixture of deionized water and an equal volume of 10% cold meta-phosphoric acid containing 2 mm EDTA. The resulting samples were vortex-mixed and the precipitate spun down by centrifugation (16,000 × g, 2 min) (35). 0.25 mm TECP was added to the samples. Freshly prepared samples were sent to the Laboratory of Biochemistry and Nuclide Analysis, China National Analytical Center, Guangzhou, China for detection.
Liquid Scintillation Counting
0.4 μCi/ml of L-[1-14C]ascorbic acid and unlabeled Vc (8 mm) were added to the medium and kept for 10 min or 50 min. Cells were then washed three times with cold DPBS and dissolved in 1 n NaOH containing 0.1% SDS. 14C activity was counted using a Tri-Carb 2800TR liquid scintillation analyzer (PerkinElmer Life Sciences).
Intracellular ROS, NAD+, and ATP Measurements and Comet Assay
Total intracellular ROS were detected using a reactive oxygen species assay kit containing dichlorofluorescein (Beyotime). Intracellular NAD+ levels and ATP levels were detected using the NAD/NADH assay kit (Abcam) and the Cell Titer-Glo® luminescent cell viability assay (Promega). An alkaline comet assay was performed using the CometAssay kit (Trevigen) and analyzed by CASP software (CASP, version 1.2.3 beta 1).
Nuclear Staining with Hoechst 33342 and PI
Cells were washed once gently with DPBS and costained with 2.5 μg/ml Hoechst 33342 and 5 μg/ml PI at 37 °C in 5% CO2 for 10 min, washed, and then visualized using an Olympus BX51 fluorescence microscope. Cell death modes (early apoptosis, late apoptosis, and necrosis) were defined as described previously (36).
Plasmids
Retroviral plasmids expressing VHL30 (the longer isoform of VHL) or lentiviral plasmids expressing constitutively active forms of HIF-1α (carrying mutations at prolines 402 and 564) and HIF-2α (carrying mutations at prolines 405 and 531) have been reported previously (37, 38). Lentiviral or retroviral supernatants containing these constructs were produced as described using HEK293T cells (34). Cells containing VHL30 or an empty vector were selected with G418 (Biovision). The efficiency of lentiviral infection was near 100% (on the basis of the use of a control GFP lentiviral vector). shRNA inserts were cloned into the lentiviral pLKO.1 vector for HIF1α and HIF2α and the retroviral pRetroSuper vector for GLUT1 and GLUT3. The sequences were as follows: HIF1α, 5′-GGCAGTAACCTTTCATCATGA-3′ and 5′-TGCTCTTTGTGGTTGGATCTA-3′; HIF2α, 5′-CAGTACCCAGACGGATTTCAA-3′ and 5′-CGACCTGAAGATTGAAGTGAT-3′; GLUT1, 5′-GCCACACTATTACCATGAGAA-3′, 5′-GGATGTCCTATCTGAGCATCG-3′, and 5′-GCTGGATGAGACTTCCAAACC-3′; and GLUT3, 5′-AGTAGCTAAGTCGGTTGAAAT-3′ and 5′-CGGTGCAGATAGATCTGGAAA-3′. A sequence that targets the firefly luciferase gene transcript was used as a control (34). These plasmids were verified by sequencing. Lentiviral or retroviral supernatants containing these shRNA constructs were produced using HEK293T cells, as reported previously (34). We added puromycin (Sigma) at day 3 post-transduction (renewed daily for 3 days) to select cells containing the shRNA vectors.
Quantitative PCR Analysis, Immunofluorescence, and Western Blotting
Quantitative PCR analysis was performed using SYBR Green (Takara) and an ABI 7300 machine. Items were run in duplicate, and values were normalized on the basis of β-actin values. Primers used in this study were as follows: GLUT1, 5′-TTTGGCTTTGTGGCCTTC-3′ (forward) and 5′-GCACATGCCCACAATGAA-3′ (reverse); GLUT3, 5′-TTTCTGGTGGAAAGGGCA-3′ (forward) and 5′-GGCCTGGTCCAATTTCAA-3′ (reverse); SVCT1, 5′-AATGTTCAGCGCCACTCTG-3′ (forward) and 5′-GCGATGATGCAGCAAATG-3′ (reverse); and SVCT2, 5′-TGCCAATGGAACAGCAGA-3′ (forward) and 5′-TGGTCAAGGGACCGATGTA-3′ (reverse). Immunofluorescence microscopy was performed using an Olympus BX51 microscope. DAPI was purchased from Sigma. Western blotting was performed using ECL (Beyotime). Antibodies were purchased from the following suppliers. HIF2α, GLUT1, and GLUT3 were from Abcam. HIF1α was from BD Biosciences. γH2A was from Millipore. Actin and β-tubulin were from Sigma.
Statistical Analysis
Student's t test was used throughout.
RESULTS
Activation of the HIF Pathway Potentiates Vc-induced Cell Toxicity
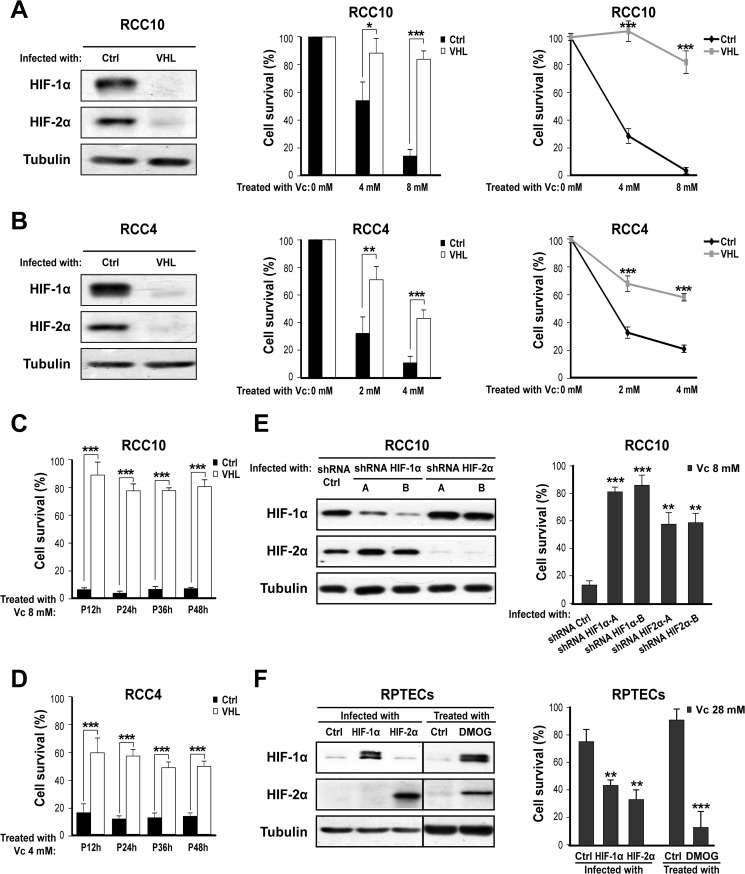
Solid cancers commonly show an imbalance between oxygen supply and consumption because of uncontrolled cell proliferation, thus becoming hypoxic (8). When this happens, HIFα subunits become stabilized as a result of inadequate oxygen-dependent hydroxylation by the HIF prolyl hydroxylases (39). Stabilized HIF1α or HIF2α subunits partner with the constitutively expressed β subunit and modulate the transcription of a large number of genes that are relevant for normal cell function as well as malignant progression (40). Because of the generality of this phenomenon and its relevance to cancer, we studied whether activation of the HIF transcriptional pathway influences the susceptibility of cancer cell lines to Vc-induced toxicity. We initially chose VHL-defective renal clear cancer cells as a tool because they show constitutive activation of the HIF pathway (41). VHL protein is part of the ubiquitin ligase complex that targets hydroxylated HIFα subunits for proteasomal degradation and is frequently absent in renal cancer because of loss of function of the corresponding gene (42). VHL-defective renal cancer cells are also attractive because reintroduction of the VHL gene restores many aspects of normal renal tubular cell function (37). We employed two well characterized VHL-defective renal cancer cell lines (RCC10 and RCC4) (37) that were infected with retroviruses producing VHL or an empty vector. Effective down-regulation of HIFα subunits (HIF1α and HIF2α) by re-expressed VHL was confirmed by Western blotting (Fig. 1, A and B, left panels). We then treated these two isogenic cell pairs with increasing concentrations of Vc for 1 h and evaluated cell toxicity after 24 h. VHL-defective RCC10 cells were significantly more sensitive to 4 and 8 mm of Vc than their VHL-expressing counterparts, as measured by counting the viable cells or using the MTT assay (Fig. 1A, center and right panels). This selectivity was observed as well with the RCC4 cell pair (Fig. 1B, center and right panels). We also measured cell toxicity at four time points (12, 24, 36, and 48 h) after Vc treatment and observed that the differential toxic effect between VHL-defective and VHL-expressing cells is sustainable (Fig. 1, C and D). Next, we clarified the specific contribution of HIFα subunits by infecting VHL-defective RCC10 cells with two pairs of shRNA lentiviral vectors that were compared with shRNA for a control sequence. This experiment was important because VHL has both HIF-dependent and HIF-independent functions (43). Interestingly, down-regulation of either HIFα subunit substantially prevented Vc-induced toxicity, although the effect was more obvious with shRNA for HIF1α (Fig. 1E, both panels). Together, these experiments demonstrate that VHL loss of function in renal cancer cell lines increases their sensitivity to Vc-induced toxicity via an HIFα-dependent mechanism.
FIGURE 1.
Activation of HIF increases the susceptibility to Vc-induced cell toxicity. A, left panel, representative Western blot analysis showing normalization of HIF1α and HIF2α protein levels after reintroduction of VHL into RCC10 cells. An empty vector was used as a control (Ctrl). Tubulin was used as a loading control (also hereafter in similar experiments unless indicated otherwise). Center panel, VHL reintroduction protects RCC10 cells from Vc-induced toxicity. Vc was added for 1 h (also in A, right panel; B, center and right panels; C; D; E, right panel; and F, right panel). Cell survival (in percent) was measured by counting viable cells and is represented as relative to untreated cells (0 mm Vc; also in B, center panel; C; D; E, right panel; and F, right panel). The mean ± S.D. of four independent experiments is shown (also in B, center panel; C; D; and F, right panel). Right panel, a similar experiment as in A, center panel, but using an MTT assay and represented as relative to untreated cells (also in B, right panel). A representative experiment is shown (also in B, right panel). B, similar experiments as in A but using RCC4 cells. C and D, cell survival (in percent) was measured at four time points (12, 24, 36, and 48 h) after Vc treatment using RCC10 (C) and RCC4 (D). P, time post-treatment with Vc. E, left panel, representative Western blot analysis showing the specific reduction of HIF1α and HIF2α levels after transduction of VHL-defective RCC10 cells with two shRNA vectors (A and B). shRNA for firefly luciferase was used as a control. Right panel, shRNA for HIFα subunits reduces Vc-induced toxicity in VHL-defective RCC10 cells. The mean ± S.D. of three independent experiments is shown. F, left panel, representative Western blot analysis for HIFα subunits using lysates of RPTECs overexpressing constitutively active forms of HIF1α and HIF2α or treated with 0.5 mm DMOG for 24 h. GFP lentiviruses or untreated cells were used as a control. Right panel, induction of HIFα subunits by lentiviruses or DMOG increases the sensitivity of RPTECs to very high doses of Vc. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
We also studied the effect of megadose Vc on RPTECs and observed that these cells were relatively insensitive to Vc even with 28 mm Vc (Fig. 1F, right panel). However, use of lentiviruses producing constitutively active forms of HIF1α or HIF2α enhanced the toxic effect of Vc on RPTECs compared with the empty vector (Fig. 1F, both panels). Similar results were observed by activating the endogenous HIF pathway with DMOG (Fig. 1F, both panels), a compound that inhibits the activity of HIF prolyl hydroxylases (12). Hence, although normal renal tubular cells are relatively insensitive to megadose Vc, its toxic effect is potentiated as well by HIFα subunits.
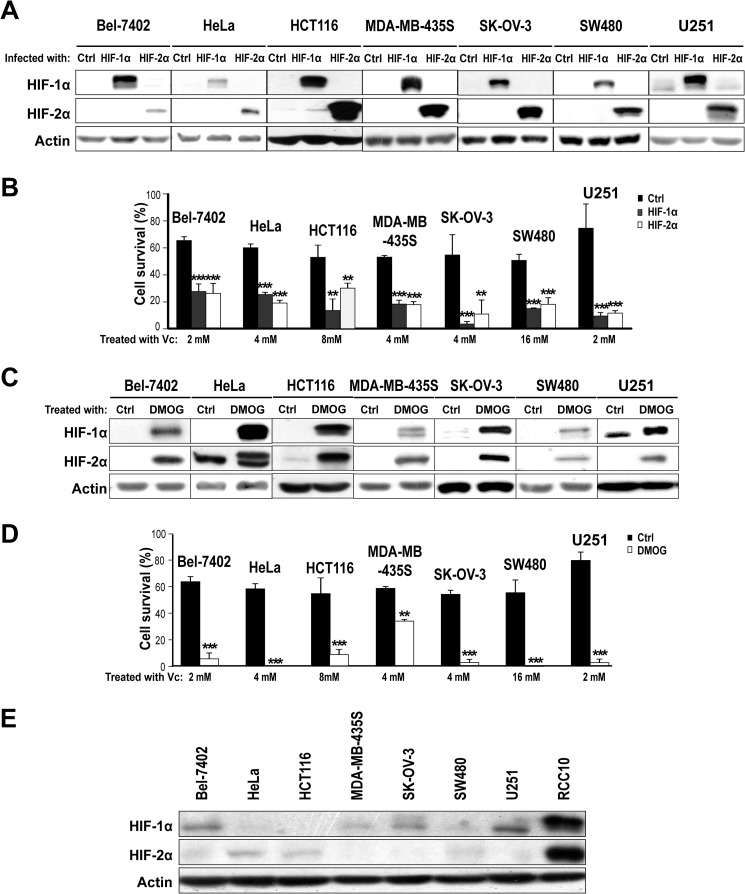
To assess whether HIFα also sensitizes other cancer cell lines to megadose Vc, we selected a panel of non-renal cancer cell lines without mutations in VHL. These cell lines included Bel-7402 (hepatocellular carcinoma), HeLa (cervix carcinoma), HCT116 (colon adenocarcinoma), MDA-MB-435S (breast carcinoma), SK-OV-3 (ovarian carcinoma), SW480 (colon adenocarcinoma), and U251 (glioblastoma). Overexpression of constitutively active HIFα subunits by means of lentiviral vectors (Fig. 2A) enhanced Vc-induced toxicity in all cell lines, although there was variability in the dose needed to achieve an optimal synergistic effect (Fig. 2B). Treatment with DMOG increased the sensitivity to Vc as well (Fig. 2, C and D). Of note, we could not observe any clear correlation between the levels of endogenous HIF1α/HIF2α in normoxia in these cancer cell lines and their basal or induced (upon HIF activation) susceptibility to megadose Vc (Fig. 2E). This supports the idea that, besides HIF, other mechanisms regulate the sensitivity to Vc in cancer cells, in agreement with findings by others (26, 27). Therefore, the synergistic effect of HIF in promoting the toxicity of Vc is not restricted to renal cancer cell lines and happens in cancer cell lines of different origins.
FIGURE 2.
Activation of HIF renders non-renal VHL-competent cancer cells more sensitive to Vc-induced toxicity. A, representative Western blot analysis for HIFα subunits using lysates from non-renal VHL-competent cancer cell lines overexpressing constitutively active forms of HIF1α and HIF2α showing the expected increase in protein levels. Actin was used as a loading control (Ctrl). Lentiviruses producing GFP were used as a control. B, overexpression of HIF1α and HIF2α increases Vc-induced toxicity in non-renal VHL-competent cancer cell lines. Vc was added for 1 h to all cell lines (also in D). The concentrations were 2 mm for Bel-7402 and U251, 4 mm for HeLa, MDA-MB-435S and SK-OV-3, 8 mm for HCT116, and 16 mm for SW480 cells. Cell survival (percent) was measured by counting viable cells and is represented as relative to untreated cells (also in D). The mean ± S.D. of four independent experiments is shown (also in D). C, representative Western blot analysis for HIFα subunits using lysates from non-renal VHL-competent cancer cell lines treated or not treated with 0.5 mm DMOG for 24 h. D, pretreatment with 0.5 mm DMOG for 24 h increases Vc-induced toxicity in non-renal VHL-competent cancer cell lines. E, representative Western blot analysis comparing the basal levels (in normoxia) of HIF1α and HIF2α in a panel of non-renal VHL-competent cancer cell lines. Lysates from VHL-defective RCC10 cells were used as a control. **, p < 0.01; ***, p < 0.001.
HIF Promotes the Toxicity of Vc in Cancer Cells by Enhancing DHA Uptake through GLUT1
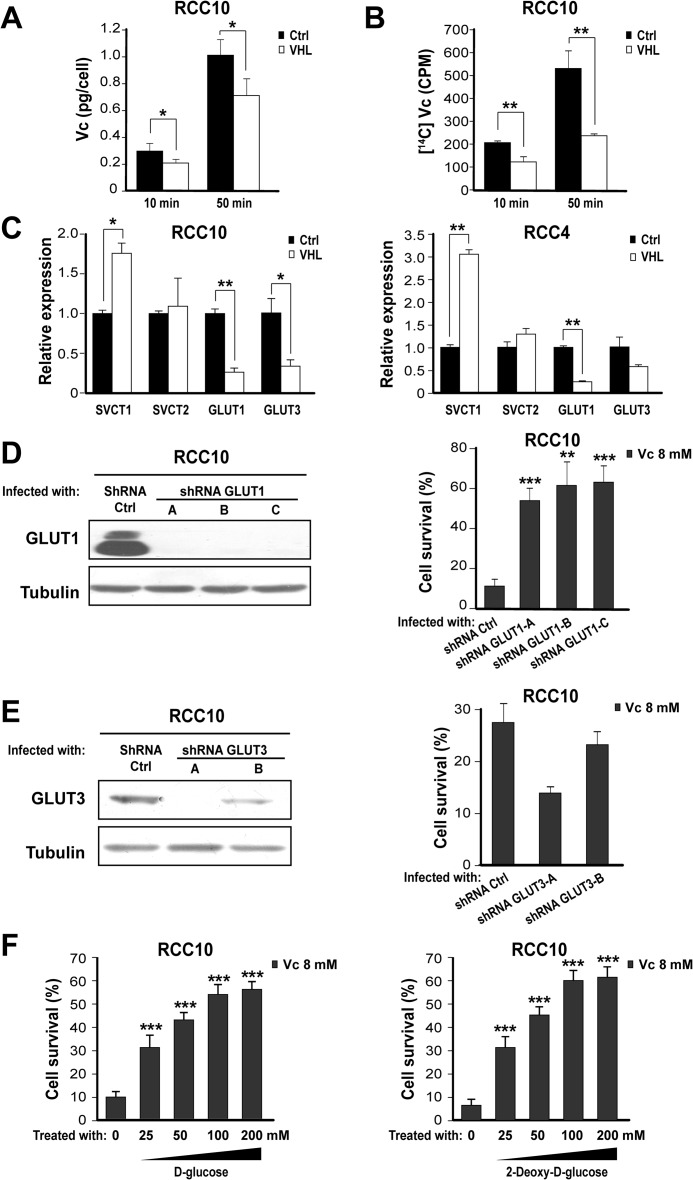
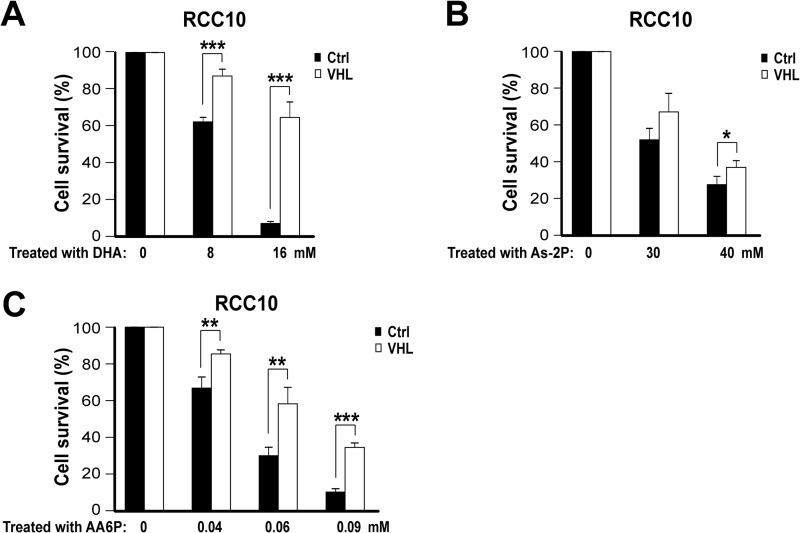
We wished to determine how HIF renders cancer cell lines more sensitive to Vc-induced toxicity. We focused on VHL-defective renal cancer cell lines because of their constitutive activation of HIF. We postulated that increased DHA intracellular uptake through the activation of GLUT1 might potentiate Vc-induced toxicity. Our specific hypothesis was that the sum of DHA taken through GLUT1, and of reduced Vc through SVCT transporters, would increase intracellular Vc levels above a tolerable threshold. The rationale for this idea includes the following. First, Vc spontaneously converts to DHA in the culture medium, but inside cells it is quickly converted back into reduced Vc (1). Second, the DHA transporter GLUT1 is a well known HIF target (8). To test our hypothesis, we performed an HPLC analysis of cell lysates to detect intracellular levels of the reduced form of Vc. Notably, VHL-defective RCC10 cells treated with Vc showed higher values than their VHL-expressing counterparts (Fig. 3A), suggestive of an increased uptake of either reduced Vc, DHA, or both. This was further confirmed by using 14C-labeled Vc, whose uptake was detected by liquid scintillation counting of cell lysates (Fig. 3B). We then examined whether DHA transport through GLUT1 is the critical component determining Vc-induced toxicity in VHL-defective cells. First, we verified by quantitative PCR that GLUT1 mRNA is more abundant in VHL-defective RCC10 and RCC4 cells than in VHL-expressing cells (Fig. 3C). In contrast, SVCT transporters displayed similar or even higher levels in VHL-expressing cells (Fig. 3C). Next, we down-regulated GLUT1 expression using shRNA retroviral vectors (Fig. 3D, left panel). This diminished Vc-induced toxicity significantly (Fig. 3D, both panels), whereas down-regulation of GLUT3 using the same approach did not (Fig. 3E, both panels). d-Glucose or its analog 2-deoxy-d-glucose can effectively compete with DHA transport through GLUT1 (6, 44). Thus, we added high levels of 2-deoxy-d-glucose or d-glucose to VHL-defective RCC10 cells treated with Vc, which significantly reduced the toxicity (Fig. 3F). We also treated VHL-defective and VHL-expressing RCC10 cells with DHA or a stable form of Vc, As-2P. The latter can be taken up through SVCT receptors after modification by cell membrane esterases but cannot be converted to DHA nor produce extracellular hydrogen peroxide (1). As anticipated, DHA killed VHL-defective RCC10 cells more effectively than VHL-expressing cells (Fig. 4A). However, it needed twice the dose of reduced Vc and a longer incubation time (4 h) (Fig. 4A). This indicates that Vc-induced toxicity in VHL-defective renal cancer cells cannot be explained by only the increased uptake of DHA. As-2P required an even higher dose (8 times) and 4 h to induce substantial toxicity, showing only a modest selective effect on VHL-defective RCC10 cells (Fig. 4B). This shows that the intracellular uptake of reduced Vc through SVCT receptors likely contributes to the selective effect of Vc on VHL-defective renal cancer cells but, on its own, cannot explain it either. Lastly, we used a lipid-soluble form of Vc, AA6P. AA6P is cell membrane-permeable and, consequently, does not need receptor-mediated transport (45). AA6P induced potent toxicity in both VHL-defective and VHL-expressing RCC10 cells, requiring a very low dose and reduced incubation time, but, as with DHA, the toxicity was more noticeable in VHL-defective cells (Fig. 4C). Taken together, these experiments demonstrate that GLUT1-mediated transport of DHA is critical for the higher sensitivity of VHL-defective renal cancer cells to Vc-induced toxicity. They also highlight the participation of additional cooperative mechanisms in this phenomenon, namely the uptake of reduced Vc through SVCT receptors (27) and, possibly, the generation of extracellular hydrogen peroxide (23).
FIGURE 3.
Increased DHA transport through GLUT1 underlies Vc-induced toxicity in VHL-defective renal cancer cells. A, HPLC shows increased intracellular Vc levels in VHL-defective RCC10 cells overexpressing VHL or the empty vector. Vc was added for 10 min or 50 min (also in B). The mean ± S.D. of four independent experiments is shown. Ctrl, control. B, liquid scintillation values after adding isotope-labeled Vc to VHL-defective RCC10 cells overexpressing VHL or the empty vector. The mean ± S.D. of three independent experiments is shown. C, representative quantitative PCR analysis showing the relative expression of different Vc transporters in VHL-defective RCC10 and RCC4 overexpressing VHL or the empty vector. D, left panel, representative Western blot analysis showing reduction of GLUT1 protein after transduction of VHL-defective RCC10 cells with three shRNA constructs (A, B, and C). Right panel, shRNA for GLUT1 reduces Vc-induced toxicity in VHL-defective RCC10 cells. Vc was added for 1 h (also in E and F). Cell survival (percent) was measured by counting viable cells and is represented as relative to untreated cells (also in E right and F). The mean ± S.D. of three independent experiments is shown. E, similar experiments as in D using two shRNA vectors (A and B) for GLUT3, which fail to reduce Vc-induced toxicity in VHL-defective RCC10 cells. The mean ± S.D. of four independent experiments is shown (also in F). F, 2-deoxy-d-glucose and d-glucose reduce Vc-induced toxicity in VHL-defective RCC10 cells. The two compounds were added simultaneously with Vc. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FIGURE 4.
Toxicity of three different forms of Vc in VHL-defective renal cancer cells. A–C, differential sensitivity of VHL-defective RCC10 cells overexpressing VHL or the empty vector to DHA (administered for 4 h), As-2P (administered for 4 h), and AA6P (administered for 30 min). Cell survival (percent) was measured by counting viable cells and is represented as relative to untreated cells. The mean ± S.D. of four independent experiments is shown. Ctrl, control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
High Intracellular Levels of Vc Induce an Oxidative DNA Damage Response in VHL-defective Renal Cancer Cells
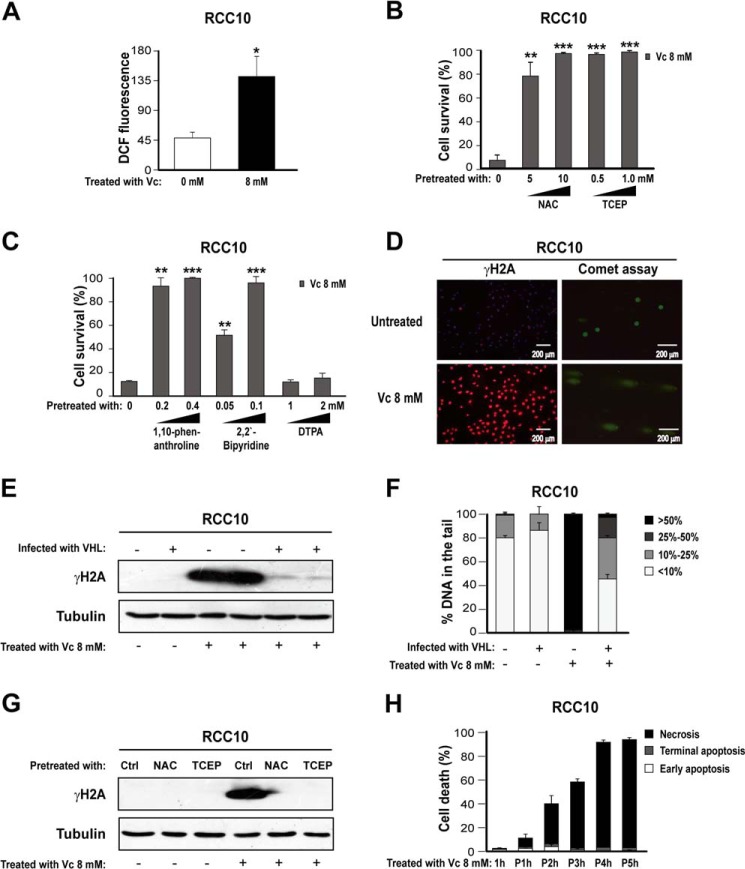
We also sought to understand the mechanism by which increased intracellular Vc ultimately kills VHL-defective renal clear cancer cells. Vc is known to produce ROS upon interaction with proteins containing transition metals, including iron (29). We measured ROS with a sensitive dye and detected enhanced generation in VHL-defective RCC10 cells treated with Vc compared with a control (Fig. 5A). Moreover, the antioxidants NAC and TCEP effectively diminished Vc-induced toxicity in VHL-defective RCC10 cells (Fig. 5B), implying a causal effect of increased ROS. Likewise, the cell permeable-iron chelators 1,10-phenanthroline and 2,2′-bipyridine prevented Vc-induced toxicity in VHL-defective RCC10 cells, whereas a non-permeable one, diethylenetriaminepentaacetic acid, had no effect (Fig. 5C). This further supports that the accumulation of intracellular Vc is fundamental to the increased toxicity in VHL-defective renal cancer cells. The increase of ROS correlated with high levels of DNA damage, as detected by Western blotting and immunofluorescence for γH2A (Fig. 5, D and E) and by comet assay (Fig. 5, D and F). Moreover, NAC and TCEP strongly protected cells from DNA damage, as shown by Western blotting for γH2A (Fig. 5G). In addition, evaluation of the type of cell death using Hoechst 33342/PI double staining showed that necrosis instead of apoptosis is the dominant form of cell death induced by Vc in VHL-defective RCC10 cells (Fig. 5H). Therefore, the excessive accumulation of intracellular Vc in VHL-defective renal cancer cells generates ROS through interaction with iron, and this, subsequently, triggers an oxidative DNA damage response that produces necrosis.
FIGURE 5.
High intracellular levels of Vc increase ROS and trigger necrosis in VHL-defective renal cancer cells. A, dichlorofluorescin (DCF) labeling shows increased ROS production in VHL-defective RCC10 cells treated with Vc. Vc was added for 1 h (also in B–H). The mean ± S.D. of three independent experiments is shown (also in B and C). B and C, the indicated antioxidants or iron chelators reduce Vc-induced toxicity in VHL-defective RCC10 cells. Cells were pretreated for 2 h before adding Vc. Cell survival (percent) was measured by counting viable cells and is represented as relative to untreated cells. DTPA, diethylenetriaminepentaacetic acid. D, representative immunofluorescence shows DNA damage after Vc treatment of VHL-defective RCC10 cells. Cells were either stained for γH2A (left column) or using the comet assay (right column). E, representative Western blot analysis for γH2A after Vc treatment of VHL-defective RCC10 cells overexpressing VHL or the empty vector. F, quantification of the comet assay after Vc treatment of VHL-defective RCC10 cells overexpressing VHL or the empty vector. A representative experiment is shown (also in H). G, representative Western blot analysis for γH2A after Vc treatment of VHL-defective RCC10 cells pretreated or not treated with NAC and TCEP. Ctrl, control. H, dual labeling with Hoechst 33342 and PI shows increased necrosis in VHL-defective RCC10 cells treated with Vc. P, time post-treatment with Vc. **, p < 0.01; ***, p < 0.001.
Activation of the PARP Pathway by Vc Depletes Intracellular ATP Reserves in VHL-defective Renal Cancer Cells
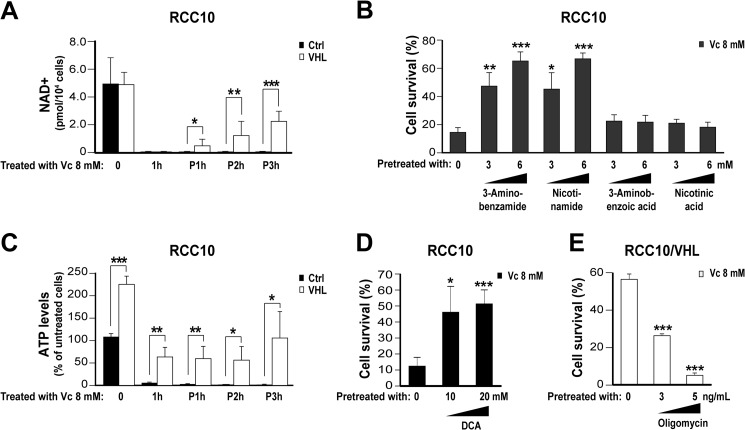
To explain how the occurrence of DNA damage produces necrosis in VHL-defective renal cancer cells treated with Vc, we considered the involvement of the poly(ADP-ribose) polymerase (PARP). PARP plays a role in DNA damage detection/repair and uses the key metabolic regulator NAD+ as a substrate (46). There are two major NAD+-dependent cellular mechanisms for generating ATP: one through the glycolytic pathway that uses cytosolic NAD+ as substrate and the other through the electron respiratory chain using mitochondrial NAD+ (47). It is known that the activation of PARP, because of DNA damage, has a more potent effect on depleting cytosolic than mitochondrial NAD+ because the two sources are not interchangeable (46). Accordingly, cells that mainly use glycolysis rather than mitochondrial respiration, a phenomenon termed the Warburg effect (47), are more sensitive to death caused by PARP overactivation (46). Notably, the activation of HIF produces a Warburg effect in VHL-defective renal cancer cells because of increased expression of target genes, including GLUT1 (which increases glucose transport), multiple glycolytic enzymes (e.g. hexokinase 2, phosphofructokinase, and phosphoglycerate kinase) (8), and pyruvate dehydrogenase kinase 1 (which blocks access of pyruvate into the mitochondria) (48). Hence, we speculated that activation of PARP in Vc-treated VHL-defective renal cancer cells depletes the ATP reserves more quickly than in VHL-expressing cells because their proglycolytic metabolic status makes them unable to compensate the deficit quickly enough. We measured NAD+ levels in VHL-defective RCC10 cells and their VHL-expressing counterparts. Basal NAD+ was comparable in both cell types, dropping drastically and similarly upon addition of Vc (Fig. 6A). Moreover, the PARP inhibitors 3-aminobenzamide and nicotinamide reduced Vc-induced cell death significantly, whereas the control 3-aminobenzoic acid and nicotinic acid did not (Fig. 6B). We also measured ATP basal levels in VHL-defective and VHL-expressing RCC10 cells, finding that they are substantially lower in the former (Fig. 6C), as reported by others (49). Moreover, treatment with Vc reduced intracellular ATP more extensively in VHL-defective RCC10 cells than VHL-expressing ones (Fig. 6C). Next, we used DCA and oligomycin to show that reduced mitochondrial utilization by VHL-defective RCC10 cells, and the consequent reduced ATP levels, contribute to their enhanced sensitivity to the toxicity of Vc. DCA blocks pyruvate dehydrogenase kinase 1 (50), activating the Krebs cycle, whereas oligomycin impairs energy generation through mitochondria by inhibiting the mitochondrial ATP synthase (51). Pretreatment of VHL-defective cells with DCA reduced Vc-induced toxicity in VHL-defective RCC10 cells (Fig. 6D). Conversely, pretreatment with oligomycin enhanced Vc-induced toxicity in their VHL-expressing counterparts (Fig. 6E). These findings show that the oxidative DNA damage response caused by high levels of intracellular Vc induces necrosis in VHL-defective renal cancer cells by consuming their limited ATP reserves. They help explain as well why VHL-defective renal cancer cells are more sensitive than VHL-expressing cells to equally high concentrations of Vc (see above, Fig. 4C).
FIGURE 6.
Oxidative DNA damage kills VHL-defective renal cancer cells by exhausting the cellular pool of ATP. A, reduced NAD+ levels at different time points after treating VHL-defective RCC10 cells overexpressing VHL or the empty vector with Vc. Vc was added for 1 h (also in B–E). The mean ± S.D. of four independent experiments is shown (also in C–E). Ctrl, control; P, time post-treatment with Vc. B, the PARP inhibitors 3-aminobenzamide and nicotinamide (added 2 h before Vc) reduce Vc-induced toxicity in VHL-defective RCC10 cells. Cell survival (percent) was measured by counting viable cells and is represented as relative to untreated cells (also in D and E). The mean ± S.D. of three independent experiments is shown. C, reduced ATP levels at different time points after treating VHL-defective RCC10 cells overexpressing VHL or the empty vector with Vc. D, DCA (added 48 h before Vc) reduces Vc-induced toxicity in VHL-defective RCC10 cells. E, oligomycin (added 3 h before Vc) increases Vc-induced toxicity in VHL-defective RCC10 cells overexpressing VHL. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
The potential effects of antioxidants on human health have been the subject of intense debate for several decades and have prompted multiple studies, including large clinical trials (28). Among these antioxidants, Vc stands out as a natural compound that reportedly acts on a variety of processes ranging from the common cold to Alzheimer disease (9). Clarifying the effects of Vc on human health is important because large doses are consumed (orally) by countless individuals worldwide without prescription and administered (most frequently intravenously) by thousands of alternative practitioners to patients with advanced cancer (52). The controversy regarding Vc and human health started in the 1970s with the debated anticancer claims by Pauling and Cameron (17–20). More recent studies have demonstrated that a possible reason for the discrepancies between independent reports is the route of administration (oral plus intravenous versus oral only) (21, 22). This has led to the reconsideration that megadose Vc might indeed be useful to treat at least some types of cancer (28), likely in combination with other cytotoxic compounds (53, 54). In this regard, routine anticancer compounds and Vc are supposed to act through different mechanisms, supporting the idea that their combination should indeed be synergistic (28). Testing megadose Vc for treating cancer is attractive because the side effects are often minimal (28). However, a major challenge for the comprehensive evaluation of the anticancer clinical efficacy of Vc is to define optimal criteria for patient selection. Among other things, this would require an improved understanding of how Vc kills cancer cells, in particular of those mechanisms that are cancer cell type-specific and those that are general. This is important because human cancers are highly heterogeneous.
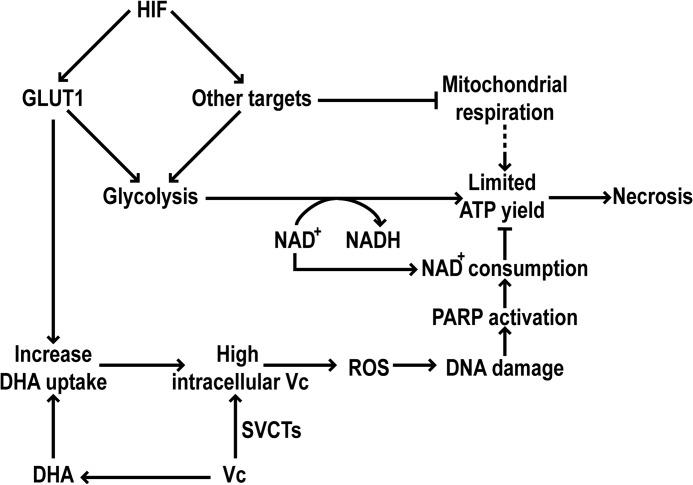
We have identified a novel mechanism that modulates the susceptibility of cancer cells to the toxicity of Vc: the HIF transcriptional pathway (see schematic in Fig. 7). We focused on this pathway because its activation is a widespread phenomenon in solid cancers in vivo, which is mostly (although not exclusively) due to hypoxia (7). Our results show that activation of HIF through genetic (VHL ablation or HIF overexpression) or chemical manipulation (using DMOG) increases the toxic effects of Vc on a panel of cancer cell lines from different tissues. Using VHL-defective renal cancer cells as a tool, we also demonstrated that HIF enhances the toxicity of Vc by promoting DHA uptake through the HIF transcriptional target GLUT1, which is a high-capacity but low-affinity transporter for DHA (2). DHA is produced spontaneously from Vc in solution, a circumstance that may be exacerbated by tumor inflammation in vivo (1). This mechanism cooperates with the simultaneous uptake of reduced Vc through SVCT transporters, increasing intracellular Vc over a tolerable threshold. Intracellular Vc then interacts with transition metals, including iron, to generate ROS (27). VHL-defective renal cancer cells display increased iron accumulation because of increased transferrin uptake (55), which, thus, may contribute to the toxicity of Vc. Moreover, the intracellular conversion of DHA to Vc is known to require glutathione, which may further facilitate ROS accumulation by reducing the cellular antioxidant defenses (56). The formation of hydrogen peroxide in the extracellular space likely contributes to increase intracellular ROS as well, as proposed by Chen et al. (23). Together, this produces cell death, which is supported by the observation that cell-permeable antioxidants and iron chelators effectively prevent Vc-induced toxicity in VHL-defective renal cancer cells. Verrax et al. (33) also employed a cell-permeable iron chelator, desferrioxamine, to prevent Vc-induced toxicity in prostate cancer cell lines.
FIGURE 7.
Schematic showing how HIF enhances Vc-induced toxicity in cancer cell lines.
Interestingly, DHA alone can preferentially kill VHL-defective renal cancer cells compared with VHL-expressing ones, but it requires a higher concentration and longer incubation time than using Vc. Heaney et al. (57) reported that DHA has a protective, rather than cytotoxic, effect on cancer cell lines treated with certain cytotoxic compounds that produce oxidative stress. However, the authors used lower doses than those employed here and reduced the incubation time. More importantly, they employed hematopoietic cancer cell lines that rely exclusively on GLUT1 for the uptake of Vc in its DHA form. On the other hand, Hong et al. (27) failed to detect a toxic effect of DHA on a panel of cancer cell lines that did not include VHL-defective cells. The discrepancy with our study may be related to differences in the expression of GLUT1 and/or differences in the dose and incubation time.
ROS generated by Vc treatment of VHL-defective cells induce massive DNA damage that is repaired by the PARP pathway. In this process, NAD+ is consumed, thus reducing the cellular reserves of ATP and killing VHL-defective renal cancer cells through necrosis but not apoptosis. Both necrosis and apoptosis have been shown to play a role in Vc-induced toxicity, depending on the cancer cell line (33, 58). Other groups have reported that autophagy can be a contributing mechanism (25, 27, 59). In this regard, we could not prevent Vc-induced toxicity after pretreatment of VHL-defective RCC10 cells with the autophagy inhibitor 3-methyladenine (27) (data not shown). The consumption of NAD+ as a cause for the induced toxicity of Vc had been postulated by others, but was not formally demonstrated (29). Supporting this idea, Chen et al. (59) showed a potent ATP reduction after treating prostate cancer cell lines with Vc. On the other hand, Du et al. (25) showed that overexpression of mitochondrial catalase reverses ATP depletion without reducing Vc-induced toxicity in prostate cancer cell lines as well. This suggests that the contribution of ATP depletion to Vc-induced toxicity likely varies between cancer cell lines. VHL-defective renal cancer cells are particularly sensitive to ATP depletion because activation of HIF shifts their metabolism toward energy production through glycolysis rather than mitochondrial respiration (48). This effect, termed the Warburg effect, allows quick energy production with poor ATP yield as opposed to the more lengthy but efficient ATP generation through access of pyruvate to the mitochondrial Krebs cycle (7). Under most circumstances, the Warburg effect is advantageous for rapidly growing cells, including cancer cells, which frequently have access to vast amounts of glucose because of increased angiogenesis (47). However, this phenomenon is also thought to represent an Achilles heel through which cancer can be attacked (47).
Our findings are relevant for several reasons. First, metabolic alterations are a hallmark of cancer, and HIF is an important player but not the only one. In fact, inactivation of tumor suppressor genes like P53 or activation of oncogenes, including PI3K/AKT and RAS, also contribute to the Warburg effect (7). Further studies in this direction may thus help to understand the preferential toxic effect of Vc on cancer cells in the absence of HIF activation. Second, one could argue that selecting cancer patients with an abundance of HIF-positive cancer cells might increase the efficacy of megadose Vc. A caveat to this is that the activation of HIF is normally patchy, partial, and transient in most solid cancers (60). Nevertheless, hypoxia is a frequent event in most cancers, and it is known to induce drug resistance in tumors, which is in part mediated by HIF (61). Therefore, Vc may actually help target cancer cells that are otherwise difficult to kill. Another consideration is that HIF prolyl hydroxylases are among the dioxygenases targeted by Vc as a cofactor (12). Consequently, treatment with modest doses of Vc can reduce HIF levels in vitro by increasing HIF prolyl hydroxylase activity (62). In fact, Gao et al. (63) proposed that Vc prevents cancer growth in vivo by reducing HIF levels. This suggests that, in specific contexts, the administration of megadose Vc could be controlling cancer cell growth through different mechanisms, depending on which tissue concentration is achieved, how homogeneously it is distributed inside the tumor, and the state of HIF activation in those cells.
VHL-defective renal cancers bear constitutive activation of HIF that is not influenced significantly by hypoxia or HIF prolyl hydroxylase activity (41). Because of this, they represent an exception to some of the caveats described above. Interestingly, Riordan et al. (64) reported a complete regression of metastatic lesions in a patient with primary renal cell cancer. Chen et al. (23) showed as well that VHL-deficient 769-P renal cancer cells are sensitive to megadose Vc, but the authors did not assess whether VHL re-expression reduces this effect. Moreover, Alexander et al. (65) demonstrated that ACHN renal cancer cells are highly sensitive to a combination of the D-fraction of the Maitake mushroom and Vc, although it should be noted that this cancer cell line is VHL-competent. VHL-defective renal cancers compose the vast majority of renal cancers in the adult (42), and a proportion of these cancers is familial in the context of the VHL syndrome (42). In the latter, the tumors likely arise from loss of heterozygosity in small, initially benign lesions that display HIF activation (66). Activation of HIF is also thought to play a role in other types of hereditary renal cancers (67). Hence, it is tempting to speculate that periodic administration of megadose Vc in VHL syndrome patients, and perhaps other hereditary renal cancer cell patients, might selectively kill premalignant early lesions and prevent the appearance of renal cancer. In this regard, a high local concentration of Vc may be more easily achieved in kidney tubular cells because of the circumstance that Vc is reabsorbed in the kidney tubules and excreted in the urine (2). Unfortunately, the lack of appropriate animal models for studying the development of VHL-defective renal cancer makes it difficult to evaluate these ideas at this time. We failed to produce xenografts with the two VHL-defective renal cancer cell lines used in this study (data not shown). In addition, overexpression (by means of lentiviruses) of HIF1α/HIF2α in non-renal cancer cells (HCT116) did not increase their susceptibility to megadose Vc when grown as xenografts (data not shown). This is presumably because HIF levels achieved by lentiviral transduction are abnormally high. In fact, HIF is known to promote tumor progression through multiple mechanisms besides the Warburg effect (e.g. by enhancing cell invasion) (68), which may thus tend to increase the tumor mass and counteract the toxic effects of Vc.
In summary, we report a new mechanism modulating the sensitivity of cancer cells to Vc-induced toxicity. Future studies will be necessary to clarify whether these findings are relevant in vivo and whether they can help select cancer patients showing an enhanced response to megadose Vc.
Acknowledgments
We thank Jianyong Xu for advice and Xingyan Li for technical assistance.
This work was supported by National Natural Science Foundation of China Grants 31071309 and 81202010.
- Vc
- vitamin C
- DHA
- dehydroascorbic acid
- HIF
- hypoxia-inducible factor
- ROS
- reactive oxygen species
- SVCT
- sodium-dependent vitamin C transporter
- RPTEC
- renal proximal tubule epithelial cell
- DMOG
- dimethyloxaloylglycine
- As-2P
- sodium L-ascorbyl-2-phosphate
- AA6P
- ascorbic acid 6-palmitate
- NAC
- N-acetyl-L-cysteine
- TCEP
- tris(2-carboxyethyl) phosphine hydrochloride
- PI
- propidium iodide
- DCA
- sodium dichloroacetate
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- VHL
- von Hippel-Lindau
- PARP
- poly(ADP-ribose) polymerase
- GLUT
- glucose transporter.
REFERENCES
- 1. Du J., Cullen J. J., Buettner G. R. (2012) Ascorbic acid. Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 1826, 443–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. May J. M. (2011) The SLC23 family of ascorbate transporters. Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 164, 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corpe C. P., Eck P., Wang J., Al-Hasani H., Levine M. (2013) Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 288, 9092–9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michels A. J., Hagen T. M., Frei B. (2013) Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 33, 45–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frolova A. I., Moley K. H. (2011) Glucose transporters in the uterus. An analysis of tissue distribution and proposed physiological roles. Reproduction 142, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montel-Hagen A., Kinet S., Manel N., Mongellaz C., Prohaska R., Battini J. L., Delaunay J., Sitbon M., Taylor N. (2008) Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 132, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 7. Ward P. S., Thompson C. B. (2012) Metabolic reprogramming. A cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semenza G. L. (2012) Hypoxia-inducible factors. Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 33, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng F., Cappai R., Ciccotosto G. D., Svensson G., Multhaup G., Fransson L. Å., Mani K. (2011) Suppression of amyloid β A11 antibody immunoreactivity by vitamin C. Possible role of heparan sulfate oligosaccharides derived from glypican-1 by ascorbate-induced, nitric oxide (NO)-catalyzed degradation. J. Biol. Chem. 286, 27559–27572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esteban M. A., Wang T., Qin B., Yang J., Qin D., Cai J., Li W., Weng Z., Chen J., Ni S., Chen K., Li Y., Liu X., Xu J., Zhang S., Li F., He W., Labuda K., Song Y., Peterbauer A., Wolbank S., Redl H., Zhong M., Cai D., Zeng L., Pei D. (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 [DOI] [PubMed] [Google Scholar]
- 11. Esteban M. A., Pei D. (2012) Vitamin C improves the quality of somatic cell reprogramming. Nat. Genet. 44, 366–367 [DOI] [PubMed] [Google Scholar]
- 12. Schofield C. J., Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 [DOI] [PubMed] [Google Scholar]
- 13. Wang T., Chen K., Zeng X., Yang J., Wu Y., Shi X., Qin B., Zeng L., Esteban M. A., Pan G., Pei D. (2011) The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 9, 575–587 [DOI] [PubMed] [Google Scholar]
- 14. He Y. F., Li B. Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L., Sun Y., Li X., Dai Q., Song C. X., Zhang K., He C., Xu G. L. (2011) Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minor E. A., Court B. L., Young J. I., Wang G. (2013) Ascorbate induces ten-eleven translocation (Tet) Methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 288, 13669–13674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cameron E., Pauling L., Leibovitz B. (1979) Ascorbic acid and cancer. A review. Cancer Res. 39, 663–681 [PubMed] [Google Scholar]
- 17. Cameron E., Pauling L. (1976) Supplemental ascorbate in the supportive treatment of cancer. Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 73, 3685–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cameron E., Pauling L. (1978) Supplemental ascorbate in the supportive treatment of cancer. Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 75, 4538–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Creagan E. T., Moertel C. G., O'Fallon J. R., Schutt A. J., O'Connell M. J., Rubin J., Frytak S. (1979) Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 301, 687–690 [DOI] [PubMed] [Google Scholar]
- 20. Moertel C. G., Fleming T. R., Creagan E. T., Rubin J., O'Connell M. J., Ames M. M. (1985) High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 312, 137–141 [DOI] [PubMed] [Google Scholar]
- 21. Graumlich J. F., Ludden T. M., Conry-Cantilena C., Cantilena L. R., Jr., Wang Y., Levine M. (1997) Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 14, 1133–1139 [DOI] [PubMed] [Google Scholar]
- 22. Padayatty S. J., Sun H., Wang Y., Riordan H. D., Hewitt S. M., Katz A., Wesley R. A., Levine M. (2004) Vitamin C pharmacokinetics. Implications for oral and intravenous use. Ann. Intern. Med. 140, 533–537 [DOI] [PubMed] [Google Scholar]
- 23. Chen Q., Espey M. G., Krishna M. C., Mitchell J. B., Corpe C. P., Buettner G. R., Shacter E., Levine M. (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells. Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U.S.A. 102, 13604–13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Q., Espey M. G., Sun A. Y., Pooput C., Kirk K. L., Krishna M. C., Khosh D. B., Drisko J., Levine M. (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 11105–11109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du J., Martin S. M., Levine M., Wagner B. A., Buettner G. R., Wang S. H., Taghiyev A. F., Du C., Knudson C. M., Cullen J. J. (2010) Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 16, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J., Lee S. D., Chang B., Jin D. H., Jung S. I., Park M. Y., Han Y., Yang Y., Il Kim K., Lim J. S., Kang Y. S., Lee M. S. (2012) Enhanced antitumor activity of vitamin C via p53 in cancer cells. Free Radic. Biol. Med. 53, 1607–1615 [DOI] [PubMed] [Google Scholar]
- 27. Hong S. W., Lee S. H., Moon J. H., Hwang J. J., Kim D. E., Ko E., Kim H. S., Cho I. J., Kang J. S., Kim D. J., Kim J. E., Shin J. S., Jung D. J., Jeong Y. J., Cho B. J., Kim T. W., Lee J. S., Kang J. S., Hwang Y. I., Noh D. Y., Jin D. H., Lee W. J. (2013) SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene 32, 1508–1517 [DOI] [PubMed] [Google Scholar]
- 28. Parrow N. L., Leshin J. A., Levine M. (2013) Parenteral ascorbate as a cancer therapeutic. A reassessment based on pharmacokinetics. Antioxid. Redox Signal. 19, 2141–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Q., Espey M. G., Sun A. Y., Lee J. H., Krishna M. C., Shacter E., Choyke P. L., Pooput C., Kirk K. L., Buettner G. R., Levine M. (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 8749–8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohno S., Ohno Y., Suzuki N., Soma G., Inoue M. (2009) High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 29, 809–815 [PubMed] [Google Scholar]
- 31. Levine A. J., Oren M. (2009) The first 30 years of p53. Growing ever more complex. Nat. Rev. Cancer 9, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gasco M., Shami S., Crook T. (2002) The p53 pathway in breast cancer. Breast Cancer Res. 4, 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verrax J., Calderon P. B. (2009) Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 47, 32–40 [DOI] [PubMed] [Google Scholar]
- 34. Xu J., Wang B., Xu Y., Sun L., Tian W., Shukla D., Barod R., Grillari J., Grillari-Voglauer R., Maxwell P. H., Esteban M. A. (2012) Epigenetic regulation of HIF-1α in renal cancer cells involves HIF-1α/2α binding to a reverse hypoxia-response element. Oncogene 31, 1065–1072 [DOI] [PubMed] [Google Scholar]
- 35. Lykkesfeldt J. (2000) Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods. Reliable reduction with tris[2-carboxyethyl]phosphine hydrochloride. Anal. Biochem. 282, 89–93 [DOI] [PubMed] [Google Scholar]
- 36. Shimizu S., Eguchi Y., Kamiike W., Itoh Y., Hasegawa J., Yamabe K., Otsuki Y., Matsuda H., Tsujimoto Y. (1996) Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 56, 2161–2166 [PubMed] [Google Scholar]
- 37. Esteban M. A., Tran M. G., Harten S. K., Hill P., Castellanos M. C., Chandra A., Raval R., O'Brien T, S., Maxwell P. H. (2006) Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 66, 3567–3575 [DOI] [PubMed] [Google Scholar]
- 38. Xu J., Li H., Wang B., Xu Y., Yang J., Zhang X., Harten S. K., Shukla D., Maxwell P. H., Pei D., Esteban M. A. (2010) VHL inactivation induces HEF1 and Aurora kinase A. J. Am. Soc. Nephrol. 21, 2041–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 40. Kaelin W. G., Jr., Ratcliffe P. J. (2008) Oxygen sensing by metazoans. The central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 41. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 42. Kaelin W. G., Jr. (2008) The von Hippel-Lindau tumour suppressor protein. O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y., Roche O., Yan M. S., Finak G., Evans A. J., Metcalf J. L., Hast B. E., Hanna S. C., Wondergem B., Furge K. A., Irwin M. S., Kim W. Y., Teh B. T., Grinstein S., Park M., Marsden P. A., Ohh M. (2009) Regulation of endocytosis via the oxygen-sensing pathway. Nat. Med. 15, 319–324 [DOI] [PubMed] [Google Scholar]
- 44. Vera J. C., Rivas C. I., Fischbarg J., Golde D. W. (1993) Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364, 79–82 [DOI] [PubMed] [Google Scholar]
- 45. Pokorski M., Marczak M., Dymecka A., Suchocki P. (2003) Ascorbyl palmitate as a carrier of ascorbate into neural tissues. J. Biomed. Sci. 10, 193–198 [DOI] [PubMed] [Google Scholar]
- 46. Zong W. X., Ditsworth D., Bauer D. E., Wang Z. Q., Thompson C. B. (2004) Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 18, 1272–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kroemer G., Pouyssegur J. (2008) Tumor cell metabolism. Cancer's Achilles' heel. Cancer Cell 13, 472–482 [DOI] [PubMed] [Google Scholar]
- 48. Kim J. W., Tchernyshyov I., Semenza G. L., Dang C. V. (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase. A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 [DOI] [PubMed] [Google Scholar]
- 49. Zhang H., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K. I., Dang C. V., Semenza G. L. (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 [DOI] [PubMed] [Google Scholar]
- 50. Michelakis E. D., Webster L., Mackey J. R. (2008) Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 99, 989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hao W., Chang C. P., Tsao C. C., Xu J. (2010) Oligomycin-induced bioenergetic adaptation in cancer cells with heterogeneous bioenergetic organization. J. Biol. Chem. 285, 12647–12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Padayatty S. J., Sun A. Y., Chen Q., Espey M. G., Drisko J., Levine M. (2010) Vitamin C. Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 5, e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurbacher C. M., Wagner U., Kolster B., Andreotti P. E., Krebs D., Bruckner H. W. (1996) Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 103, 183–189 [DOI] [PubMed] [Google Scholar]
- 54. Abdel-Latif M. M., Raouf A. A., Sabra K., Kelleher D., Reynolds J. V. (2005) Vitamin C enhances chemosensitization of esophageal cancer cells in vitro. J. Chemother. 17, 539–549 [DOI] [PubMed] [Google Scholar]
- 55. Alberghini A., Recalcati S., Tacchini L., Santambrogio P., Campanella A., Cairo G. (2005) Loss of the von Hippel-Lindau tumor suppressor disrupts iron homeostasis in renal carcinoma cells. J. Biol. Chem. 280, 30120–30128 [DOI] [PubMed] [Google Scholar]
- 56. Bevan R. J., Mistry N., Patel P. R., Halligan E. P., Dove R., Lunec J. (2010) Can vitamin C induce nucleotide excision repair? Support from in vitro evidence. Br. J. Nutr. 103, 686–695 [DOI] [PubMed] [Google Scholar]
- 57. Heaney M. L., Gardner J. R., Karasavvas N., Golde D. W., Scheinberg D. A., Smith E. A., O'Connor O. A. (2008) Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 68, 8031–8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carosio R., Zuccari G., Orienti I., Mangraviti S., Montaldo P. G. (2007) Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol. Cancer 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen P., Yu J., Chalmers B., Drisko J., Yang J., Li B., Chen Q. (2012) Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 23, 437–444 [DOI] [PubMed] [Google Scholar]
- 60. Harris A. L. (2002) Hypoxia. A key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 [DOI] [PubMed] [Google Scholar]
- 61. Wilson W. R., Hay M. P. (2011) Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 [DOI] [PubMed] [Google Scholar]
- 62. Knowles H. J., Raval R. R., Harris A. L., Ratcliffe P. J. (2003) Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 63, 1764–1768 [PubMed] [Google Scholar]
- 63. Gao P., Zhang H., Dinavahi R., Li F., Xiang Y., Raman V., Bhujwalla Z. M., Felsher D. W., Cheng L., Pevsner J., Lee L. A., Semenza G. L., Dang C. V. (2007) HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12, 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Riordan H. D., Jackson J. A., Schultz M. (1990) Case study. High-dose intravenous vitamin C in the treatment of a patient with adenocarcinoma of the kidney. J. Ortho. Med. 5, 5–7 [Google Scholar]
- 65. Alexander B., Fishman A. I., Eshghi M., Choudhury M., Konno S. (2013) Induction of cell death in renal cell carcinoma with combination of D-fraction and vitamin C. Integr. Cancer Ther. 12, 442–448 [DOI] [PubMed] [Google Scholar]
- 66. Mandriota S. J., Turner K. J., Davies D. R., Murray P. G., Morgan N. V., Sowter H. M., Wykoff C. C., Maher E. R., Harris A. L., Ratcliffe P. J., Maxwell P. H. (2002) HIF activation identifies early lesions in VHL kidneys. Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1, 459–468 [DOI] [PubMed] [Google Scholar]
- 67. Linehan W. M., Srinivasan R., Schmidt L. S. (2010) The genetic basis of kidney cancer. A metabolic disease. Nat. Rev. Urol. 7, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gilkes D. M., Bajpai S., Chaturvedi P., Wirtz D., Semenza G. L. (2013) Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]