Background: The human innate immune system can discriminate between Candida albicans yeast and hyphal forms.
Results: C. albicans hyphae possess glucan structures that are unique to the hyphae and are not found in yeast.
Conclusion: Hyphal glucan elicits robust immune responses.
Significance: These data provide a structural basis for differential immune recognition of C. albicans yeast versus hyphae.
Keywords: Candida albicans, Carbohydrate Structure, Host-Pathogen Interactions, Immunology, NMR, Glucan, Hyphae, Immune Function
Abstract
The innate immune system differentially recognizes Candida albicans yeast and hyphae. It is not clear how the innate immune system effectively discriminates between yeast and hyphal forms of C. albicans. Glucans are major components of the fungal cell wall and key fungal pathogen-associated molecular patterns. C. albicans yeast glucan has been characterized; however, little is known about glucan structure in C. albicans hyphae. Using an extraction procedure that minimizes degradation of the native structure, we extracted glucans from C. albicans hyphal cell walls. 1H NMR data analysis revealed that, when compared with reference (1→3,1→6) β-linked glucans and C. albicans yeast glucan, hyphal glucan has a unique cyclical or “closed chain” structure that is not found in yeast glucan. GC/MS analyses showed a high abundance of 3- and 6-linked glucose units when compared with yeast β-glucan. In addition to the expected (1→3), (1→6), and 3,6 linkages, we also identified a 2,3 linkage that has not been reported previously in C. albicans. Hyphal glucan induced robust immune responses in human peripheral blood mononuclear cells and macrophages via a Dectin-1-dependent mechanism. In contrast, C. albicans yeast glucan was a much less potent stimulus. We also demonstrated the capacity of C. albicans hyphal glucan, but not yeast glucan, to induce IL-1β processing and secretion. This finding provides important evidence for understanding the immune discrimination between colonization and invasion at the mucosal level. When taken together, these data provide a structural basis for differential innate immune recognition of C. albicans yeast versus hyphae.
Introduction
Candida albicans is a polymorphic fungal pathogen that can grow as a yeast, as pseudohyphae, or true hyphae (1). The ability of C. albicans to undergo morphogenic transformation from yeast to hyphae is a critical step in the pathogenicity of this opportunistic fungus (1). Specifically, yeast forms of C. albicans colonize the epithelium followed by hyphal penetration and invasion of the tissues (1). Epithelial invasion is followed by vascular dissemination, which involves hyphal penetration of blood vessels and seeding of the blood with yeast forms (1). Blood-borne C. albicans adhere to the vascular endothelium and form colonies followed by hyphal penetration into the tissues. Hyphae also play a role in biofilm development. Nett and Andes (2) have noted that the ability of C. albicans to form biofilms “has a profound impact” on the ability of the organisms to cause disease (3). Evidence also suggests that Candida biofilm development and maturation depends, in part, on yeast-to-hyphae transition (2). Despite the importance of C. albicans as a pathogen and the relevance of C. albicans hyphae to pathogenicity, there are very few reports on the structure and composition of C. albicans hyphal cell walls. Most of the published data has focused on ultrastructural analysis (electron microscopy) or immune responses to hyphae (1, 4).
We have shown that the innate immune system effectively discriminates between yeast and hyphal forms of C. albicans (4). We speculated that the differential recognition of C. albicans yeast versus hyphae is due to “differences in cell wall architecture” (4). Glucans are major structural components of the fungal cell wall (1, 5) and they are also major fungal pathogen-associated molecular patterns (6–8). The basic structure of glucan has been extensively investigated (9–11). In general, glucans are composed of a polymer backbone containing (1→3)-β-d-linked anhydroglucose repeat units (12–14). Some, but not all, glucan polymers exhibit side chain anhydroglucose repeat units that branch from the 6-position of the backbone anhydroglucose repeat units (9–11). Most of the reports on the physicochemical/structural analysis of glucans have focused on glucans derived from yeast such as Saccharomyces cerevisiae and C. albicans (9–11). There are only a handful of reports describing the physicochemical and structural characterization of hyphal cell wall glucans (15–17).
We have determined that the method of extraction can impact the higher structure of the glucan (18). Therefore, we developed a “modified” extraction method that is much less harsh than methods previously described for the isolation of blastospore/hyphal cell wall carbohydrates. This extraction method minimizes degradation and maintains more of the native glucan structure found in the cell wall. Using this approach we compared and contrasted the structure of C. albicans blastospore and hyphal extracts. We discovered that glucan is a major component of the C. albicans hyphal cell wall and that hyphal glucan contains macromolecular structures that have never been previously identified and are unique to hyphal glucan, i.e. these structures are not present in C. albicans blastospore glucan. We also found that this unique hyphal glucan structure elicits functionally distinct cytokine induction profiles in human peripheral blood mononuclear cells and macrophages when compared with yeast glucan.
EXPERIMENTAL PROCEDURES
Ethics Statement
Human peripheral blood mononuclear cells (PBMCs)3 were obtained after written informed consent. This study has been approved by the Ethics Committee of the region Arnhem-Nijmegen under the number NL32357.091.10.
C. albicans Hyphae
Wild type strain SC5314 was grown at 30 °C on yeast extract/peptone/dextrose. For hyphal production, 5 × 105 cells/ml were inoculated into prewarmed medium 199, pH 7.5, and grown for 4 h for well developed hyphae at 37 °C. For hyphal growth, six 4-liter flasks containing 2.5 liters of M199 prewarmed to 37 °C were inoculated with 5 × 105 cells/ml of overnight grown C. albicans. Cells were grown for 4 h with gentle shaking (70 rpm). Fully developed hyphae were confirmed microscopically before harvesting each flask by filtration. Cells (15 liters) were harvested by filtration. The average yield of pooled hyphal mass was 10 g before lyophilization. Yeast were grown in 2 liters of yeast extract/peptone/dextrose in a 40-liter flask grown overnight at 30 °C and harvested by centrifugation for glucan extraction. Yeast morphology was confirmed microscopically before harvesting each flask by filtration. The average cell mass harvested was 10 g before lyophilization.
Glucan Isolation
Glucan was isolated from C. albicans hyphae or yeast using modifications of the methods of Lowman et al. (16, 19) and Mueller et al. (18). Briefly, hyphal or yeast cell walls were extracted with 0.1 n NaOH (1× for 15 min at 100 °C) followed by neutralization to pH 7.0. The neutral residue was extracted with 0.1 n H3PO4 (1× for 15 min at 100 °C). The residue was neutralized to pH 7.0. The majority of the lipids were removed from the hyphal cell wall with boiling absolute ethanol (1× for 15 min) as previously described (20). The glucan extractions from hyphal or yeast forms were performed in parallel using the same reagents and extraction apparatus, i.e. a multiposition heating mantle with stirring and temperature control. S. cerevisiae glucan was isolated and characterized as described by our laboratory (21). The glucans isolated using this approach are water-insoluble microparticulates (1–5 μm). The particle distribution was similar between yeast and hyphal glucan. Protein was not detected in the glucans.
NMR Analysis
Proton and 13C NMR spectra were collected on a Bruker Avance II 800 NMR spectrometer using a 5-mm TCI inverse cryoprobe operating at 343 K. Approximately 25 mg of glucan was dissolved in 950 μl of DMSO-d6 (Sigma; “100”, p/n 156914, CAS 2206-27-1) with 40 μl of trifluoroacetic acid-d (Cambridge Isotope Laboratories, 99.8+% deuterated, p/n DLM-46, CAS 599-00-8) to shift the exchangeable proton resonances downfield. Approximately 550 μl of the solution was placed into a 5-mm NMR tube. Trifluoroacetic acid was added just before NMR analysis. Proton and 13C one-dimensional and COSY (22, 23), NOESY (24, 25) with a 150-ms mixing time, HSQC (26–28), HSQC-TOCSY (29, 30) with a 60-ms mixing time, and HMBC (31) two-dimensional NMR spectra were obtained in this study. For one-dimensional NMR experiments, chemical shift referencing used absolute referencing based on the 2H DMSO-d6 lock signal and 1H and 13C gyromagnetic ratios. For two-dimensional NMR experiments, chemical shift referencing was accomplished relative to the anomeric resonance of the (1→3)-β-linked backbone repeat unit at 4.54 ppm for 1H and 102.49 for 13C relative to DMSO-d6 residual protons as referenced above. One-dimensional and two-dimensional NMR spectra were collected and processed under conditions similar to those used previously (19). NMR spectra were processed using TOPSPIN 2.1 running on the Avance II 800 NMR and TOPSPIN 3.0.b.8 running on Windows XP Professional operating system under VMWare Fusion version 2.0.5 (VMWare, Inc., Palo Alto, CA) on a Macintosh MacBook Pro.
Monosaccharide Composition and Linkage Analysis
To analyze the sugar composition of the C. albicans hyphal glucan by GC-MS, the alditol acetate derivatives of the monosaccharides were analyzed (32). Polysaccharide was hydrolyzed in 4 m trifluoroacetic acid (TFA) for 4.5 h at 105 °C with frequent stirring followed by reduction with NaBD4 in water overnight. The sample was then acetylated using acetic anhydride at 105 °C for 90 min. The alditol acetate derivatives were analyzed by GC using a Varian 3400 gas chromatograph equipped with a 30-m DB-17 capillary column (210 °C (30 min), then 240 °C at 2 °C/min) and by GC-MS in the electron-impact mode on a ThermoFinnigan PolarisQ instrument. Methylation linkage analysis was performed using the DMSO/NaOH/CH3I procedure, and the permethylated alditol acetate derivatives were characterized by GC-MS in the electron-impact mode (DB-17 column, isothermal at 190 °C for 100 min) (33).
Smith Degradation
Polysaccharide (10 mg) was dissolved in a 5-ml solution of 0.1 m sodium acetate, 0.04 m NaIO4 at pH 4 and left at 4 °C in the dark for 3 days to oxidize the glucose units with vicinal hydroxyls (terminal and 6-linked glucose residues). The oxidized material was dialyzed against water (cut-off 1000 Da) and lyophilized. The oxidized material was reduced with NaBH4 at room temperature for 5 days. The sample was treated with1 m TFA at 45 °C for 1 h to cleave the acyclic acetal moieties and then dialyzed against water and freeze-dried.
Powder X-ray Diffraction Analysis
Powder diffraction measurements were conducted at room temperature on a SuperNova Agilent diffractometer equipped with a microfocus CuKα (λ = 1.54184 Å) radiation source and Atlas CCD detector. Microsamples of glucans sized between 0.2 and 0.4 mm were mounted on glass fibers in such a way that only the glucan substance was exposed to the x-ray beam. The diffraction images were recorded in the 5–60º 2θ-range, but no features above 30º were observed. The images were processed using CrysAlisPro software v.1.171.35.8 (Agilent Technologies, Santa Clara, CA). The diffraction pattern did not show any noticeable changes for different samples of the same material or after the samples were exposed to the atmosphere, suggesting the materials investigated were structurally homogeneous and air-stable.
Human PBMC and Macrophage Stimulation
Human PBMCs were isolated by density centrifugation of buffy coats (Sanquin Biobank, Nijmegen), diluted 1:1 in pyrogen-free saline over Ficoll-Paque (GE Healthcare). Cells were washed twice in saline and resuspended in culture medium (RPMI, Invitrogen) supplemented with gentamicin 10 μg/ml, l-glutamine 10 mm, and pyruvate 10 mm. Cells were counted in a Coulter counter (Coulter Electronics), and the number was adjusted to 5 × 106 cells/ml. A total of 5 × 105 mononuclear cells in a 100-μl volume was added to round-bottom 96-well plates (Greiner) with RPMI and thereafter incubated at 37 °C with either 10 μg/ml β-glucan particles in suspension or culture medium with the final volume of 200 μl per well. After 24 h, supernatants were collected and stored at −20 °C until assayed. For macrophage differentiation, adherent human monocytes were cultured in complete RPMI 1640 medium (ICN Biomedicals) supplemented with 100 ng/ml human macrophage colony-stimulating factor and 10% pooled human serum for 6 days.
To assess the role of Dectin-1 for cytokine induction by hyphal glucan, PBMCs isolated from either volunteers expressing the normal Dectin1 gene or a defective premature stop-codon variant (34) were stimulated with similar amounts of the yeast or hyphal extract.
Cytokine Measurements
IL-6, TNF, and IL-1β concentrations were measured by commercial sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. For intracellular IL-1β measurement, after collection of supernatant for extracellular cytokine measurement, 200 μl of fresh RPMI was added to the macrophages. Three freeze-thaw cycles were used to disrupt the cell membranes, and intracellular IL-1β was determined by ELISA.
Quantitative IL-1β Polymerase Chain Reaction
Macrophages were stimulated as described above. The supernatant was removed after 4 h, and the cells were resuspended in 200 μl of RNAzolB RNA isolation solvent (Campro Scientific) and stored at −80 °C. mRNA was isolated according to the instruction of manufacturer. cDNA was synthesized from 1 μg of total RNA by use of SuperScript reverse transcriptase (Invitrogen). Relative mRNA levels were determined using the Bio-Rad i-Cycler and the SYBR Green method (Invitrogen). The following primers were used: IL-1β forward primer (5′-GCCCTAAACAGATGAAGTGCTC-3′) and reverse primer (5′-GAACCAGCATCT TCCTCAG-3′) and β2M forward primer (5′-ATGAGTATGCCTGCCGTGTG-3′) and reverse primer (5′-CCAAATGCGGCATCTTCAAAC-3′) (Biolegio). Values are expressed as -fold increases in mRNA levels relative to those in unstimulated cells. The expression of IL-1β was normalized to the expression of HPRT-1.
Statistics
Cytokine expression data are presented as means ± S.E. The Wilcoxon sign ranked test was used to compare differences between groups. The level of significance was set at p < 0.05.
RESULTS
Evidence for a Cyclic Glucan Structure in C. albicans Hyphae
Recently we reported the complete 1H and 13C NMR chemical shift assignments for a linear (1→3)-β-d-glucan with long (1→6)-β-linked glucosyl side chains (SCs) isolated from Candida glabrata based upon extensive one- and two-dimensional NMR studies (19). The average side chain length was 4.7 (1→6)-linked repeat units with an average side chain branching frequency of 21 (1→3)-linked backbone repeat units (RUs). NMR analysis of this branched, linear (1→3,1→6)-β-d-glucan exhibited clear evidence for the presence of reducing (RT) and non-reducing (NRT) termini as well as the backbone polymer repeat unit for the glycosyl repeat unit second from RT (SRT). In addition, repeat units for the first (SC1), internal (SCInt), next to last (SC SNRT), and last (SCNRT) (1→6)-linked repeat units in SC as well as the SC branch point (Br) within the backbone chain were clearly defined. Using the chemical shift assignments derived from Lowman et al. (19), we were able to critically examine hyphal glucan using one-dimensional and two-dimensional NMR techniques.
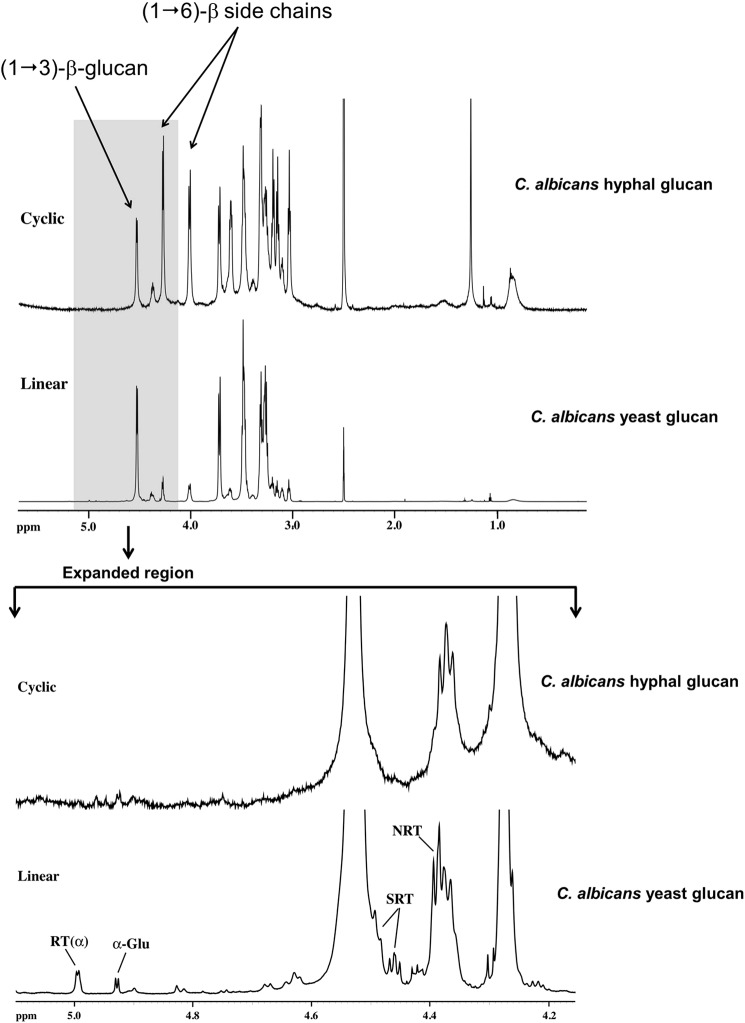
The one-dimensional 1H NMR spectrum of hyphal glucan (Fig. 1, top) clearly shows only carbohydrate resonances between 3.0 and 5.0 ppm with residual lipid resonances between 0.7 and 1.5 ppm. Examination of the anomeric 1H spectral region of the hyphal glucan versus the linear C. albicans yeast glucan isolated under the same conditions (Fig. 1, bottom) clearly shows the absence of resonances assigned to the α-anomer of RT, SRT, and NRT RUs in cyclic glucan when compare with the linear glucan. Only resonances for repeat units assignable to unbranched and branched (1→3)-β-linked repeat units in the backbone were observed in the two-dimensional NMR spectra. There was no evidence for resonances assignable to SRT and NRT in the linear backbone based upon comparison of the HSQC two-dimensional NMR spectra of the linear and cyclic glucans (Fig. 2). These observations are consistent with data reported for cyclic (1→3)-β-glucans, such as the glucan isolated from the basidiomycete Phanerochaete chrysosporium (35). Resonances for (1→6)-β-linked side chain-related repeat units assigned to Br, SC1, SCInt, SC SNRT, and SCNRT were readily assigned by NOESY, HSQC-TOCSY, and HMBC two-dimensional NMR spectra. Resonances for the first side chain RU (1→6)-linked at Br (SC1) are only observed for the anomeric 1H and its neighboring 1H due to resonance overlap of the remaining 1H atoms in that RU with internal (1→6)-linked side chain RUs. Protons of the internal RUs are clearly assignable. Methylene (H6 and H6′) 1H atoms of the RU (1→6)-linked to SC NRT and the anomeric 1H and its neighboring 1H of the SC NRT are observed, whereas the remaining 1H atoms of these two RUs are obscured due to resonance overlap. As noted above, this detailed analysis of the two-dimensional NMR side chain spectra were compared with side chain resonances from a branched C. glabrata glucan (19) to confirm the 1H and 13C chemical shift assignments presented in Table 1 for the cyclic glucan.
FIGURE 1.
1H NMR analysis indicates that C. albicans hyphal glucans lack reducing and non-reducing termini. Specifically, there is no evidence for anomeric proton resonances (expanded region) for repeat units next to the RT, α, and β anomers of RT, NRT, or SRT side chains (arrows).
FIGURE 2.
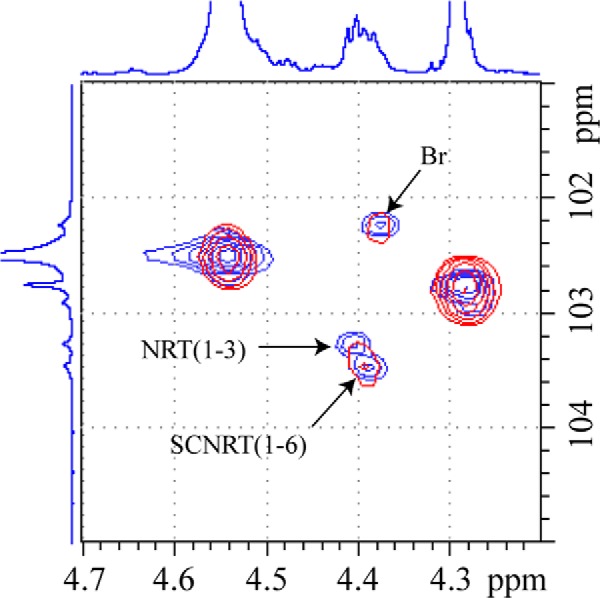
Comparison of HSQC two-dimensional NMR spectra of the branched, linear glucan (blue) and the branched, cyclic glucan (red). The one-dimensional spectra on the borders are from the linear, branched glucan. Cross-peak for resonances assigned to NRT in the linear backbone (NRT(1–3)) are labeled (blue spectrum). The absence of this resonance in the spectrum of the cyclic glucan (red spectrum) is evident. Br, branch point; SCNRT, side chain non-reducing terminus.
TABLE 1.
Proton (1H) and 13C NMR chemical shift assignments for C. albicans hyphal glucan
SC SNRT indicates side chain second glucose residue from the non-reducing terminus, and SC NRT indicates side chain non-reducing terminus.
| Chemical shift |
||||||
|---|---|---|---|---|---|---|
| (1→3)-β-Linked backbone chain | Br | SC1 | SC internal (1→6)-β-linked side chain | SC SNRT (1→6) | SC NRT (1→6) | |
| ppm | ||||||
| Proton assignment | ||||||
| H1 | 4.542 | 4.374 | 4.255 | 4.282 | a | 4.364 |
| H2 | 3.321 | 3.258 | 3.038 | 3.045 | a | 3.107 |
| H3 | 3.502 | 3.408 | a | 3.202 | a | a |
| H4 | 3.265 | 3.327 | a | 3.163 | a | a |
| H5 | 3.322 | 3.225 | a | 3.328 | a | a |
| H6 | 3.729 | 4.012 | a | 4.021 | 4.012 | a |
| H6′ | 3.483 | 3.642 | a | 3.619 | 3.640 | a |
| Carbon assignment | ||||||
| C1 | 102.49 | 102.16 | 102.91 | 102.73 | a | 103.28 |
| C2 | 72.93 | 71.75 | a | 72.89 | a | 73.31 |
| C3 | 85.72 | 86.70 | a | 76.01 | a | a |
| C4 | 67.95 | 67.71 | a | 69.57 | 69.71 | 69.70 |
| C5 | 74.96 | 74.50 | a | 75.01 | a | a |
| C6 | 60.41 | 67.67 | a | 68.03 | a | 60.60 |
a Resonance was not observed due to resonance overlap.
For the cyclic glucan, the average side chain length was 13.9 (1→6)-linked repeat units with an average side chain branching frequency of 12.5 (1→3)-linked backbone repeat units, both calculated previously reported (19). Compared with the previously characterized linear, branched glucan (19), the cyclic, branched glucan exhibits a higher concentration of side chains that are on average three times longer and occur at twice the frequency along the linear chain, i.e. the branching frequency of hyphal glucan is twice that of blastopore glucan.
To confirm the reproducibility of this observation we conducted a separate experiment in which four C. albicans hyphal preparations were extracted simultaneously. The hyphal glucan was analyzed by NMR as described above. We identified the cyclical nature of the hyphal glucan in all four samples (data not shown), thus demonstrating that this is a consistent observation.
Monosaccharide Composition and Linkage Analysis of Hyphal Glucan
Sugar composition analysis revealed that the C. albicans hyphae polysaccharide isolate contains predominantly glucose (Glc) (Fig. 3A). In addition to the glucose, traces of mannose and N-acetyl-glucosamine (GlcNAc) were also detected. Linkage-type analysis showed two major glucose linkages, 3-substituted and 6-substituted linear units, and in lower amounts, 2,3-disubstituted and 3,6-disubstituted branch units (Fig. 3B). A very small quantity of terminal glucose was also detected.
FIGURE 3.
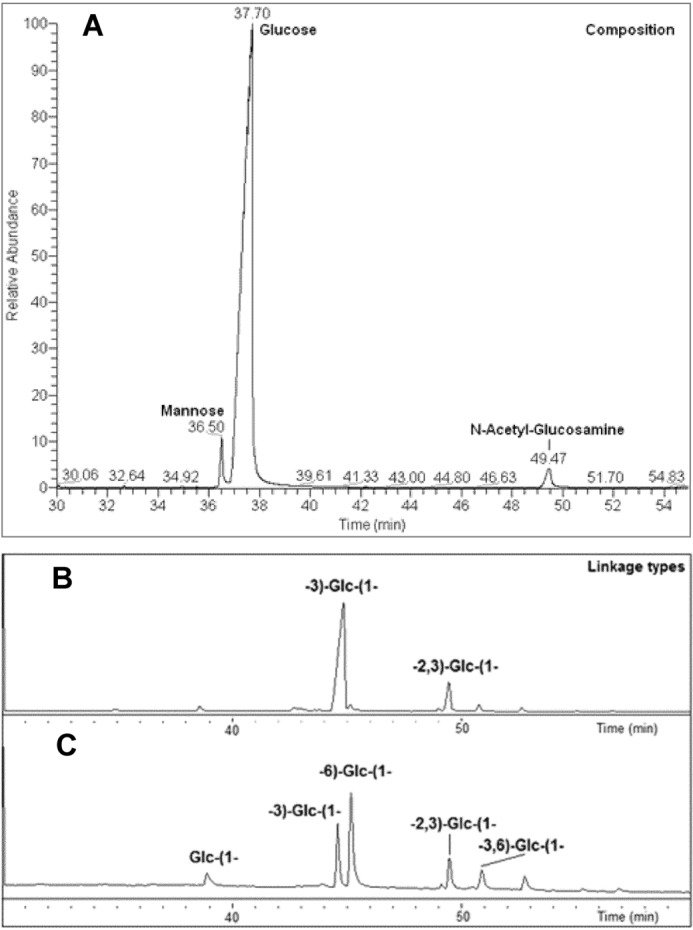
Monosaccharide composition and linkage analysis of C. albicans hyphal glucan. A, GC profile showing glucose as the dominant monosaccharide unit in the C. albicans hyphal glucan with traces of mannose and N-acetyl-glucosamine. B, Smith degradation of C. albicans hyphal glucan confirms the presence of the 2,3 linkage. C, GC profile of the linkage analysis of the Smith degradation product shows the elimination of 6-linked, 3,6-linked, and terminal glucose compared with the parent polysaccharide (bottom). These data confirm the backbone of the polysaccharide is composed of the 3-linked glucose and that the 6-linked chains branch off the 6-position of the 3,6-linked units.
Smith Degradation Analysis of C. albicans Glucan
The Smith degradation resulted in the elimination of terminal, 6-linked (linear) and 3,6-linked (branch) glucose units (Fig. 3C). The 3-linked and 2,3-linked glucose units remained intact. Also, the amount of 3-linked glucose in the Smith-degraded product increased due to the conversion of the 3,6-linked glucose branch unit to a 3-linked glucose linear residue, which indicated that the constituent attached to the 6-position of the 3,6-linked glucoses was a 6-linked glucose unit. The Smith degradation results suggest that the hyphal glucan is composed of a 3-linked backbone with 6-linked glucose chains branching from the 6 position of intermittent 3,6-linked RUs. The survival of the 2,3-linked branch glucose unit points to the fact that the components attached to the 2 and 3 position of this branch glucose are periodate-resistant. The survival of 2,3-linked glucose also confirmed that the 6-linked glucans are attached solely to the 6-position of the 3,6-linked glucose.
Powder X-ray Diffraction Analysis of C. albicans Hyphal Glucan
Powder x-ray diffraction analysis patterns reveal crystallinity in the hyphal and yeast glucans from C. albicans and glucan from S. cerevisiae (Fig. 4). The presence of peaks on the diffractograms indicates that there are crystal-like fragments (“crystallites”) with repeat periods of ∼13.8 Å (from peaks at 6.4º, 12.8º, and 19.2º) and ∼9.8 Å (peak at ∼9.4º). The broad peaks are consistent with small and imperfect crystallites. Although all three glucans show similarity of their crystal structure motifs, variations in the peak intensities suggest different shapes or mutual orientation of the crystallites within these glucans that may result from different length of the polymeric chains building the crystallites or different orientation of these chains with respect to each other.
FIGURE 4.
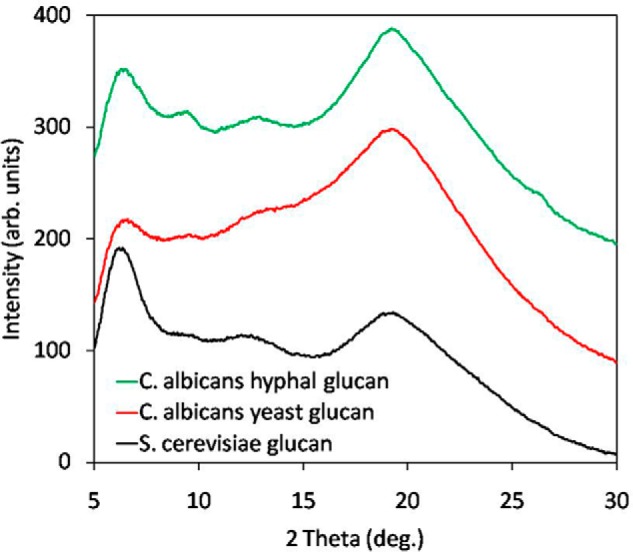
Powder x-ray diffractograms of the hyphal and yeast glucans from C. albicans with glucan from S. cerevisiae used as a reference. Although all three glucans show similarity of their crystal structure motifs, the variations in the peak intensities suggest different shape or mutual orientation of the crystal-like fragments (crystallites) in these glucans. This in turn may result from different length of the polymeric chains building the crystallites or their different orientation with respect to each other.
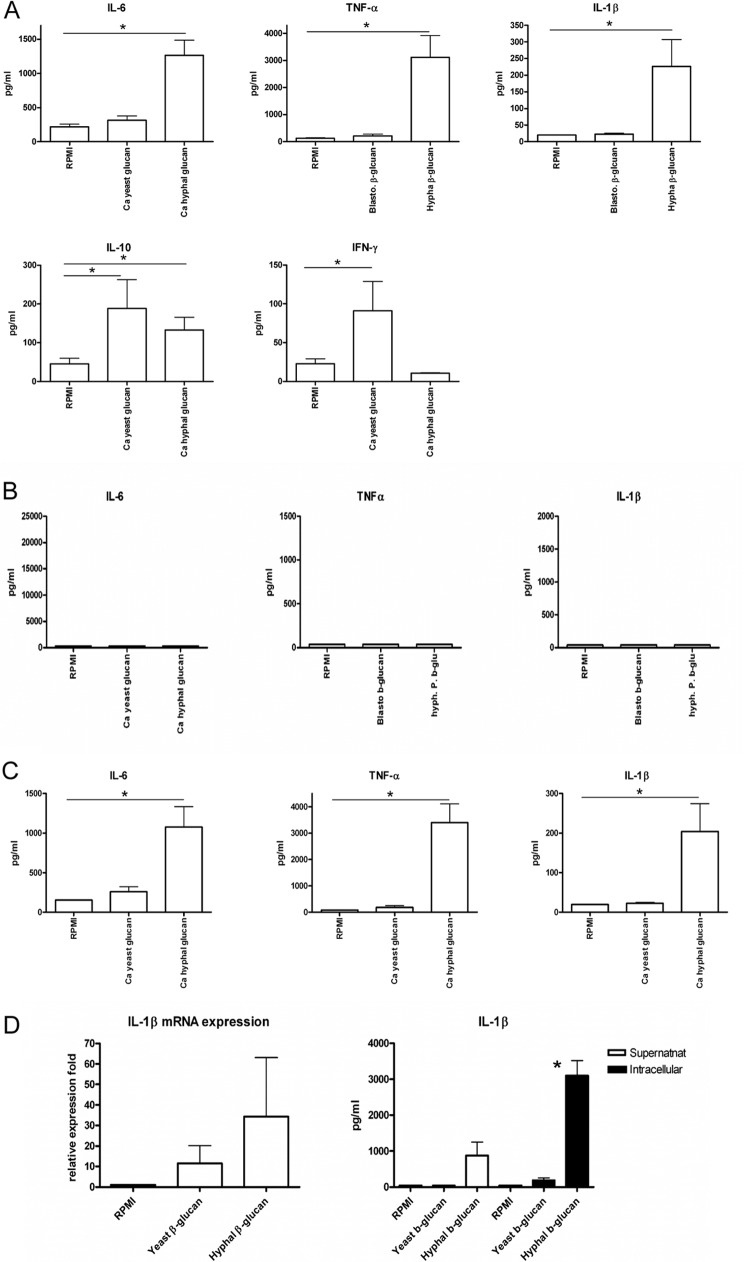
Human PBMCs Show Differential Cytokine Expression in Response to C. albicans Yeast and Hyphal Glucan
β-Glucan is a major cell wall component of C. albicans, and extensive work has been carried out to examine the functional effect of β-glucan in terms of its receptor and downstream signaling (36–40). However, most of the β-glucan studied in the past was extracted from yeasts, and few studies have been performed with hyphal glucans. Based on the fundamental structural differences between C. albicans yeast and hyphal glucan, we hypothesized that differential recognition of the two different glucans by the host might lead to different downstream responses. To critically evaluate this hypothesis, cytokine profiles induced by yeast and hyphal glucan in human PBMCs were assessed (Fig. 5A). In agreement with previous results (4), C. albicans yeast glucan is not a potent stimulus for human PBMCs by itself and induces only small amounts of TNF-α, IL-6, and IL-β. In contrast, hyphal glucan is able to induce strong proinflammatory cytokine production (Fig. 5A). Interestingly, yeast glucan induces higher IFN-γ than hyphal glucan, thus emphasizing the differential response of PBMCs to hyphal versus yeast glucan. The hyphal glucan-induced cytokine production is fully dependent on Dectin-1 signaling. Specifically, human PBMCs from Dectin-1-deficient patients failed to produce cytokines upon hyphal glucan stimulation (Fig. 5B).
FIGURE 5.
Differential cytokine responses to C. albicans (Ca) hyphal versus yeast glucan is mediated through a Dectin-1-dependent mechanism. A, hyphal glucan stimulates cytokine expression from normal human PBMCs, whereas yeast glucan does not. B, PBMCs from Dectin-1-deficient patients do not respond to C. albicans hyphal or yeast glucan, indicating that the hyphal glucan response is Dectin-1-dependent. C, C. albicans hyphal glucan stimulates cytokine expression in human monocyte-derived-macrophages. Monocyte-derived macrophages were incubated with hyphal or yeast glucan. Media alone served as control. Supernatant was collected for TNFα, IL-6, and IL-1β ELISA after 24 h stimulation. *, p < 0.05 versus RPMI control. D, C. albicans hyphal glucan is a more potent stimulus for IL-1β production in human monocyte-derived macrophages than yeast glucan. IL-1β mRNA expression was measured by RT-PCR after 4 h of stimulation. Extracellular and intracellular IL-1β production was measured by ELISA after 24 h of stimulation. Data are presented as the mean ± S.E. Results are from at least three sets of experiments, with a minimum of six volunteers were pooled and analyzed using GraphPad Prism software. *, p < 0.05.
Human Monocyte-derived Macrophages Show Differential Cytokine Expression in Response to C. albicans Yeast and Hyphal Glucan
We next examined whether the differential cytokine induction observed in PBMCs holds true when human monocyte-derived macrophages were used as the target cells. Similar patterns were observed with almost no induction of cytokine by yeast glucan and strong induction by hyphal glucan (Fig. 5C).
C. albicans Hyphal Glucan Is a Potent Stimulus for Human Macrophage IL-1β Responses
We have demonstrated previously that yeast-to-hyphae transition triggers inflammasome activation in macrophages and induces IL-1β processing and secretion (4). The differential induction of IL-1β by yeast and hyphal β-glucan could, therefore, be an alternative recognition mechanism at the level of the mucosae. We assessed IL-1β mRNA expression in human macrophages. Yeast glucan is able to induce IL-1β mRNA expression, although the mRNA level is lower than with the hyphal glucan-treated group (Fig. 5D). Intracellular IL-1β measurement (including the inactive proIL-1β) clearly demonstrated that although there is intracellular IL-1β in yeast glucan-treated macrophages, no extracellular IL-1β was detectable. In contrast, IL-1β was present both intracellularly and extracellularly when macrophages were stimulated with C. albicans hyphal glucans. In fact, intracellular IL-1β was significantly (p < 0.05) greater in the hyphal glucan-treated cells. This demonstrates that although both yeast and hyphal glucans can induce the first steps of transcription and translation of proIL-1β, only the hyphal glucan can induce processing and release of bioactive IL-1β by macrophages.
DISCUSSION
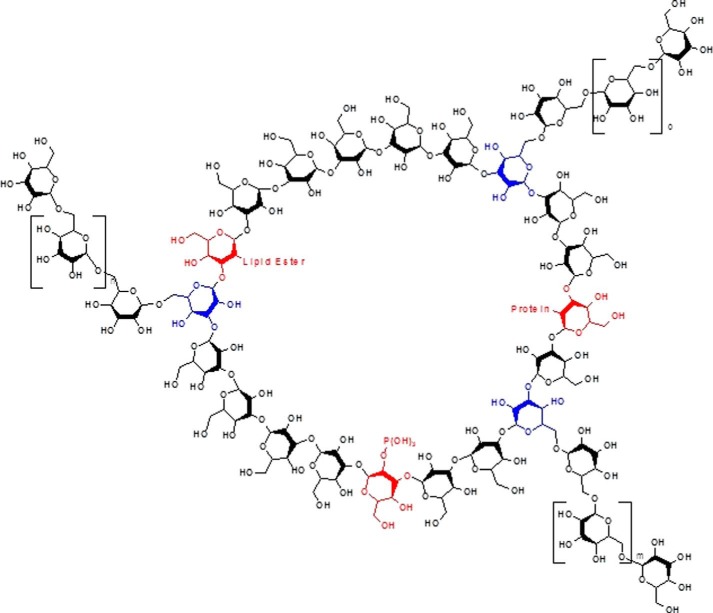
Several new and important observations have emerged from this study. First, using a new, milder extraction approach, we have isolated and purified the glucan from C. albicans hyphae. Our data are consistent with a glucan that is composed of a cyclic (1→3)-β-linked polymer backbone with long (1→6)-β-linked side chains stemming from the 6-position of 3,6 branches that occur at twice the frequency observed for linear blastospore glucan (Fig. 6). Second, glucan appears to be a major structural carbohydrate in the cell wall of C. albicans hyphae. Third, C. albicans hyphal glucan does not exhibit the reducing termini that are routinely observed in (1→3)-β-glucans isolated from C. albicans blastospores. Consequently, C. albicans hyphal glucan is structurally distinct from C. albicans blastospore glucan. NMR data indicate that the hyphal glucan structure is consistent with a cyclical (1→3)-β-glucan. To the best of our knowledge, this is the first report of a cyclical glucan in C. albicans. Fourth, in addition to the (1→3), (1→6), and 3,6 linkages, we identified a 2,3 linkage in hyphal glucan that has not been reported previously in C. albicans. Finally, we compared and contrasted immune responsiveness to yeast and hyphal glucans. We found that hyphal glucan induces stronger proinflammatory cytokine production in human PBMCs and macrophages than does C. albicans yeast glucan. In addition, hyphal glucan can induce processing and release of bioactive IL-1β by human macrophages, but yeast glucan does not. This indicates that C. albicans hyphal glucan dictates structure-specific immune responses distinct from those elicited by blastospore glucan. We conclude that hyphal glucan is a major pathogen-associated molecular pattern in hyphae.
FIGURE 6.
Conceptual model of C. albicans hyphal glucan showing the cyclical (1→3)-β-linked polymer backbone with long (1→6)-β-linked side chains. In addition to the expected (1→3), (1→6), and 3,6 linkages (blue), we identified a 2,3 linkage (red) in hyphal glucan that has not been reported previously in C. albicans or any other fungi. Specifically, the 2,3 structure is a glucose subunit in the (1→3)-β-linked polymer backbone that also has a linkage point at the 2-position (hence, the 2,3 designation) rather than the 6-position, i.e. the (1→6)-β-linked side chains. The model does not represent the molar mass, i.e. molecular weight, of the hyphal glucan; rather the model is intended to be a conceptual representation of the cyclical or closed chain nature of the hyphal glucan.
At the present time we do not fully understand the structural significance of the cyclical hyphal glucan. However, we know that hyphae are produced rapidly, and our data clearly show that glucan is a major structural carbohydrate in C. albicans hyphal cell wall. We speculate that hyphal cell wall glucan is structurally different from blastospore cell wall glucan due to the different kinetics of its induction during hyphal growth, with hyphal cell wall integrity being almost entirely dependent on the glucan.
It is well established that the C. albicans yeast cell walls contain substantial amounts of mannan (41). Using single-molecule atomic force probe microscopy Beaussart et al. (42) have recently reported that mannans are more abundant on C. albicans hyphae versus yeast. As part of this investigation we attempted to isolate mannan from C. albicans hyphae using mannan specific extractions methods previously reported by our group (43–45). We were not able to isolate hyphal mannans, except in trace quantities that were too small to be analyzed. The difference between our results and those of Beaussart et al. (42) may reflect differences in methodology. It is also possible that hyphal mannan has a lower molecular weight than yeast mannan. This may make it more difficult to isolate using standard mannan extraction methods. Whatever the case, our results indicate that mannans are present in low abundance on the hyphal cell wall.
The discovery of the 2,3 linkage may provide important insights into hyphal cell wall organization, inter-connections, and structural integrity. It is currently thought that the (1→6)-glucan side chains serve as attachment points for cell wall proteins through GPI remnants (46). However, the (1→6) side chains terminate in non-reducing termini that are not reactive. This raises the question of how proteins and other macromolecules are attached to non-reactive side chain termini. In contrast, the 2,3 structure may be ideally suited for linkage of the hyphal glucan with other macromolecules, such as cell wall proteins or lipids or perhaps as bridges between the 3-linked cyclic glucans forming a macromolecular structure (Fig. 6). Additional studies will be required to unambiguously determine the identification of components attached to the two position. The linkage analysis data indicate that the 2,3 linkage is present in hyphal glucan at levels that should be adequate for linking to other macromolecules, i.e. carbohydrates, proteins, or lipids, within the hyphal cell wall.
Only a few types of β-glucans showing crystallinity have been reported in the literature (47–49). Only (1→2)-β glucans have been reported to form cyclic molecules, but no diffraction data have been reported for these glucans (50–53). In the present study, the powder x-ray diffraction analysis data (Fig. 4) make it possible to compare the studied glucans with other crystalline or semi-crystalline glucans reported previously (48, 49, 54–58). The linear (1→4)-β glucan, cellulose, exhibits several crystalline forms with the polymeric chains combined in H-bonded sheets (54, 55). These forms are monoclinic with the longest repeat distance of ∼10 Å (54, 55). Two linear natural product (1→3)-β glucans, paramylon (Euglena species) and curdlan (Alcaligenes bacteria), also exhibit a number of crystalline forms, but the polymeric molecules form helices (48, 56, 57). Paramylon forms a structure where the longest repeat period is 13.6 Å (48, 56, 57). The most recent report confirms a hexagonal structure with a = 15.547 Å and c = 18.587 Å for never dried and a = 14.543 Å and c = 5.853 Å for dried paramylon (57). Curdlan was isolated in three forms (47), two being identical to the never dried and dried forms of paramylon (48, 59). The third form is reported to be hexagonal with a = 17.01 Å and c = 22.70 Å (47, 58). A recent study reported two forms of curdlan with repeat periods of 15.4 and 13.6 Å (49). To the best of our knowledge, no diffraction data were reported for this linear (1→6)-β glucan. Pelosi et al. (60) have reported powder x-ray diffraction analysis images of (1→3,1→6)-β glucan from Saprolegnia monoïca and Rubus fructicosus, with the longest repeat distances of ∼13.5 and ∼15 Å, respectively. Although naturally occurring cyclic glucans have been isolated and studied, no evidence of their crystallinity was reported.
From these comparisons, the crystallites in the glucans we studied were formed by the helices of either (1→3) or (1→6) chains but not by cyclic fragments. If the 13.82 ± 0.14 Å repeat period is the a parameter of a hexagonal unit cell, then the distance between the helices (and hence the diameter of the helix) is ∼16 Å. The crystallites in hyphal, and yeast glucans seem to have a similar crystal structure, but they differ in size and mutual orientation. This is consistent with a different orientation of side chains attached to either cyclic or linear backbone.
Evidence indicates that the innate immune system effectively discriminates between yeast and hyphal forms of C. albicans (4). However, the mechanistic basis for the ability of the immune system to differentiate between C. albicans yeast and hyphal forms is the subject of debate. Several immune mechanisms and pattern recognition receptors have been proposed to mediate this discriminatory activity, including C-type lectin receptors Dectin-1 (4), TLR2 (61), or Dectin-2 (62–64), and/or MAPK (63). Recent data have also documented differential inflammasome activation by C. albicans yeast and hyphae (1, 4, 46, 65). We have previously demonstrated that the inflammasome is differentially activated by C. albicans yeast and hyphae in macrophages by comparing wild type and yeast-locked C. albicans strains (4). In the present study we provide a structural explanation for this differential recognition of hyphae versus yeast. By demonstrating the capacity of hyphal glucan to induce IL-1β processing and secretion, we provide an important piece of evidence for understanding the immune discrimination between C. albicans colonization and invasion at the mucosal level (1).
It is well established that the innate immune system recognizes and responds to fungal glucans based on structural features including, but not limited to, the (1→3)-β-linked backbone and/or the presence, frequency, and length of (1→6)-β-linked side chains (40, 66–68). The present data clearly demonstrate that hyphal glucan has at least three unique structural features, i.e. cyclical structure, 2,3 linkages, and (1→6)-β-linked side chains that are longer and of greater frequency, any or all of which may contribute to the immune recognition of hyphae. We found that hyphal glucan, but not yeast glucan, induces IL-1β secretion by macrophages, implying that the unique structure of this glucan might serve as a hyphal specific pathogen-associated molecular pattern to activate inflammasomes in macrophages. Moreover, hyphal glucan induces higher levels of proinflammatory cytokines in PBMCs. This indicates that the recognition of hyphal glucan represents a danger signal for the innate immune system to respond in a more robust way. Dectin-1 recognition is involved in this process; however, the stronger cell stimulation by hyphal glucan suggests that a different receptor complex containing Dectin-1 is engaged when cells are stimulated by hyphae. Presumably, this not only happens at the site of the mucosal barrier where tissue macrophages are located but also in the blood where monocytes are present. Dectin-1 is known to interact with a variety of other pattern recognition receptors (69–71). We speculate that differential engagement of pattern recognition receptors by hyphal versus yeast glucan may explain, in part, the difference in immunological responses that we observed. It also possible that the other pattern recognition receptors may recognize hyphal proteins and/or lipids present at very low levels in the hyphal glucan.
In summary, our data indicate that C. albicans hyphal glucan contains structures that have not been previously identified and appear to be unique to hyphal glucan, i.e. these structures are not present in C. albicans blastospore glucan. In fact, these structures have not been previously identified in any Candida species or other pathogenic fungus for that matter. We have shown that the innate immune system effectively discriminates between yeast and hyphal forms of C. albicans (4). We speculated that the differential recognition of C. albicans yeast versus hyphae was due to differences in cell wall architecture (4). The present data provide compelling evidence that the structural differences in hyphal glucan induces greater proinflammatory cytokine production in PBMCs and inflammasome activation in macrophages. We conclude that hyphal glucan is a major pathogen-associated molecular pattern in C. albicans hyphae. To the best of our knowledge, these are entirely new and novel findings which advance our understanding of hyphal cell wall structure and composition. These data also shed light on the biology and immune recognition of hyphae, and they provide a structural basis for the differential recognition of C. albicans yeast versus hyphae.
This work was supported, in whole or in part, by National Institutes of Health Grant NIHGM53522 (to D. L. W.). This was also supported by the Natural Science and Engineering Research Council (NSERC; to M. A. M.) and a CFI/MRI grant for x-ray diffractometer (to D. S.). D. W.L. is an employee of AppRidge International; however, no funds were provided by AppRidge for this research, nor does D. W. L. have any competing interests or conflicts of interest related to this research.
- PBMC
- peripheral blood mononuclear cell
- HSQC
- heteronuclear single quantum correlation
- TOCSY
- two-dimensional total correlation spectroscopy
- HMBC
- heteronuclear multiple bond coherence
- SC
- side chain
- RU
- repeat unit
- RT
- reducing terminus
- NRT
- non-reducing terminus
- SRT
- second glucose subunit from the reducing terminus.
REFERENCES
- 1. Gow N. A., van de Veerdonk F. L., Brown A. J., Netea M. G. (2012) Candida albicans morphogenesis and host defence. Discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nett J., Andes D. (2006) Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 9, 340–345 [DOI] [PubMed] [Google Scholar]
- 3. Ramage G., Rajendran R., Sherry L., Williams C. (2012) Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng S. C., van de Veerdonk F. L., Lenardon M., Stoffels M., Plantinga T., Smeekens S., Rizzetto L., Mukaremera L., Preechasuth K., Cavalieri D., Kanneganti T. D., van der Meer J. W., Kullberg B. J., Joosten L. A., Gow N. A., Netea M. G. (2011) The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 90, 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams D. L., Lowman D. W., Ensley H. E. (2004) Introduction to the chemistry and immunobiology of β-glucans. In Toxicology of 1→3-β-glucans. Glucans as a marker for fungal exposure (Young S. H., Castranova V., eds), pp. 1–34, Taylor & Francis, New York [Google Scholar]
- 6. Brown G. D., Gordon S. (2003) Fungal β-glucans and mammalian immunity. Immunity 19, 311–315 [DOI] [PubMed] [Google Scholar]
- 7. Brown G. D., Gordon S. (2005) Immune recognition of fungal β-glucans. Cell. Microbiol. 7, 471–479 [DOI] [PubMed] [Google Scholar]
- 8. Brown G. D., Williams D. L. (2009) (1,3)-β-Glucans in innate immunity. Mammalian systems. In Chemistry, Biochemistry, and Biology of (1,3)-β-Glucans and Related Polysaccharides (Bacic A., Fincher G. B., Stone B. A., eds), p. 677, Academic Press, Elsevier Inc., San Diego, CA [Google Scholar]
- 9. Ensley H. E., Tobias B., Pretus H. A., McNamee R. B., Jones E. L., Browder I. W., Williams D. L. (1994) NMR spectral analysis of a water-insoluble (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 258, 307–311 [DOI] [PubMed] [Google Scholar]
- 10. Kim Y. T., Kim E. H., Cheong C., Williams D. L., Kim C. W., Lim S. T. (2000) Structural characterization of β-d-(1→3, 1→6) glucans using NMR spectroscopy. Carbohydr. Res. 328, 331–341 [DOI] [PubMed] [Google Scholar]
- 11. Lowman D. W., Williams D. L. (2001) A proton nuclear magnetic resonance method for the quantitative analysis on a dry weight basis of (1→3)-β-d-glucans in a complex, solvent-wet matrix. J. Agric. Food Chem. 49, 4188–4191 [DOI] [PubMed] [Google Scholar]
- 12. Kapteyn J. C., Hoyer L. L., Hecht J. E., Müller W. H., Andel A., Verkleij A. J., Makarow M., Van Den Ende H., Klis F. M. (2000) The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 35, 601–611 [DOI] [PubMed] [Google Scholar]
- 13. Chauhan N., Li D., Singh P., Calderone R., Kruppa M. (2002) The Cell Wall of Candida spp. In Candida and Candidiasis (Calderone R. A., ed), pp. 159–175, American Society for Microbiology, Washington, D. C. [Google Scholar]
- 14. Klis K. M., de Groot P., Hellingwerf K. (2001) Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39, 1–8 [PubMed] [Google Scholar]
- 15. Gopal P. K., Shepherd M. G., Sullivan P. A. (1984) Analysis of wall glucans from yeast, hyphal, and germ-tube forming cells of Candida albicans. J. Gen. Microbiol. 130, 3295–3301 [DOI] [PubMed] [Google Scholar]
- 16. Lowman D. W., Ferguson D. A., Williams D. L. (2003) Structural characterization of (1→3)-β-d-glucans isolated from blastospore and hyphal forms of Candida albicans. Carbohydr, Res. 338, 1491–1496 [DOI] [PubMed] [Google Scholar]
- 17. Shibata N., Suzuki A., Kobayashi H., Okawa Y. (2007) Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem. J. 404, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller A., Ensley H., Pretus H., McNamee R., Jones E., McLaughlin E., Chandley W., Browder W., Lowman D., Williams D. (1997) The application of various protic acids in the extraction of (1→3)-β-d-glucan from. Saccharomyces cerevisiae. Carbohydr. Res. 299, 203–208 [DOI] [PubMed] [Google Scholar]
- 19. Lowman D. W., West L. J., Bearden D. W., Wempe M. F., Power T. D., Ensley H. E., Haynes K., Williams D. L., Kruppa M. D. (2011) New insights into the structure of (1→3, 1→6)-β-d-glucan side chains in the Candida glabrata cell wall. PLoS ONE 6, e27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller A., Mayberry W., Acuff R., Thedford S., Browder W., Williams D. (1994) Lipid content of macroparticulate (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Microbios 79, 253–261 [PubMed] [Google Scholar]
- 21. Williams D. L., McNamee R. B., Jones E. L., Pretus H. A., Ensley H. E., Browder I. W., Di Luzio N. R. (1991) A method for the solubilization of a (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 219, 203–213 [DOI] [PubMed] [Google Scholar]
- 22. Shaw A. A., Salaun C., Dauphin J.-F., Ancian B. (1996) Artifact-free PFG-enhanced double-quantum-filtered COSY experiments. J. Magn. Reson. A 120, 110–115 [Google Scholar]
- 23. Ancian B., Bourgeois I., Dauphin J.-F., Shaw A. A. (1997) Artifact-free pure absorption PFG-enhanced DQF-COSY spectra including a gradient pulse in the evolution period. J. Magn. Reson. 125, 348–354 [Google Scholar]
- 24. Jeener J., Meier B. H., Bachmann P., Ernst R. R. (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 71, 4546–4553 [Google Scholar]
- 25. Wagner R., Berger S. (1996) Gradiant-selected NOESY. A fourfold reduction of the measurement time for the NOESY experiment. J. Magn. Reson. A. 123, 119–121 [DOI] [PubMed] [Google Scholar]
- 26. Palmer A. G., III, Cavanagh J., Wright P. E., Rance M. (1993) Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J. Magn. Reson. 93, 151–170 [Google Scholar]
- 27. Kay L. E., Keifer P., Saarinen T. (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 114, 10663–10665 [Google Scholar]
- 28. Schleucher J., Schwendinger M., Sattler M., Schmidt P., Schedletzky O., Glaser S. J., Sørensen O. W., Griesinger C. (1994) A general enhancement scheme in heteronuclear multidimentional NMR employing pulsed field gradients. J. Biomol NMR 4, 301–306 [DOI] [PubMed] [Google Scholar]
- 29. Norwood T. J., Boyd J., Heritage J. E., Soffe N., Campbell I. D. (1990) Comparison of techniques for 1H-detected heteronuclear 1H-15N spectroscopy. J. Magn. Reson. 87, 488–501 [Google Scholar]
- 30. Cavanagh J., Palmer A. G., III, Wright P. E., Rance M. (1991) Sensitivity improvement in proton-detected two-dimensional heteronuclear relay spectroscopy. J. Magn. Reson. 91, 429–436 [Google Scholar]
- 31. Bax A., Summers M. F. (1986) Proton and carbon-13 assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108, 2093–2094 [Google Scholar]
- 32. Sawardeker J. S., Sloneker J. H., Jeanes A. (1965) Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 37, 1602–1604 [Google Scholar]
- 33. Ciucanu I., Kerek F. (1984) A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131, 209–217 [Google Scholar]
- 34. Ferwerda B., Ferwerda G., Plantinga T. S., Willment J. A., van Spriel A. B., Venselaar H., Elbers C. C., Johnson M. D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morré S. A., Vriend G., Williams D. L., Perfect J. R., Joosten L. A., Wijmenga C., van der Meer J. W., Adema G. J., Kullberg B. J., Brown G. D., Netea M. G. (2009) Human Dectin-1 deficiency and mucocutaneous fungal Infections. N. Engl. J. Med. 361, 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vasur J., Kawai R., Jonsson K. H., Widmalm G., Engström A., Frank M., Andersson E., Hansson H., Forsberg Z., Igarashi K., Samejima M., Sandgren M., Ståhlberg J. (2010) Synthesis of cyclic β-glucan using laminarinase 16A glycosynthase mutant from the basidiomycete Phanerochaete chrysosporium. J. Am. Chem. Soc. 132, 1724–1730 [DOI] [PubMed] [Google Scholar]
- 36. Brown G. D., Taylor P. R., Reid D. M., Willment J. A., Williams D. L., Martinez-Pomares L., Wong S. Y. C., Gordon S. (2002) Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 296, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. (2003) Collaborative induction of inflammatory responses by Dectin-1 and toll-like receptor 2. J. Exp. Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 [DOI] [PubMed] [Google Scholar]
- 39. Dennehy K. M., Ferwerda G., Faro-Trindade I., Pyz E., Willment J. A., Taylor P. R., Kerrigan A., Tsoni S. V., Gordon S., Meyer-Wentrup F., Adema G. J., Kullberg B. J., Schweighoffer E., Tybulewicz V., Mora-Montes H. M., Gow N. A., Williams D. L., Netea M. G., Brown G. D. (2008) Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 38, 500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams E. L., Rice P. J., Graves B., Ensley H. E., Yu H., Brown G. D., Gordon S., Monteiro M. A., Papp-Szabo E., Lowman D. W., Power T. D., Wempe M. F., Williams D. L. (2008) Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J. Pharmacol. Exp. Ther. 325, 115–123 [DOI] [PubMed] [Google Scholar]
- 41. Cambi A., Netea M. G., Mora-Montes H. M., Gow N. A., Hato S. V., Lowman D. W., Kullberg B. J., Torensma R., Williams D. L., Figdor C. G. (2008) The interaction of human dendritic cells with Candida albicans occurs through specific recognition of the fungal N-linked mannan by C-type lectins. J. Biol. Chem. 283, 20590–29599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beaussart A., Alsteens D., El-Kirat-Chatel S., Lipke P. N., Kucharíková S., Van Dijck P., Dufrêne Y. F. (2012) Single-molecule imaging and functional analysis of Als adhesins and mannans during Candida albicans morphogenesis. ACS Nano 6, 10950–10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li D., Williams D., Lowman D., Monteiro M. A., Tan X., Kruppa M., Fonzi W., Roman E., Pla J., Calderone R. (2009) The Candida albicans histidine kinase Chk1p. Signaling and cell wall mannan. Fungal Genet. Biol. 46, 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kruppa M., Greene R. R., Noss I., Lowman D. W., Williams D. L. (2011) C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum, and cultivation at physiological temperature. Glycobiology 21, 1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowman D. W., Ensley H. E., Greene R. R., Knagge K. J., Williams D. L., Kruppa M. D. (2011) Mannan structural complexity is decreased when Candida albicans is cultivated in blood or serum at physiological temperature. Carbohydr. Res. 346, 2752–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gow N. A., Hube B. (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 15, 1–7 [DOI] [PubMed] [Google Scholar]
- 47. Marchessault R. H., Deslandes Y., Ogawa K., Sundararajan P. R. (1977) X-ray diffraction data for β-(1→3)-d-glucan. Can. J. Chem. 55, 300–303 [Google Scholar]
- 48. Chuah C. T., Sarko A., Deslandes Y., Marchessault R. H. (1983) Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 16, 1375–1382 [Google Scholar]
- 49. Pelosi L., Bulone V., Heux L. (2006) Polymorphism of curdlan and (1→3)-β-d-glucans synthesized in vitro. A 13C CP-MAS and x-ray diffraction analysis. Carbohydr. Polym. 66, 199–207 [Google Scholar]
- 50. Hisamatsu M., Amemura A. (1983) Structural studies on cyclic (1→2)-β-d-glucans (cyclosophoraoses) produced by Agrobacterium and Rhizobium. Carbohydr. Res. 121, 31–40 [Google Scholar]
- 51. Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. (1990) Excessive excretion of cyclic β-(1,2)-glucan by Rhizobium trifolii TA-1. Appl. Environ. Microbiol. 56, 2080–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williamson G., Damani K., Devenney P., Faulds C. B., Morris V. J., Stevens B. J. (1992) Mechanism of action of cyclic β-(1,2)-glucan synthetase from Agrobacterium tumefaciens. Competition between cyclization and elongation reactions. J. Bacteriol. 174, 7941–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Breedveld M. W., Miller K. J. (1994) Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 58, 145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishiyama Y., Langan P., Chanzy H. (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron x-ray and neutron fiber diffraction. J. Am. Chem. Soc. 124, 9074–9082 [DOI] [PubMed] [Google Scholar]
- 55. Wada M., Nishiyama Y., Chanzy H., Forsyth T., Langan P. (2008) The structure of celluloses. Powder Diffraction 23, 92–95 [Google Scholar]
- 56. Clarke A. E., Stone B. A. (1960) Structure of the paramylon from Euglena gracilis. Biochim. Biophys. Acta 44, 161–163 [DOI] [PubMed] [Google Scholar]
- 57. Kobayashi K., Kimura S., Togawa E., Wada M., Kuga S. (2010) Crystal transition of paramylon with dehydration and hydation. Carbohydr. Polym. 80, 491–497 [Google Scholar]
- 58. Fulton W. S., Adkins E. D. T. (1980) The gelling mechanism and relationship to molecular structure of microbial polysaccharide curdlan in Fiber Diffraction Methods. (French A. D., Gardner K. H., ed.), pp. 385–410, American Chemical Society, Washington, D. C [Google Scholar]
- 59. Deslandes Y., Marchessault R. H., Sarko A. (1980) Triple-helical structure of (1→3)-d-glucan. Macromolecules 13, 1466–1471 [Google Scholar]
- 60. Pelosi L., Imai T., Chanzy H., Heux L., Buhler E., Bulone V. (2003) Structural and morphological diversity of (1→3)-β-d-glucans synthesized in vitro by enzymes from Saprolegnia monoica. Comparison with a corresponding in vitro product from blackberry (Rubus fruticosus). Biochemistry 42, 6264–6274 [DOI] [PubMed] [Google Scholar]
- 61. Gil M. L., Gozalbo D. (2006) TLR2, but not TLR4, triggers cytokine production by murine cells in response to Candida albicans yeasts and hyphae. Microbes Infect. 8, 2299–2304 [DOI] [PubMed] [Google Scholar]
- 62. Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. (2010) Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 1–11 [DOI] [PubMed] [Google Scholar]
- 63. Moyes D. L., Murciano C., Runglall M., Islam A., Thavaraj S., Naglik J. R. (2011) Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 6, e26580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bi L., Gojestani S., Wu W., Hsu Y. M., Zhu J., Ariizumi K., Lin X. (2010) CARD9 mediates dectin-2 induced IkBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hypahl form of Candida albicans. J. Biol. Chem. 285, 25969–25977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van de Veerdonk F. L., Joosten L. A., Shaw P. J., Smeekens S. P., Malireddi R. K., van der Meer J. W., Kullberg B. J., Netea M. G., Kanneganti T. D. (2011) The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur. J. Immunol. 41, 2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kulicke W.-M., Lettau A. I., Thielking H. (1997) Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-β-d-glucans. Carbohydr. Res. 297, 135–143 [DOI] [PubMed] [Google Scholar]
- 67. Mueller A., Raptis J., Rice P. J., Kalbfleisch J. H., Stout R. D., Ensley H. E., Browder W., Williams D. L. (2000) The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-d-glucan receptors in a human monocyte-like cell line. Glycobiology 10, 339–346 [DOI] [PubMed] [Google Scholar]
- 68. Brown J., O'Callaghan C. A., Marshall A. S., Gilbert R. J., Siebold C., Gordon S., Brown G. D., Jones E. Y. (2007) Structure of the fungal β-glucan-binding immune receptor dectin-1. Implications for function. Protein Sci. 16, 1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brown G. D., Herre J., Williams D. L., Willment J. A., Marshall A. S., Gordon S. (2003) Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ferwerda B., McCall M. B., Verheijen K., Kullberg B.-J., van der Ven A. J., Van der Meer J. W., Netea M. G. (2008) Functional consequences of Toll-like receptor 4 polymorphisms. Mol. Med. 14, 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Esteban A., Popp M. W., Vyas V. K., Strijbis K., Ploegh H. L., Fink G. R. (2011) Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. U.S.A. 108, 14270–14275 [DOI] [PMC free article] [PubMed] [Google Scholar]