Abstract
Background
Patients with residual poliomyelitis can have advanced degenerative arthritis of the hip in the paralytic limb or the nonparalytic contralateral limb. Although THA is a treatment option for some of these patients, there are few studies regarding THA in this patient population.
Questions/purposes
We therefore reviewed a group of patients with residual poliomyelitis who underwent cementless THA on either their paralytic limb or nonparalytic limb to assess (1) Harris hip scores, (2) radiographic results, including implant loosening, (3) complications, including dislocation, and (4) limb length discrepancy after recovery from surgery.
Methods
From January 2000 to December 2009, 10 patients with residual poliomyelitis (10 hips, four paralytic limbs and six nonparalytic contralateral limbs) underwent THA using cementless prostheses. Harris hip scores, complications, and leg length discrepancy were determined by chart review, and confirmed by questionnaire and examination; radiographs were reviewed by two observers for this study. Followup was available for all 10 patients at a minimum of 3 years (median, 7 years; range, 3.4–13 years). Surgery was done at the same side of the paralytic limb in four hips and contralateral to the paralytic limb in six.
Results
All patients had pain relief and improvement in function; the Harris hip score improved from mean of 68 preoperatively to 92 at last followup (p = 0.043). However, only three patients had complete pain relief. One hip dislocated, which was treated successfully with closed reduction and a hip spica cast for 2 months. There was no loosening or osteolysis in this series. Leg length discrepancy improved after the index operation, but only in the THAs performed in the paralytic limbs.
Conclusions
Cementless THA may be suitable for painful hips in adult patients with residual poliomyelitis. Nonetheless, these patients should be informed of the possibility of mild residual pain and persistent leg length discrepancy, particularly patients whose THA is performed on the limb that was not affected by polio (ie, the nonparalytic contralateral limb).
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Widespread vaccination has dramatically reduced the number of poliomyelitis cases [37]. Although poliomyelitis has been nearly eradicated, orthopaedic surgeons still occasionally see patients who had the disease [34]. Some have asserted that joints in the paralytic limb are protected from the development of osteoarthritis in patients with residual poliomyelitis, perhaps from a reduction in the forces across the joint [16]. However, when the gluteus maximus and medius muscles are paralyzed and the hip flexors and adductors are of normal strength, subluxation of the hip affected by poliomyelitis can occur [22], as painful arthritis of the hip [8, 27, 40]. In addition, although patients who had poliomyelitis may be less active owing to sequelae of this condition, degenerative arthritis in the previously normal contralateral hip can be caused by leg length discrepancy, pelvic obliquity, or severe deformities of the affected hip [2, 6, 33]. Therefore, an arthritic hip may be encountered in either the paralytic or normal contralateral limb. Treatment options for a painful degenerated hip in adult patients with residual poliomyelitis include periacetabular or femoral osteotomy and THA [22, 25]. Periacetabular or femoral osteotomy can be used in paralytic hip dislocations if the arthritic change is not advanced. For patients presenting with advanced degenerative change, THA often is the best option [2, 16, 33].
There are few studies regarding THA in patients with residual poliomyelitis, and most of these are case reports or descriptions of THAs for polio in mixed series on surgery for diverse neuromuscular conditions [2, 3, 20, 36, 41]. To our knowledge, there has been no case series of THA in adult patients with residual poliomyelitis, and we could not find any report comparing the results of arthroplasties between the affected and the nonparalytic contralateral limbs.
We therefore reviewed a group of patients with residual poliomyelitis who underwent cementless THA on either their paralytic limb or nonparalytic limb to assess (1) Harris hip scores, (2) radiographic results, including implant loosening, (3) complications, including dislocation, and (4) limb length discrepancy after recovery from surgery.
Patients and Methods
From January 2000 to December 2009, 20 patients (20 hips) underwent surgery at our institute for painful advanced arthritis with residual poliomyelitis that limited functional activity and in which previous nonoperative treatment had failed. Seven patients (seven hips) who had arthrodesis, osteotomy, or resection arthroplasty were excluded. Three (three hips) who had a followup less than 3 years also were excluded. Finally, 10 adult patients (10 hips), seven men and three women, who underwent THA at our institute from January 2000 to December 2009 were retrospectively reviewed. The design and protocol of the study were approved by the institutional review board. Patients were informed that their medical data could be used in a scientific study. The indication for surgery for all patients was advanced arthritis of the hip that limited functional activity and in which previous nonoperative treatment had failed. The average age of the patients at the time of surgery was 48 years (median, 50 years; range, 32–59 years) and all patients were followed for a minimum of 3 years (median, 7 years; range, 3.4–13 years). The right side was affected in five patients and the left in five. Surgery was done on the side of the paralytic limb in four patients and on the contralateral side of the paralytic limb in six. We divided patients into two groups depending on the site of the operation (paralytic side or nonparalytic contralateral side) and compared the results. Five patients had previous surgery on the paralytic limb. All five patients underwent Achilles tendon lengthening and three of these five patients also had foot arthrodesis during adolescence before undergoing THA.
The physical examination for each patient included checking preoperative and postoperative average range of motion (ROM) and strength of the hip. Preoperative muscle grade was checked on hip flexors, hip extensors, hip abductors, and hip adductors by manual muscle testing according to the Medical Research Council Scale [30]. Osteoarthritis graded using the Tönnis classification [39] was Grade 2 in five hips preoperatively, Grade 3 in one, and Grade 4 in four. Crowe et al. [5] classified dysplastic hips radiographically into four categories according to the extent of proximal migration of the femoral head; the preoperative classification was Group I in eight hips, Group II in one, and Group III in one (Table 1). There was no correlation between preoperative muscle power and disease severity.
Table 1.
Preoperative demographic details of patients
| Patient number | Sex/age at surgery | BMI (kg/m2) | Followup duration (years) | Side | Prior surgery on paralytic limb | Osteoarthritis grade | Crowe classification | Hip abductor strength* |
|---|---|---|---|---|---|---|---|---|
| 1 | F/51 | 27.2 | 5.8 | Contralateral | No | 2 | 1 | 5 |
| 2 | M/59 | 22.2 | 3.4 | Paralytic | Achilles tendon lengthening | 2 | 1 | 4 |
| 3 | F/57 | 24.8 | 3.8 | Contralateral | No | 3 | 1 | 5 |
| 4 | M/32 | 22.2 | 12.8 | Paralytic | Achilles tendon lengthening | 2 | 3 | 4 |
| 5 | M/56 | 22.0 | 11.9 | Contralateral | No | 4 | 1 | 5 |
| 6 | M/43 | 28.3 | 8.8 | Contralateral | No | 4 | 1 | 5 |
| 7 | M/48 | 25.9 | 7 | Paralytic | No | 2 | 1 | 4 |
| 8 | M/37 | 29.2 | 7.4 | Contralateral | Achilles tendon lengthening, foot arthrodesis | 4 | 1 | 5 |
| 9 | F/56 | 23.6 | 6.5 | Paralytic | Achilles tendon lengthening, foot arthrodesis | 2 | 2 | 4 |
| 10 | M/41 | 26.5 | 5.4 | Contralateral | Achilles tendon lengthening, foot arthrodesis | 4 | 1 | 5 |
* Muscle strength graded from 0 to 5.
All operations were performed directly or under the supervision of one of four high-volume arthroplasty surgeons (YMK, HJK, KHK, JJY) at our institutions. Nine THAs were performed with the patient under spinal anesthesia and one under general anesthesia. Depending on the surgeon’s preference, the posterolateral approach was used in nine hips and the transgluteal direct lateral approach in one.
We set the goal of cup position at 40° abduction and 15° anteversion as previously suggested [23]. Before implantation of the final prosthesis, trial prostheses were implanted and we tested the stability. Posterior stability was tested at 90° flexion and anterior stability at extension and external rotation of the hip. In cases of instability, abduction and anteversion of the acetabular component were adjusted to a more stable position.
All components were cementless. The acetabular components were the Plasmacup® SC (Aseculap, Tuttlingen, Germany; n = 5), Pinnacle® (DePuy Orthopaedics, Warsaw, IN, USA; n = 2), Triology® (Zimmer, Warsaw, IN, USA; n = 2), and Coren® cup (Corentec, Cheon-An, Korea; n = 1). Femoral components were the Bicontact® stem (Aseculap; n = 5), Corail® stem (DePuy Orthopaedics; n = 2), S-ROM® (DePuy Orthopaedics; n = 1), Wagner Cone prosthesis™ (Zimmer; n = 1), and Bencox ID® stem (Corentec; n = 1). The S-ROM stem® was used because of a high degree of femoral anteversion in one hip. Screws were used in the porous-coated acetabular component in nine hips.
In eight hips, an alumina head and liner (BIOLOX® forte; CeramTec AG, Plochingen, Germany) were used. A metal-on-metal bearing surface (Metasul; Zimmer) was used in one hip and metal on highly crosslinked polyethylene was used in one hip. Twenty-eight millimeter heads were used in seven hips and 32 mm were used in three hips. For the posterior approach, the posterior capsular flap and the external rotator tendons were repaired using the technique described by Ji et al. [19]. After removal of the drain 2 or 3 days postoperatively, the patients were encouraged to walk with toe-touch weightbearing using two crutches for 6 weeks and then were allowed weightbearing as tolerated. We strongly recommended abductor strengthening exercises especially for patients in the paralytic group.
The 6-week AP and cross-table lateral radiographs were the baseline for radiographic evaluations of implant stability, wear, osteolysis, and loosening [28]. Fixation of the femoral and acetabular components was classified as previously described [9, 21]. Liner wear was calculated as described previously [24]. Osteolytic lesions were defined according to criteria described by Engh et al. [11]. The lesions were recorded according to three previously described zones [7] on the acetabular side and seven zones on the femoral side [15].
Pain was assessed by a VAS from 0 to 10; pain and function were assessed using the Harris hip score [17]. The pain and function data obtained through chart review were confirmed through a questionnaire. Leg length difference was determined by measuring the distance from the umbilicus to the medial malleoli of the ankle (apparent true leg length discrepancy) and from the anterosuperior iliac spine to the medial malleoli of the ankle (true leg length discrepancy). Error is susceptible in these patients owing to pelvic obliquity, therefore we used true leg length discrepancy [18]. The preoperative discrepancies in our patients ranged from 0 to 4 cm (median, 2 cm; mean, 2.1 cm). Leg length discrepancy is common in this population, and we tried to correct it intraoperatively within a range of 2.5 cm in which palsy did not occur. After trial reduction, we checked sciatic nerve tension and adjusted length by keeping the tension moderate.
Radiographs obtained 6 weeks after surgery were evaluated for implant position by two independent observers (BHY, YKL) who had not participated in the operations. One of these observers had finished his residency and fellowship and worked as an adult hip reconstruction specialist for 5 years (YKL) and the other (BHY) was a second-year fellow. Abduction and anteversion angles of the acetabular component and alignments of the femoral stems were measured on the 6-week postoperative AP radiographs. The abduction angle of the acetabular component was measured as previously described [10]. The anteversion of the acetabular component was calculated using the method of Woo and Morrey [42]. Cups with an abduction angle of 30° or less or 50° or greater with an anteversion angle of 5° or less or 25° or greater were considered as outliers of the optimal cup position [23, 43].
The Wilcoxon signed-rank test was used to compare preoperative and postoperative clinical outcomes. Chi-square, descriptive, and Mann-Whitney U tests were used to compare demographics and results between the surgeries on the paralytic side and the contralateral side. When there were fewer than five patients in any cell, we used Fisher’s exact test. Statistical analyses were conducted using SPSS Version 20 (IBM, Armonk, NY, USA). Differences were considered significant at a p less than 0.05.
Results
The average Harris hip score improved from 70 preoperatively (median, 70; range, 55–78) to 92 postoperatively (median, 90; range, 84–100; p = 0.043). The mean VAS decreased from 7 preoperatively (median, 7; range, 6–8) to 0.9 postoperatively (median, 1; range, 0–3; p = 0.004) at the latest followup. However, only three patients had complete pain relief and seven patients had mild pain occasionally at the end of the day. These seven patients had THAs performed in three of the four paralytic limbs and four of the six nonparalytic contralateral limbs. We could not correlate pain and weakness, but there seemed to be a pattern that the weaker limbs were more likely to have some measure of residual pain. With the numbers available, there were no significant differences in hip scores or VAS pain between the patients in the paralytic and nonparalytic contralateral groups.
None of the acetabular or femoral components was classified as loose (Fig. 1). No hip showed radiographic signs of wear or osteolysis (Fig. 2). The cup abduction angle was 34º to 48º (median, 43º) and the cup anteversion was 15° to 33° (median, 29º). In all 10 hips, radiographic abduction was within the safe range (40° ± 10°) as defined by Lewinnek et al. [23]. In five (including one dislocated hip) of 10 hips, radiographic anteversion was within the safe range (15° ± 10°). One patient (Patient 8) in whom the index operation was performed on the nonparalytic contralateral limb experienced hip dislocation 10 weeks after the surgery during squatting. The dislocated head was reduced manually with the patient under general anesthesia, and a hip spica cast was applied for 2 months because of persistent instability detected using fluoroscopy. There was no recurrence of the dislocation as of the latest followup (7 years after surgery).
Fig. 1A–B.
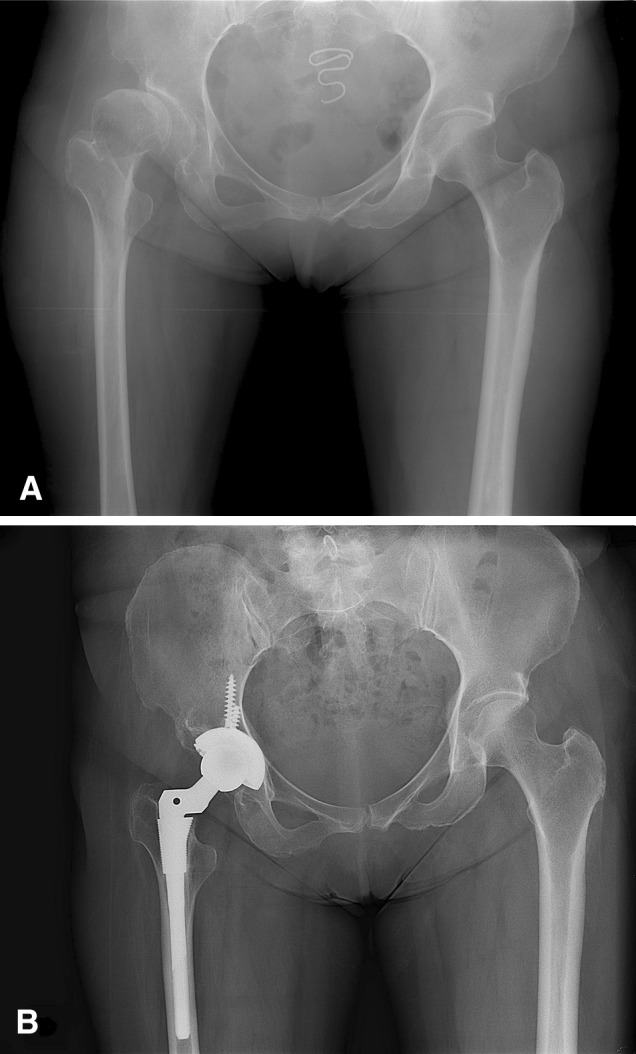
(A) A 56-year-old woman (Patient 9) had residual poliomyelitis in the right lower leg. Her preoperative AP radiograph shows a subluxated femoral head with a dysplastic acetabulum in the right hip. Her leg length discrepancy was 4 cm. (B) Her AP radiographs obtained 6.5 years after THA show no evidence of implant loosening or osteolysis. Her leg length discrepancy decreased by 2 cm. She reported mild pain at night.
Fig. 2A–B.
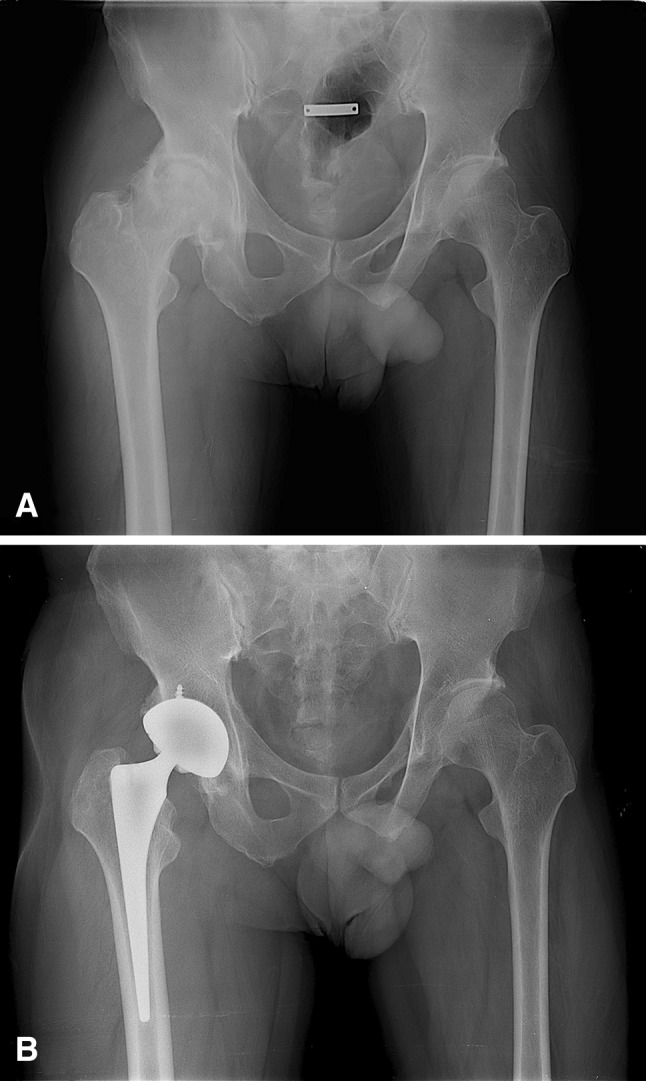
(A) A 41-year-old man (Patient 10) had residual poliomyelitis in his left lower leg. His preoperative AP radiograph shows severe arthritic change in the right hip (contralateral limb). His leg length discrepancy was 1 cm. (B) Postoperative AP radiographs obtained 5.4 years after THA shows no evidence of implant loosening or osteolysis. The postoperative leg length discrepancy was still 1 cm, and he reported mild pain after working.
The mean true leg length discrepancy decreased from 2.1 ± 1.3 cm (median, 2 cm; range, 0–4 cm) to 1.7 ± 1.3 cm (median, 2 cm; range, 0–4 cm) (Table 2). The true leg length discrepancy decreased after the index operation only for patients in the paralytic group, because limbs of patients in the nonparalytic contralateral group typically were long before surgery and shortening them was not considered safe because of the risk of dislocation.
Table 2.
Preoperative and postoperative parameters of patients
| Variable | VAS | Harris hip score | Leg length discrepancy (cm) | ROM (degrees) | |||
|---|---|---|---|---|---|---|---|
| Flexion | Rotation | Abduction | Adduction | ||||
| Preoperative | 7.3 ± 0.9 | 67.8 ± 8.7 | 2.1 ± 1.3 | 109° ± 19° | 78° ± 34° | 53° ± 29° | 30° ± 13° |
| Postoperative | 0.9 ± 0.7 | 91.8 ± 5.5 | 1.7 ± 1.1 | 113° ± 13° | 63° ± 15° | 44° ± 22° | 23° ± 5° |
| p value | < 0.001 | 0.003 | 0.226 | 0.766 | 0.145 | 0.121 | 0.184 |
Data are expressed as mean ± SD.
Discussion
Some orthopaedic surgeons are reluctant to perform THA in adult patients with residual poliomyelitis because of their concern for the risk of postoperative complications such as dislocation resulting from flaccid paralysis of muscle. There have been few studies on the results of THA in adult patients with residual poliomyelitis [2, 3, 20, 36, 41]. In our small, retrospective series, we found an improvement in Harris hip scores and pain scores, but only a few patients (three of 10) reported being pain free at final followup. Implant loosening was not found to be a problem at short term, but one of the 10 patients did experience a hip dislocation. Finally, we found that leg length discrepancies improved when the THAs were performed on the affected limb, but not when they were performed on the nonparalytic contralateral limb, because those limbs typically were long before surgery, and shortening them was not considered safe because of the risk of dislocation.
There were several limitations in our study. First, our study was retrospective and involved a small number of patients. This limitation was unavoidable owing to the rarity of poliomyelitis and the low number of patients treated by THA. Second, the length of followup was not uniform for all patients. However, all patients with implants had a minimum of 3 years followup (some for as much as 13 years) and the average followup was 7 years. This length of followup allowed us to determine mid-term results in this population. Third, numerous surgeons and various implants and bearings were involved because THA in patients with residual poliomyelitis is infrequent. This limitation also is inevitable. Fourth, leg length discrepancy was evaluated by tape measurement. Radiographic measurement is preferred, but preoperative full-length radiographs were not available for several patients. Difficulty in identifying bony prominences is the major factor of inaccuracy, however, all our patients had a BMI less than 30 and six of them had a BMI less than 25.
We observed improvement in Harris hip scores and VAS pain in this population, but seven of the 10 patients in this series had some amount of residual pain. Cabanela and Weber reported on five hip replacements contralateral to hips affected by poliomyelitis, and the results in their patients (after 2–8 years of followup) appeared to be no different from those in the general population of patients who have a hip arthroplasty [2]. Laguna and Barrientos reported that only THA enabled a successful return to the initial functional level [20]. With the numbers available, we observed no differences in Harris hip or VAS pain scores between THAs performed in paralytic and nonparalytic contralateral limbs. The mild pain at the end of the day that patients reported could be related to postpolio muscle pain in the paralytic group and remnant leg length discrepancy in the contralateral group, but this is speculative. We recommend abductor strengthening exercises in the paralytic group [4, 12, 38].
Our radiologic results were comparable to those in other reports of THA in adult patients with residual poliomyelitis (Table 3). At a mean of 7 years, we saw no aseptic loosening in this population. In addition, our findings, although not significant with the numbers available on this point, suggested more severe dysplasia in hips on the paralytic side and more severe arthritic changes in hips on the nonparalytic contralateral side. These are identified by preoperative classification. A high percentage of patients in the contralateral group had high Tönnis grades of OA, whereas in the paralytic group patients had more Group 1 dysplasia according to the Crowe classification (Table 4). Patients with residual poliomyelitis tend to favor their intact leg during daily activities, therefore the nonaffected leg experiences an increased load. We believe this may explain the apparent increase in degenerative changes observed in those limbs, while the muscular imbalances render the paralytic limbs more prone to dysplasia. Dysplastic arthritic hips are more prevalent on the paralytic side and overuse-induced degenerative changes are more susceptible in hips on the nonparalytic contralateral limb.
Table 3.
Studies of hip arthroplasty in patients with residual poliomyelitis
| Study | Number of hips (patients) | Side | Followup (years) | Pain | Complications (including implant loosening) |
|---|---|---|---|---|---|
| Laguna & Barrientos [20] 2008 | 1 (1) | Paralytic | 3.8 | No | No |
| Spinnickie & Goodman [36] 2007 | 1 (1) | Paralytic | 0.6 | Dissociation of the femoral head and trunnion | |
| Cabanela & Weber [2] 2000 | 5 (5) | Contralateral | 2 to 8 | No | No |
| Wicart et al. [41] 1999 | 2 (2) | Paralytic | 5 | No | Anterior dislocation in one hip |
| Cameron [3] 1995 | 1 (1) | Paralytic | 3 | No | No |
| Current study | 10 (10) | Paralytic and contralateral | 7.0 | Improved | Anterior dislocation in one hip |
Table 4.
Comparison of demographics and results between groups
| Variable | Operation on paralytic side (n = 4 patients) | Operation on contralateral side (n = 6 patients) |
|---|---|---|
| Average age (years) | 48.8 ± 12.0 | 47.5 ± 8.3 |
| BMI (g/cm2) | 23.4 ± 1.7 | 26.4 ± 2.7 |
| Length of followup | 7.4 ± 3.9 | 7.2 ± 2.9 |
| Preoperative OA grade | ||
| Grade 2 | 4 | 1 |
| Grade 3 | 0 | 1 |
| Grade 4 | 0 | 4 |
| Preoperative Crowe classification | ||
| Group I | 2 | 6 |
| Group II | 1 | 0 |
| Group III | 1 | 0 |
| Preoperative VAS | 7.5 ± 0.5 | 7.22 ± 0.9 |
| Postoperative VAS | 0.75 ± 0.5 | 1.0 ± 1.1 |
| Preoperative Harris hip score | 67.7 ± 11.7 | 68 ± 5.7 |
| Postoperative Harris hip score | 92.0 ± 3.5 | 91.7 ± 8.0 |
| Preoperative leg length discrepancy (cm) | 2.3 ± 1.3 | 2.0 ± 1.4 |
| Postoperative leg length discrepancy (cm) | 0.9 ± 0.8 | 2.3 ± 1.3 |
| Inclination of cup | 44.8 ± 2.5 | 39.8 ± 4.2 |
| Anteversion of cup | 27.5 ± 7.1 | 27.7 ± 7.4 |
Data are shown as mean ± SD.
Postoperative instability is the main concern in this patient population [31]. In our series, one hip was dislocated and was reduced nonsurgically; it did not recur. The rate of dislocation (one of 10) seems higher than that seen in THAs for degenerative arthritis or avascular necrosis (range, 1%–3%) [29, 32, 42], but seems comparable to rates in reports of patients who had developmental dysplasia of the hip (range, 5%–11%) [13, 14, 26].
In our series there was a significant difference in the improvement of true leg length discrepancy between the groups (1.3 cm versus 0 cm). Because paralytic limbs are shorter than the normal side, leg length discrepancy could be corrected relatively easily by surgery for patients in the paralytic group; shortening the already long nonparalytic contralateral limb was not considered safe because of the risk of dislocation, and therefore we made no specific efforts to equalize leg length in patients in this group. Falls resulting in fractures in patients who are polio survivors occur more easily and the majority of the fractures occur in the affected limb [35]. Maintaining balance is associated with falls and changes of leg length can cause an unfamiliar environment in balancing, therefore patients should be informed of these changes [1].
To our knowledge, our study may be the first to report results of a comparison of THAs in polio-affected and nonaffected contralateral limbs. Results at a mean of 7 years showed improvement of hip scores (although a high proportion of patients still reported some residual pain), and the absence of loosening, wear, or osteolysis. Dislocation occurred in one patient, and leg length differences could be improved, but only in hips affected by poliomyelitis; shortening the nonparalytic contralateral side is not recommended. Nonetheless, patients should be informed of the possibilities of mild muscle pain and residual leg length discrepancy after surgery. The leg length discrepancy may be a concern for some patients who are undergoing THA on the nonparalytic contralateral limb, and the likely outcome should be mentioned when counseling these patients before surgery.
Acknowledgments
We gratefully acknowledge Young Min Kim MD, our teacher, who also contributed several patients to this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Department of Orthopaedic Surgery, Seoul National University College of Medicine, Seoul, Korea.
References
- 1.Bickerstaffe A, Beelen A, Nollet F. Circumstances and consequences of falls in polio survivors. J Rehabil Med. 2010;42:908–915. doi: 10.2340/16501977-0620. [DOI] [PubMed] [Google Scholar]
- 2.Cabanela ME, Weber M. Total hip arthroplasty in patients with neuromuscular disease. Instr Course Lect. 2000;49:163–168. [PubMed] [Google Scholar]
- 3.Cameron HU. Total hip replacement in a limb severely affected by paralytic poliomyelitis. Can J Surg. 1995;38:386. [PubMed] [Google Scholar]
- 4.Chan KM, Amirjani N, Sumrain M, Clarke A, Strohschein FJ. Randomized controlled trial of strength training in post-polio patients. Muscle Nerve. 2003;27:332–338. doi: 10.1002/mus.10327. [DOI] [PubMed] [Google Scholar]
- 5.Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61:15–23. [PubMed] [Google Scholar]
- 6.Delaunay CP, Bonnomet F, Clavert P, Laffargue P, Migaud H. THA using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop Relat Res. 2008;466:340–346. doi: 10.1007/s11999-007-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Emr J. [Study of surgical treatment of paralytic dislocation of the hip after poliomyelitis][in Czech] Acta Chir Orthop Traumatol Cech. 1959;26:233–237. [PubMed] [Google Scholar]
- 9.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants: the femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 10.Engh CA, Griffin WL, Marx CL. Cementless acetabular components. J Bone Joint Surg Br. 1990;72:53–59. doi: 10.1302/0301-620X.72B1.2298795. [DOI] [PubMed] [Google Scholar]
- 11.Engh CA, Hooten JP, Jr, Zettl-Schaffer KF, Ghaffarpour M, McGovern TF, Macalino GE, Zicat BA. Porous-coated total hip replacement. Clin Orthop Relat Res. 1994;298:89–96. [PubMed] [Google Scholar]
- 12.Farbu E, Gilhus NE, Barnes MP, Borg K, de Visser M, Driessen A, Howard R, Nollet F, Opara J, Stalberg E. EFNS guideline on diagnosis and management of post-polio syndrome: report of an EFNS task force. Eur J Neurol. 2006;13:795–801. doi: 10.1111/j.1468-1331.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 13.Fredin H, Sanzen L, Sigurdsson B, Unander-Scharin L. Total hip arthroplasty in high congenital dislocation: 21 hips with a minimum five-year follow-up. J Bone Joint Surg Br. 1991;73:430–433. doi: 10.1302/0301-620X.73B3.1670444. [DOI] [PubMed] [Google Scholar]
- 14.Garvin KL, Bowen MK, Salvati EA, Ranawat CS. Long-term results of total hip arthroplasty in congenital dislocation and dysplasia of the hip: a follow-up note. J Bone Joint Surg Am. 1991;73:1348–1354. [PubMed] [Google Scholar]
- 15.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 16.Haddad FS, Masri BA, Garbuz DS, Duncan CP. Primary total replacement of the dysplastic hip. Instr Course Lect. 2000;49:23–39. [PubMed] [Google Scholar]
- 17.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 18.Herring JA. Leg length discrepancy Tachdjian’s Pediatric Orthopaedics. 4. Philadelphia, PA: WB Saunders Co; 2007. pp. 1194–1196. [Google Scholar]
- 19.Ji HM, Kim KC, Lee YK, Ha YC, Koo KH. Dislocation after total hip arthroplasty: a randomized clinical trial of a posterior approach and a modified lateral approach. J Arthroplasty. 2012;27:378–385. doi: 10.1016/j.arth.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Laguna R, Barrientos J. Total hip arthroplasty in paralytic dislocation from poliomyelitis. Orthopedics. 2008;31:179. doi: 10.3928/01477447-20080201-16. [DOI] [PubMed] [Google Scholar]
- 21.Latimer HA, Lachiewicz PF. Porous-coated acetabular components with screw fixation: five to ten-year results. J Bone Joint Surg Am. 1996;78:975–981. doi: 10.2106/00004623-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Lau JH, Parker JC, Hsu LC, Leong JC. Paralytic hip instability in poliomyelitis. J Bone Joint Surg Br. 1986;68:528–533. doi: 10.1302/0301-620X.68B4.3733824. [DOI] [PubMed] [Google Scholar]
- 23.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 24.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–528. [PubMed] [Google Scholar]
- 25.MacDonald SJ, Hersche O, Ganz R. Periacetabular osteotomy in the treatment of neurogenic acetabular dysplasia. J Bone Joint Surg Br. 1999;81:975–978. doi: 10.1302/0301-620X.81B6.9700. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie JR, Kelley SS, Johnston RC. Total hip replacement for coxarthrosis secondary to congenital dysplasia and dislocation of the hip: long-term results. J Bone Joint Surg Am. 1996;78:55–61. doi: 10.2106/00004623-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Mallet J. [Paralytic dislocation of the hip caused by poliomyelitis][in French] Rev Chir Orthop Reparatrice Appar Mot. 1956;42:85–98. [PubMed] [Google Scholar]
- 28.Martell JM, Pierson RH, 3rd, Jacobs JJ, Rosenberg AG, Maley M, Galante JO. Primary total hip reconstruction with a titanium fiber-coated prosthesis inserted without cement. J Bone Joint Surg Am. 1993;75:554–571. doi: 10.2106/00004623-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop Relat Res. 2002;405:46–53. doi: 10.1097/00003086-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Medical ResearchCouncil. Aid to the Examination of the Peripheral Nervous System Memorandum no. 45. London, UK: Her Majesty’s Stationery Office; 1976.
- 31.Meek RM, Allan DB, McPhillips G, Kerr L, Howie CR. Epidemiology of dislocation after total hip arthroplasty. Clin Orthop Relat Res. 2006;447:9–18. doi: 10.1097/01.blo.0000218754.12311.4a. [DOI] [PubMed] [Google Scholar]
- 32.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20–26. doi: 10.1302/0301-620X.85B3.13201. [DOI] [PubMed] [Google Scholar]
- 33.Queally JM, Abdulkarim A, Mulhall KJ. Total hip replacement in patients with neurological conditions. J Bone Joint Surg Br. 2009;91:1267–1273. doi: 10.1302/0301-620X.91B10.22934. [DOI] [PubMed] [Google Scholar]
- 34.Sheth NP, Keenan MA. Orthopedic surgery considerations in post-polio syndrome. Am J Orthop (Belle Mead NJ). 2007;36:348–353. [PubMed] [Google Scholar]
- 35.Silver JK, Aiello DD. Polio survivors: falls and subsequent injuries. Am J Phys Med Rehabil. 2002;81:567–570. doi: 10.1097/00002060-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Spinnickie A, Goodman SB. Dissociation of the femoral head and trunion after constrained conversion total hip arthroplasty for poliomyelitis. J Arthroplasty. 2007;22:634–637. doi: 10.1016/j.arth.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Thompson K, Tebbens RJ. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines. 2012;11:449–459. doi: 10.1586/erv.11.195. [DOI] [PubMed] [Google Scholar]
- 38.Tiffreau V, Rapin A, Serafi R, Percebois-Macadré L, Supper C, Jolly D, Boyer FC. Post-polio syndrome and rehabilitation. Ann Phy Rehabil Med. 2010;53:42–50. doi: 10.1016/j.rehab.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Tonnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Valls J. Paralytic dislocation of the hip. Bull Hosp Joint Dis. 1960;21:346–350. [PubMed] [Google Scholar]
- 41.Wicart P, Barthas J, Guillaumat M. [Replacement arthroplasty of paralytic hip: apropos of 18 cases][in French] Rev Chir Orthop Reparatrice Appar Mot. 1999;85:581–590. [PubMed] [Google Scholar]
- 42.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed] [Google Scholar]
- 43.Woolson ST, Mow CS, Syquia JF, Lannin JV, Schurman DJ. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg Am. 2004;86:1353–1358. doi: 10.2106/00004623-200407000-00001. [DOI] [PubMed] [Google Scholar]


