Abstract
Background
The best approach for surgical treatment of an infected THA remains controversial. Two-stage revision is believed to result in lower reinfection rates but may result in significant functional impairment. Some authors now suggest that single-stage revision may provide comparable results in terms of infection eradication while providing superior functional outcomes.
Questions/purposes
We performed a systematic review to determine whether single- or two-stage revision for an infected THA provides lower reinfection rates and higher functional outcome scores.
Methods
We conducted a comprehensive search of PubMed and Embase, using the search string [Infection AND (“total hip replacement” OR “total hip arthroplasty”) AND revision]. All studies comparing reinfection rates or functional scores for single- and two-stage revision were retrieved and reviewed. A systematic review was performed according to the PRISMA checklist.
Results
The initial search retrieved 1128 studies. Following strict exclusion criteria, we identified nine comparative studies comparing reinfection rates (all nine studies) or functional scores (four studies) between single- and two-stage revisions. The overall quality of studies was poor with no randomized studies being identified. Groups often varied in their baseline characteristics. There was no consensus among the studies regarding the relative incidence of reinfection between the two procedures. There was a trend toward better functional outcomes in single-stage surgery, but this reached significance in only one study.
Conclusions
In appropriate patients, single-stage revision appears to be associated with similar reinfection rates when compared with two-stage revision with superior functional outcomes. This concurs with earlier studies, but given the methodologic quality of the included studies, these findings should be treated with caution. High-quality randomized studies are needed to compare the two approaches to confirm these findings, and, if appropriate, to determine which patients are appropriate for single-stage revision.
Introduction
The best treatment strategy for deep prosthetic infection after THA has been debated since the widespread introduction of THA in the 1970s [1, 18, 24]. Two-stage revision, in which the removal of infected components and reimplantation of revision components are separated by a period with no prosthesis in situ, has become the gold standard [18]. However, two-stage revision is associated with significant morbidity and mortality, is poorly tolerated by patients, and the tissue changes associated with a period without a hip implant can lead to important functional deficits after reimplantation [1, 16–18]. As a result, there has been increasing interest in the use of single-stage revision surgery in some patients [6, 9, 12, 22, 29]. Proponents of single-stage revision contend that the quality of the initial debridement is the most important factor in resolving the infection, and that as long as strict protocols are followed, reinfection is uncommon even after single-stage revision [9, 29]. Single-stage revision may be associated with significantly less morbidity, mortality, and functional impairment; it may eliminate a second hospital stay (and a period of disability between stages), and therefore may confer a large cost savings [1, 3, 9, 12, 13, 19, 30].
As primary and revision THAs become more common, it is increasingly important to reach consensus regarding the best approach to manage deep prosthetic infection after THAs [5, 13, 14, 19]. Although studies have been published on this subject, the results have varied, and there is a shortage of good-quality primary research in this area. Previous attempts to summarize the data in this area either have attempted to pool results from single-arm studies using either one- or two stage-techniques [2, 15] or have been a narrative review [25]. No previous study has attempted to pool the results of functional outcomes after each procedure. The aim of our systematic review was to appraise the current comparative literature to provide an evidence-based assessment of the merits of single- and two-stage revisions for management of infected THAs. We asked whether outcomes differed in terms of reinfection rate and functional outcome between single- and two-stage revision surgeries for an infected THA.
Search Strategy and Criteria
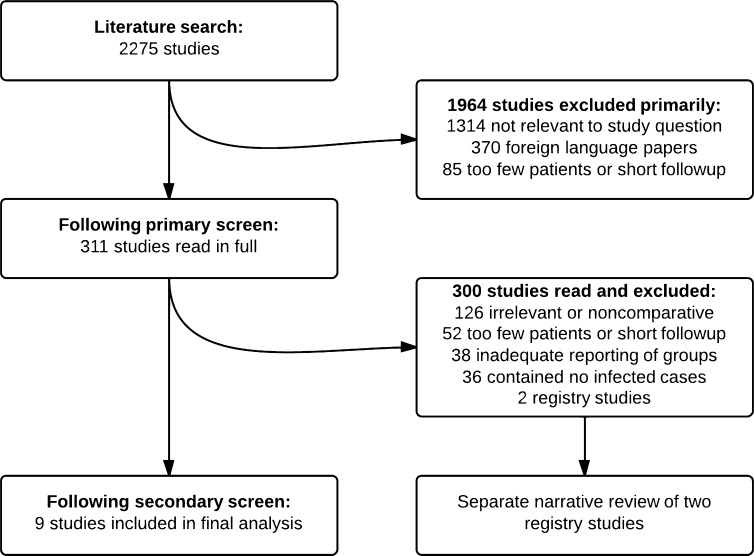
A thorough literature search was performed using the PubMed and EMBASE databases in April 2012 using the search string [Infection AND (“total hip replacement” OR “total hip arthroplasty”) AND revision]. The study protocol was registered with PROSPERO, an international database of prospectively registered systematic reviews (www.crd.york.ac.uk/prospero). Preexisting knowledge of the available literature suggested that there would be few or no randomized controlled trials available for analysis. As such, the decision was made a priori to include prospective or retrospective cohort studies in the knowledge that such studies are open to more bias, particularly selection bias and confounding [10]. The inclusion criteria were any comparative study with at least 1-year minimum followup and 2-year mean followup. Exclusion criteria included studies mixing revisions of THAs with other operations and not providing a detailed breakdown, nonbacterial infections, and foreign language papers not available in English. When different followup intervals of the same study were reported in multiple papers, data were taken from the latest published study, and previous versions were examined if additional information was required. The search process is shown in the flow diagram (Fig. 1). The primary outcome measure of interest was rate of reinfection, and the secondary outcome measure was functional outcome.
Fig. 1.
A flow chart of the study protocol is shown.
The search was performed in a stepwise manner. The initial search results underwent a primary screening based on the title and abstract. Studies deemed relevant to the study question were retrieved in full and examined further by two authors (ADL, HACL) independently of one another. The bibliographies of these studies were examined and any previously unexamined references were retrieved. After strict analysis of the retrieved papers, nine met the inclusion criteria for a systematic review. Of these, all had details of reinfection rates and four had functional outcomes in the form of Harris hip scores (HHS, three studies) or Merle d’Aubigné-Postel scores (one study). A total of 596 patients (333 single-stage and 263 two-stage surgeries) were assessed as part of the reinfection analysis; 228 patients had functional scores (78 single-stage and 150 two-stage surgeries) and were analyzed separately.
Reinfection rate was determined by the criteria used by each study (Table 1). Reinfections with the same organism and infections by different organisms were considered reinfections for the purposes of this review. Merle d’Aubigné-Postel and HHS scores were considered comparable; this was justified by a previous study that showed good reliability between HHS and Merle d’Aubigné-Postel scoring systems [23]. Merle d’Aubigné-Postel score was normalized to the HHS by converting it to a percentage for the purposes of comparison. Risk of bias was estimated using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [20] and methodologic quality was estimated using the Methodological Index for Non-Randomised Studies (MINORS), which is a validated instrument designed for assessment of the methodologic quality of nonrandomized studies in surgery [26]. Data were extracted from each of the selected papers independently by two assessors (ADL, HACL); there was complete agreement regarding inclusion or exclusion in all cases.
Table 1.
Included studies
| Study | Year | Number of patients | Criteria for initial diagnosis | Cement + antibiotics | Design | Mean followup (years) | Criteria for reinfection | MINORS score |
|---|---|---|---|---|---|---|---|---|
| Carlsson et al. [4] | 1985 | 72 | Not specified | Cement with gentamycin | Prospective | 3.3 | Pain, elevated CRP and ESR, loosening on radiographs or positive cultures | 13 |
| De Man et al. [6] | 2011 | 72 | At least 2 cultures or histology | Cementless or cement with gentamycin | Retrospective | 2 | Clinical or radiologic signs, CRP ≥ 10 mg/L, ESR ≥ 20 mm/hour | 15 |
| Garvin et al. [8] | 1994 | 40 | Deep aspiration and culture | Cement with gentamycin | Retrospective | 5.7 | Rerevision with positive cultures | 11 |
| Hope et al. [11] | 1989 | 91 | Deep tissue biopsy - CNS | Cement with gentamycin | Retrospective | 3 | Rerevision with positive cultures | 11 |
| Klouche et al. [13] | 2012 | 84 | Preoperative or intraoperative culture | Cementless or cement without antibiotics | Prospective | 2 | Positive culture from aspiration of joint | 19 |
| Morscher et al. [21] | 1990 | 62 | Clinical features/culture | Cementless or cement without antibiotics | Retrospective | 3 | Rerevision with positive cultures | 16 |
| Oussedik et al. [22] | 2010 | 50 | Clinical features/culture | Cementless or cement with gentamycin | Prospective | 6.8 | Rerevision with positive cultures | 19 |
| Sanzen et al. [24] | 1988 | 102 | Positive culture | Cement with gentamycin | Retrospective | 6 | Wound, pain with ESR ≥ 35 mm/hour and no other mechanical explanation | 14 |
| Wilson & Dorr [28] | 1989 | 22 | Clinical only | Cementless or cement without antibiotics | Retrospective | 3.1 | Sinus, fever, pain with ESR ≥ 35 mm/hour, radiographic changes. If doubtful aspiration performed. | 17 |
MINORS = Methodological Index for NOn-Randomized Studies, maximum MINORS score is 24; CRP = C-reactive protein (normal, < 10 mg/L); ESR = erythrocyte sedimentation rate (normal, < 30 mm/hour); CNS = coagulase-negative staphylococcus.
Given the lack of prospective randomized trials, a meta-analysis was not attempted. Our systematic review was performed in accordance with the PRISMA guidelines [20]. Although a meta-analysis was not attempted, the results and effect size of each outcome were presented graphically as forest plots. The forest plots were produced using Review Manager 5.2.1 (The Cochrane Collaboration, Oxford, UK) [10].
Results
A total of 2275 articles were identified through the initial literature search. A total of 1964 of the 2275 were excluded in the primary screening leaving 311 studies to be read in full. Of these, nine studies fulfilled the inclusion criteria and were included in the systematic review [4, 6, 8, 11, 12, 21, 22, 24, 28]. Of the nine studies, none was randomized; all but two of the studies were retrospective cohort studies. Two large registry-based studies were excluded from the main review because it was impossible to extract demographic and procedure data from such studies. The first was a study of 784 revisions from the Norwegian Arthroplasty Register [7], and the second was a study of 349 patients from 14 French teaching hospitals, originally published in abstract form in the French literature [27] and subsequently rereported in the English-language literature [16]. In addition to the problems raised by registry analysis, the second study [16, 27] was not reported in detail and contained significant (18%) loss to followup. Both studies are described separately.
In most cases (six of nine), there was a qualitative difference in the presentation of patients between the two groups: for instance, patients were selected for two-stage revision if no organism was isolated preoperatively or if there was a sinus present, depending on the study (Table 2). Overall, the quality of the studies was poor when assessed using the MINORS scale (Table 1).
Table 2.
Details of two-stage surgery
| Study | Year | Number of patients | Spacer/beads | Time between stages | Reasons for two stage |
|---|---|---|---|---|---|
| Carlsson et al. [4] | 1985 | 72 | No | Until infection resolved | Not given |
| De Man et al. [6] | 2011 | 72 | Not specified | 3–8 weeks | Difficult bacteria or reconstruction |
| Garvin et al. [8] | 1994 | 40 | Beads | Not specified | Two stage unless infirm, acute, treatable, good tissues |
| Hope et al. [11] | 1989 | 91 | Beads | Not specified | Failed previous revision, fracture, severity (1 patient) |
| Klouche et al. [13] | 2012 | 84 | Yes | Not specified | Unknown organism or bone loss |
| Morscher et al. [21] | 1990 | 62 | Not specified | 9 days–178 months | Not specified |
| Oussedik et al. [22] | 2010 | 50 | Yes | Until infection resolved | Criteria including multiple or no organism |
| Sanzen et al. [24] | 1988 | 102 | Beads | 3–233 weeks | Not specified |
| Wilson & Dorr [28] | 1989 | 22 | Not specified | Not specified | Single stage if no positive cultures |
Techniques (and details given regarding techniques) varied among studies. In most cases, reconstruction was performed with cemented implants, and antibiotic-impregnated cement was used. Although it was specified that prostheses and cement were removed in their entirety in every case, the details provided regarding the extent of débridement varied among studies. In some studies, techniques no longer in widespread use, such as continuous postoperative irrigation, were used. In two-stage revisions, the time between stages varied widely as did the use of spacers (Table 2).
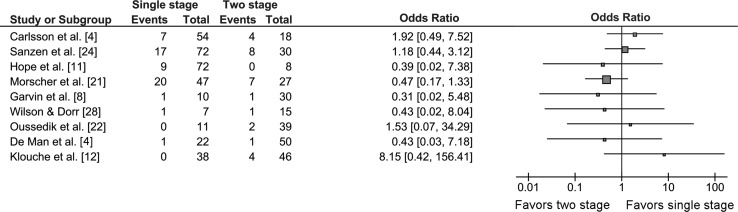
No individual study determined a statistically significant difference between single- and two-stage revisions in terms of infection-free survival. There was no consensus between studies of whether there was a trend toward increased reinfection rate with either procedure (Fig. 2). Definition of reinfection varied among studies. In four of nine studies, a hip was considered reinfected if it needed additional revision or excision arthroplasty with a confirmed infection. The remaining five studies used a combination of clinical, radiologic, and hematologic criteria along with positive culture to define reinfection (Table 1). Reinfection was a relatively uncommon event overall; 56 of 333 patients treated with single-stage revision (16.8%) and 28 of 263 (10.6%) patients treated with two-stage revision were reported to have reinfections.
Fig. 2.
The forest plot shows a comparison of single- with two-stage revisions in terms of risk of reinfection. The squares represent the overall hazard ratio; the larger squares represent larger studies. The bars on either side represent 95% CI. There is heterogeneity among the studies with no study showing a statistically significant difference between the procedures in terms of reinfection rate.
Single-stage surgery was associated with a trend to better outcomes in three of four studies with usable data for functional outcome (Fig. 3). This trend reached statistical significance only in the study of Oussedik et al. [22]. Mean HHS ranged from 76.4 to 87.8 (Fig. 3).
Fig. 3.
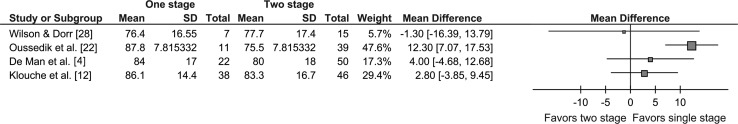
The forest plot shows a comparison of single- with two-stage revision in terms of functional outcome. The squares represent the overall hazard ratio; the larger squares represent larger studies. The bars either side represent 95% CI. There is heterogeneity among the studies but an overall trend exists toward better outcomes with single-stage revision. One study [22] shows significantly superior function with single-stage revision.
Two large comparative studies of registry data were retrieved during the literature search for this article. Although they otherwise met the inclusion criteria, they were registry-based studies and as a result, it was impossible to extract data regarding surgical techniques, therefore they were excluded from the main review. The first was a study of 784 revisions from the Norwegian joint registry [7]. Two hundred eighty-three patients underwent two-stage and 129 underwent single-stage revisions with the remainder undergoing an isolated revision of either the femoral or acetabular component. They compared survival using Cox regression and found a relative risk of revision of 2.0 in the patients treated with single-stage surgery when compared with patients treated with two-stage revision (95% CI, 1.1–3.9; p = 0.04).
The second excluded study was a multicenter retrospective review combining data from 14 units in France. It was reported in the form of an annotation in the Journal of Bone and Joint Surgery (British Volume) [16], but the study was published only in abstract form [27], which limits the conclusions that can be drawn from it (as does the 18% loss to followup). This study compared 127 patients with single-stage with 222 patients with two-stage revisions, and reported that infection was controlled in 88% of the patients who had single-stage and 85% of the patients who had two-stage surgeries. They also reported a high complication rate in patients undergoing two-stage revision, but there were few details regarding the preoperative status of the patients.
Discussion
The choice of single- or two-stage revision for prosthetic joint infection of the hip remains controversial. However, as the burden of primary and revision THAs increases, it becomes more important to reach consensus regarding the optimal management of such cases. The aims of our study were twofold: (1), to determine if there was a difference in reinfection rate, and (2) to compare functional outcomes between single- and two-stage revision surgeries for infected THAs.
This study has numerous limitations. As with any systematic review, the strength of the conclusions that can be drawn is limited by the quality of the primary data. Most studies had significant levels of loss to followup and reinfection rate may be underestimated as a result. In this case, there were no available randomized trials and few prospective studies. This raises the possibility of bias in the selection of patients in each study. In some studies, selection bias was built into study protocols with cases deemed less severe undergoing the single-stage procedure. As a result, the findings of this study may underestimate the relative risk of a poor result with the single-stage procedure. Protocols for one- and two-stage revisions have improved during recent years and the older studies may report a higher reinfection rate compared with what would be expected today. This is particularly true of single-stage revision surgery, in which “two-in-one” procedures, involving a full change of instruments after thorough débridement and removal of components, have become widespread. In addition, the overall methodologic quality of the studies was poor, and the functional outcome analysis was limited to a small number of studies. As such, it was not possible to do a meaningful meta-analysis, but rather, a systematic review was done.
Reinfection rate is the most commonly reported outcome measure in revision for infection. Although a previous single-arm study showed a significantly higher reinfection rate after single-stage surgery [30], our review of comparative studies showed relative parity between the two approaches. Future studies need to have consistent selection criteria and use modern techniques for both procedures. To avoid the problems of selection bias, patients should be randomized to single- or two-stage surgery. Many specialist units now perform large numbers of revisions for infection and such a trial should be possible. However, as reinfection remains an uncommon event, it is likely that such a study would have to be large and multicenter.
Functional outcome is much less frequently studied, and this is the first systematic review to study function in this setting. Most of the articles we reviewed reported better function after single-stage revision, but the numbers are small. In a putative future randomized trial, validated patient-reported outcome measures should be used to measure functional outcome. Such trials should be on an intention-to-treat basis, so as to take into account the substantial numbers of patients who do not undergo their second-stage surgery. The use of articulating spacers, which was inconsistent in the studies reported here, should be standardized in any future trial to accurately reflect current routine practices. A study of functional outcomes after single- and two-stage revisions would not need to be as large as one examining reinfection as an outcome: based on the means and standard deviations of the largest study [22], a study with approximately 50 patients in each arm would be adequately powered to detect a 5-point difference in HHS.
Despite its limitations, our study suggests that the two approaches may be comparable in terms of the risk of reinfection, and that single-stage revision for an infected THA may have an advantage in terms functional outcomes. However, because the literature lacks high-quality comparative trials, both of our questions merit further study. Whereas the presumed superiority of two-stage revision may have discouraged randomized trials in the past, many centers are now performing single-stage revision on a routine basis. This should have the effect of decreasing the barrier to performing such trials which are necessary to determine optimal management in these challenging cases.
Footnotes
One of the authors (ADL) received funding from a Clinical Research Fellowship from the Royal College of Surgeons of England. The institution of the authors has received, during the study period, funding from Biomet (Bridgend, UK) and Stryker (Newbury, UK).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beswick AD, Elvers KT, Smith AJ, Gooberman-Hill R, Lovering A, Blom AW. What is the evidence base to guide surgical treatment of infected hip prostheses? Systematic review of longitudinal studies in unselected patients. BMC Med. 2012;10:18. doi: 10.1186/1741-7015-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson AS, Egund N, Gentz CF, Hussenius A, Josefsson G, Lindberg L. Radiographic loosening after revision with gentamicin-containing cement for deep infection in total hip arthroplasties. Clin Orthop Relat Res. 1985;194:271–279. [PubMed] [Google Scholar]
- 5.Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pederson AB, Karrholm J, Garellick G, Pulkkinen P, Eskelinen A, Makela K, Engesaeter LB. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83:449–458. doi: 10.3109/17453674.2012.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Man FH, Sendi P, Zimmerli W, Maurer TB, Ochsner PE, Ilchmann T. Infectiological, functional, and radiographic outcome after revision for prosthetic hip infection according to a strict algorithm. Acta Orthop. 2011;82:27–34. doi: 10.3109/17453674.2010.548025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engesaeter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011;82:530–537. doi: 10.3109/17453674.2011.623572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvin KL, Evans BG, Salvati EA, Brause BD. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Relat Res. 1994;298:97–105. [PubMed] [Google Scholar]
- 9.Gehrke T, Kendoff D. Peri-prosthetic hip infections: in favour of one-stage. Hip Int. 2012;22(suppl 8):S40–S45. doi: 10.5301/HIP.2012.9569. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Available at: http://handbook.cochrane.org/. Accessed August 18, 2013.
- 11.Hope PG, Kristinsson KG, Norman P, Elson RA. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br. 1989;71:851–855. doi: 10.1302/0301-620X.71B5.2584258. [DOI] [PubMed] [Google Scholar]
- 12.Klouche S, Leonard P, Zeller V, Lhotellier L, Graff W, Leclerc P, Mamoudy P, Sariali E. Infected total hip arthroplasty revision: one- or two-stage procedure? Orthop Traumatol Surg Res. 2012;98:144–150. doi: 10.1016/j.otsr.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res. 2010;96:124–132. doi: 10.1016/j.otsr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 15.Lange J, Troelsen A, Thomsen RW, Soballe K. Chronic infections in hip arthroplasties: comparing risk of reinfection following one-stage and two-stage revision: a systematic review and meta-analysis. Clin Epidemiol. 2012;4:57–73. doi: 10.2147/CLEP.S29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langlais F. Can we improve the results of revision arthroplasty for infected total hip replacement? J Bone Joint Surg Br. 2003;85:637–640. [PubMed] [Google Scholar]
- 17.Luu A, Syed F, Raman G, Bhalla A, Muldoon E, Hadley S, Smith E, Rao M. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty. 2013 Apr 8 [Epub ahead of print]. [DOI] [PubMed]
- 18.Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ. 2009;338:b1773. doi: 10.1136/bmj.b1773. [DOI] [PubMed] [Google Scholar]
- 19.Merollini KM, Crawford RW, Graves N. Surgical treatment approaches and reimbursement costs of surgical site infections post hip arthroplasty in Australia: a retrospective analysis. BMC Health Serv Res. 2013;13:91. doi: 10.1186/1472-6963-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PMC free article] [PubMed]
- 21.Morscher E, Babst R, Jenny H. Treatment of infected joint arthroplasty. Int Orthop. 1990;14:161–165. doi: 10.1007/BF00180122. [DOI] [PubMed] [Google Scholar]
- 22.Oussedik SI, Dodd MB, Haddad FS. Outcomes of revision total hip replacement for infection after grading according to a standard protocol. J Bone Joint Surg Br. 2010;92:1222–1226. doi: 10.1302/0301-620X.92B9.23663. [DOI] [PubMed] [Google Scholar]
- 23.Ovre S, Sandvik L, Madsen JE, Roise O. Comparison of distribution, agreement and correlation between the original and modified Merle d’Aubigne-Postel Score and the Harris Hip Score after acetabular fracture treatment: moderate agreement, high ceiling effect and excellent correlation in 450 patients. Acta Orthop. 2005;76:796–802. doi: 10.1080/17453670510045390. [DOI] [PubMed] [Google Scholar]
- 24.Sanzen L, Carlsson AS, Josefsson G, Lindberg LT. Revision operations on infected total hip arthroplasties: two- to nine-year follow-up study. Clin Orthop Relat Res. 1988;229:165–172. [PubMed] [Google Scholar]
- 25.Senthi S, Munro JT, Pitto RP. Infection in total hip replacement: meta-analysis. Int Orthop. 2011;35:253–260. doi: 10.1007/s00264-010-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 27.Vielpeau C, Lortat-Jacob A. [Management of the infected hip prostheses][in French] Rev Chir Orthop Reparatrice Appar Mot. 2002;88(suppl 1):159–216. [Google Scholar]
- 28.Wilson MG, Dorr LD. Reimplantation of infected total hip arthroplasties in the absence of antibiotic cement. J Arthroplasty. 1989;4:263–269. doi: 10.1016/S0883-5403(89)80023-3. [DOI] [PubMed] [Google Scholar]
- 29.Winkler H, Stoiber A, Kaudela K, Winter F, Menschik F. One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br. 2008;90:1580–1584. doi: 10.1302/0301-620X.90B12.20742. [DOI] [PubMed] [Google Scholar]
- 30.Wolf BR, Gu NY, Doctor JN, Manner PA, Leopold SS. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection: a Markov expected-utility decision analysis. J Bone Joint Surg Am. 2011;93:631–639. doi: 10.2106/JBJS.I.01256. [DOI] [PubMed] [Google Scholar]