Abstract
Background
Artificial bone graft substitutes are widely used to fill bony defects after curettage of benign tumors. We sought to evaluate the efficacy of one such bone graft substitute, geneX®, which contains tricalcium phosphate and calcium sulphate; however, during the course of this study we observed a high number of complications.
Questions/purposes
The primary aim of this prospective series was assessment of the effectiveness of geneX® concerning resorption profile and bone healing and remodeling after surgery. We present the types and frequencies of complications observed in patients treated for bone tumors by curettage and filling the defect using geneX®.
Methods
We planned to study 40 patients; however, after enrollment of the first 31 patients, the study was stopped as a result of serious complications. There were 20 female and 11 male patients with a mean age at surgery of 40 years (range, 6–71 years). Plain radiographs were obtained at different intervals during followup and CT scans were obtained 6 and 12 months postoperatively. Complications were assessed using a 5-point scale according to Goslings and Gouma.
Results
Five of the 31 patients (16%) had complications develop after surgery. In three cases, a sterile inflammation adjacent to the geneX® occurred, with delayed wound healing in two patients and local pain. In the third patient, geneX® produced moderate to severe skin damage in the area of the scar, needing revision surgery. In two other patients, inflammatory cystic formations developed in the soft tissues with sizes up to 15 cm, which gradually reduced in size with time. Overall, there were four Grade 1 complications and one Grade 2 according to Goslings and Gouma.
Conclusions
We concluded from this series of patients that geneX® causes soft tissue inflammation and pain with its use. Based on this experience we believe that this type of bone substitute should not be used in the treatment of bony defects.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Benign and low-grade malignant bone tumors generally are treated with intralesional curettage with or without using surgical adjuvants. Artificial bone graft substitutes have been introduced as a possible alternative to autografts and allograft material because of their complications and shortcomings [2, 5–7, 11, 12, 16]. There are several substitutes available that can be divided into demineralized bone matrix, bone graft extenders, and bone morphogenic proteins [2, 5–7, 11, 12]. Demineralized bone matrix consists of proteins gained from processed cadaver bone without minerals. Bone graft extenders include ceramics (eg, hydroxyapatite), salts (eg, calcium sulphate, tricalcium phosphate), or synthetic products like polymethylmethacrylate (PMMA). Bone morphogenic proteins induce production of new bone by stimulating osteoblastic differentiation [6, 7, 11].
The reconstructive approach after intralesional curettage is controversial and clinical practice varies. PMMA, allografts, or autografts have been used in several studies and complication rates up to 33% have been reported [5, 6, 12, 16]. Others reported voids unfilled [10].
Depending on the kind of reconstruction, the most common complications are infection, delayed wound healing, early fracture or collapse, and nonunion. In case of autografts there is also a possibility of donor-site morbidity [5, 6, 10, 12, 16].
GeneX® (Biocomposites Ltd, Staffordshire, UK) is a commercially available bone graft extender composed of calcium sulfate and β-tricalcium phosphate (β-TCP) in a weight ratio of 1:1 [1, 15]. According to the manufacturer it is a fully synthetic product with so-called “smart pores”, which produce a developing macroporosity, allowing cells and nutrients to populate the pores and the graft eventually is absorbed [1]. It is applied as an injectable paste, which can be contoured to the surgical site, and sets to a high degree of compressive strength in situ at body temperature within 15 minutes [1]. Yang et al. [15] and Walsh et al. [14] reported resorption of the calcium sulfate component of geneX® by dissolution within 12 weeks after implantation, whereas the remaining β-TCP component is known to have a longer resorption profile [7, 8, 13]. Other in vivo studies also showed the poor osteogenic effect of β-TCP [4, 9]. Saadoun et al. [11] also raised concerns regarding the safety of this product. However, the number of complications reported in their series was small [11].
We started a prospective, nonrandomized single-cohort study to investigate bone remodeling after curettage of various bone tumors and refilling the defect using the artificial bone graft substitute geneX®; however, we halted the trial prematurely because of concerns regarding side effects. The current report describes the types and incidence of complications we observed in patients treated for bone tumors by curettage and filling the defect using geneX®.
Patients and Methods
We sought to perform a prospective, nonrandomized study assessing the effectiveness of geneX® concerning resorption and bone-healing quality during a minimum followup of 12 months. Time to healing, local recurrence rates, and complications were evaluated. The study was a nonregistered single-center investigation and was approved by the Ethics Committee of the Medical University of Graz (EK 23-277 ex 10/11). Written informed consent was obtained from all patients.
In the study, we anticipated enrolling 40 patients with benign and low-grade malignant bone tumors treated by curettage and refilling of the defect using the artificial bone graft substitute geneX®. Patients with high-grade malignancies were excluded. We planned to review the patients recruited for the current series at 2 weeks postoperatively, and then at 1 month, 3 months, 6 months, 12 months, and yearly, thereafter. Patients with low-grade malignancies were investigated at 3-month intervals, including actual staging investigations (chest CT and alternately chest radiographs and abdominal sonography). Every followup included a clinical examination and a radiographic control. In addition, we planned to obtain CT scans 6 months and 12 months postoperatively to investigate bone remodeling.
All operations were performed by two experienced tumor surgeons (AL, WME). After surgical exposure of the affected bone, a window was created allowing access to the lesion. Curettage was performed under radiographic control using a c-arm. The collected tissue was sent for histopathologic analysis. GeneX® was prepared according to the manufacturer’s instructions and then injected into the void. According to the instructions, geneX® was allowed to set into strength within 15 minutes, thereafter, the bone window was reapposed if possible and wound closure was done in layers. However, after inclusion of 31 patients, further enrollment in the study was stopped owing to adverse reactions and new complications which were not known before to the users applying the new bone graft substitute. Therefore, the goal of the current report is to characterize the types and frequencies of these complications.
Complications were evaluated according to Goslings and Gouma [3] as follows: 0 (no harm), 1 (temporary disadvantage, no reoperation), 2 (recovery after reoperation), 3 (permanent damage/disability), 4 (death), and 5 (unclear as a result of untimely death).
Between September 2010 and March 2012, there were 20 female and 11 male patients with a mean age at the time of surgery of 40 years (range, 6–71 years). Nine lesions were located in the proximal humerus, seven in the femur, five in the tibia or fibula, and 10 in the small bones of the hand (eight) and foot (two). There were 16 enchondromas, four low-grade chondrosarcomas, five simple or juvenile bone cysts, and six other different benign lesions (eg, fibrous dysplasia, nonossifying fibroma, intraosseous lipoma)(Table 1). The mean mass of geneX® used for refilling after curettage was 19 cm3 (range, 5–60 cm3). In two cases of a relapsed juvenile bone cyst, strut allografts were used to refill the bony cavity and to enhance internal stability (Patients 30 and 31).
Table 1.
Patients’ demographics
| Patient number | Age at surgery (years), sex | Diagnosis, localization and side | Treatment | geneX® (cc) | Allergy | Smoker (pack years) | Alcohol use | Complication | Followup (months) | Time until event (days) | Goslings & Gouma grade |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37, F | Enchondroma, proximal humerus, R | Curettage | 50 | None | 4.5 | Yes | None | 29 | – | 0 |
| 2 | 32, F | Enchondroma, hand, fifth digit L | Curettage | 5 | Ibuprofen | No | No | Aseptic inflammation | 27 | 7 | 1 |
| 3 | 23, M | Enchondroma, proximal humerus, R | Curettage, plate | 10 | None | 8 | Yes | None | 12 | – | 0 |
| 4 | 26, M | Enchondroma, hand, fifth digit, L | Curettage | 5 | None | No | Yes | None | 20 | – | 0 |
| 5 | 52, M | Enchondroma, hand, fifth digit, L | Curettage | 5 | None | 35 | No | None | 12 | – | 0 |
| 6 | 57, F | Enchondroma, hand, second digit, R | Curettage | 5 | Tramadol hydrochloride | 20 | Yes | None | 11 | – | 0 |
| 7 | 37, F | Enchondroma, hand, fifth digit, L | Curettage | 10 | None | 4.5 | Yes | None | 33 | – | 0 |
| 8 | 58, F | Intraosseous ganglion, foot, first digit, L | Curettage | 5 | None | No | No | None | 14 | – | 0 |
| 9 | 38, F | Enchondroma, hand, fourth digit, L | Curettage | 5 | None | 23 | No | None | 31 | – | 0 |
| 10 | 42, F | Enchondroma, hand, fifth digit, L | Curettage | 5 | None | 18.75 | Yes | None | 31 | – | 0 |
| 11 | 49, F | Intraosseous ganglion, hand, third digit, L | Curettage | 5 | NA | NA | NA. | None | 30 | – | 0 |
| 12 | 71, M | Chondrosarcoma grade 1, proximal humerus, L | Curettage | 30 | None | No | Yes | None | 12 | – | 0 |
| 13 | 50, F | Chondrosarcoma grade 1, proximal humerus, L | Curettage | 20 | None | 74 | Yes | None | 19 | – | 0 |
| 14 | 6, M | Juvenile bone cyst, proximal femur, L | Curettage | 10 | None | No | No | None | 15 | – | 0 |
| 15 | 26, F | Fibrous dysplasia, distal tibia, R | Curettage | 10 | None | No | No | None | 22 | – | 0 |
| 16 | 23, F | Intraosseous ganglion, calcaneus, R | Curettage | 5 | None | No | No | Pain* | 16 | – | 1 |
| 17 | 47, F | Enchondroma, distal femur, R | Curettage, plate | 10 | Mefenamic acid | No | No | None | 14 | – | 0 |
| 18 | 13, F | Nonossifying fibroma, proximal fibula, R | Curettage | 15 | None | No | No | None | 15 | – | 0 |
| 19 | 16, M | Fibrous dysplasia, distal tibia, L | Curettage, plate | 20 | None | 0.25 | No | None | 19 | – | 0 |
| 20 | 44, F | Chondrosarcoma grade 1, distal femur, L | Curettage, plate | 50 | None | No | No | None | 25 | – | 0 |
| 21 | 44, M | Intraosseous ganglion, distal femur, L | Curettage | 15 | Potassium dichromate | No | No | Soft tissue cyst | 10 | 52 | 1 |
| 22 | 63, M | Enchondroma, distal femur, R | Curettage, plate | 30 | Hornets | 60 | Yes | None | 20 | – | 0 |
| 23 | 50, F | Enchondroma, proximal humerus, L | Curettage | 15 | None | No | Yes | None | 13 | – | 0 |
| 24 | 57, F | Enchondroma, distal femur, R | Curettage, plate | 15 | None | No | Yes | None | 12 | – | 0 |
| 25 | 57, F | Enchondroma, proximal tibia, R | Curettage, plate | 30 | Iodine, acetylsalicylic acid | 22.5 | No | Aseptic inflammation | 18 | 15 | 1 |
| 26 | 42, F | Enchondroma, proximal humerus, R | Curettage | 30 | Pollen | No | Yes | None | 14 | – | 0 |
| 27 | 63, F | Enchondroma, proximal fibula, L | Curettage | 10 | None | No | No | Pain* | 8 | – | 0 |
| 28 | 38, F | Intraosseous lipoma, proximal humerus, R | Curettage | 30 | None | 19 | No | None | 18 | – | 0 |
| 29 | 40, F | Chondrosarcoma grade 1, distal femur, L | Curettage, plate | 60 | Latex | 25 | No | None | 18 | – | 0 |
| 30 | 15, M | Juvenile bone cyst, proximal humerus, R | Curettage, allograft | 45 | None | No | No | Soft tissue cyst | 9 | 55 | 1 |
| 31 | 14, M | Juvenile bone cyst, proximal humerus, R | Curettage, allograft | 40 | None | No | No | Aseptic inflammation | 12 | 41 | 2 |
L = left; R = right; NA = not available; *unclear whether the pain was associated with geneX®.
The mean postoperative followup for all patients was 18 months (range, 8–33 months). One patient was lost to followup after 11 months and seven failed the outpatient review according to the followup protocol. Of these seven patients, six were interviewed by telephone and reported having no complications. Nevertheless, an assessment of outcome of the bone graft substitute that we used cannot be done in these cases.
Observation of healing chronology showed complete or partial resorption of the artificial bone graft substitute in 87% (n = 27) at a mean followup of 3.7 months (range, 1.5–7.1 months). During the time of the investigation, there were no local recurrences or metastases in patients with low-grade malignancies, although the followup is too short to interpret the oncologic outcome.
Results
Five of the 31 patients (16%) had adverse reactions after surgery (Table 1). Three patients (Patients 2, 25, and 31) had a sterile inflammation adjacent to the geneX® causing delayed wound healing with continuous secretion and pain; these reactions presented at 7, 15, and 41 days after the index procedure, respectively. In the third patient (Patient 31), geneX® produced moderate to severe skin damage in the area of the scar that required revision 41 days after the index surgery (Fig. 1). In two other patients (Patients 21 and 30), inflammatory cystic formations up to 15 cm developed in the soft tissues causing slight to moderate pain and swelling at an average of 54 days (52 and 55 days) after surgery. These formations reduced in size with time (reduction from 15 cm to 9 cm within 2 months) without surgical intervention (Fig. 2). Another two patients reported continuing pain 6 months and 10 months after surgery (Patients 16 and 27), however, we could not determine whether the pain was associated with geneX® or if it was caused by a secondary osteoarthritis of the adjacent joints.
Fig. 1A–B.
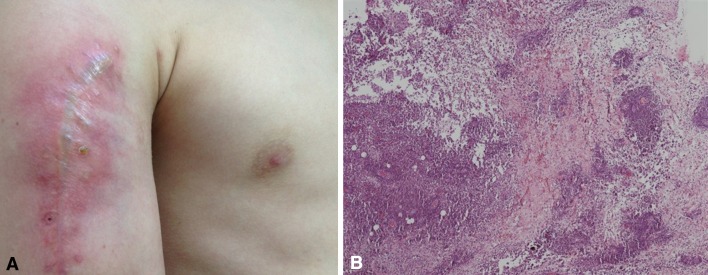
(A) The photograph shows the upper arm of a 14-year-old male patient with an aseptic inflammation resulting from geneX® . The patient needed revision surgery more than 1 month after surgery for a juvenile bone cyst of the humerus. (B) Histologic analysis of The hematoxylin and eosin-stained section showed a chronic, lymphocytic inflammation with plasma cells (Original magnification, ×100).
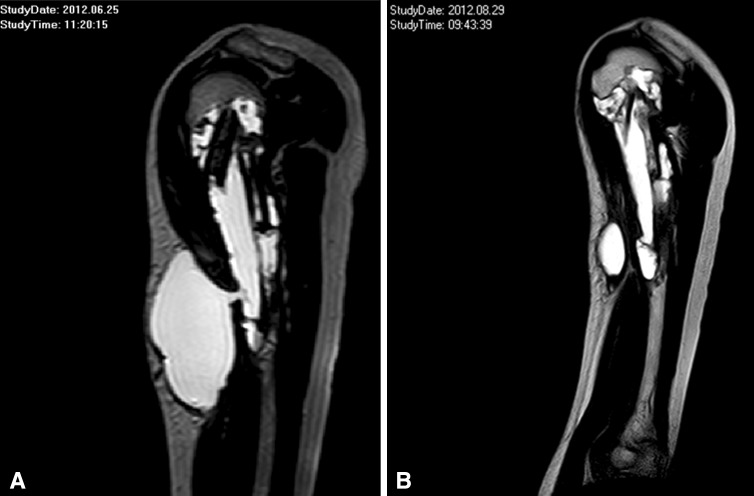
Fig. 2A–B.
MR images of the upper arm of a 15-year-old male patient obtained (A) 3 and (B) 5 months after surgery show a soft tissue cyst that developed after curettage of a juvenile bone cyst and refilling the defect with geneX® and allografts. The cystic formation reduced with time from greater than 15 cm to 9 cm.
A total of four patients had a Grade I complication according to the classification system of Goslings and Gouma [3], meaning a temporary disadvantage without revision surgery. One patient had a Grade II complication, implying recovery after reoperation. No patients had Grades III, IV, or V complications. Overall, there was one revision surgery.
Based on the frequency of complications, the prospective trial on this product was halted prematurely.
Discussion
The reconstructive approach after intralesional curettage of benign and low-grade malignant bone tumors is controversial and clinical practice varies. Refilling the void to enhance the primary strength of bone and to accelerate earlier healing and weightbearing has been reported in previous studies [2, 5, 12, 15, 16]. However, unfilled voids with a similar outcome also have been reported [10]. The complications and shortcomings associated with the use of autograft and allograft bone [5, 12, 16] have prompted interest in bone graft extenders and substitutes [2, 12]. We sought to evaluate the efficacy of one such bone graft substitute; however, during the course of our prospective, observational trial we observed a high number of complications and the prospective trial was halted prematurely. GeneX® was introduced as a fully resorbable, artificial bone graft substitute as an alternative to autografts and allografts for refilling bony defects. Because geneX® had the same technologic characteristics as other devices already on the market, and because bone graft substitutes are classified as medical devices rather than drugs, it did not need to undergo clinical safety tests before obtaining FDA 510(k) clearance for use in patients as there were no concerns regarding safety and effectiveness [11]. Numerous authors reported outcomes after curettage of benign and low-grade malignant bone tumors and using different bone graft extenders based on calcium phosphate, calcium sulfate, or tricalcium phosphate for filling the void without any complications [2, 6, 9, 12, 14]. To the best of our knowledge, this is the first report of geneX® causing wound complications after tumor surgery.
There are several limitations of the current study. From the beginning, we planned to enroll only a small number of patients in this series. Owing to the complications and increasing doubts regarding the safety of geneX®, the study was halted prematurely. Furthermore, there is a lack of control groups with unfilled voids and another bone graft substitute. Eleven patients had a followup less than 12 months with radiographic control films obtained at 2 weeks, 6 weeks, and 6 months after surgery. Therefore, it is possible that the number of complications is underestimated and that the problems with geneX® may be more extensive than we have seen. Furthermore, we cannot comment on the effectiveness of the geneX® in these cases owing to the lack of clinical and radiographic controls.
We attempted to identify cofactors such as smoking behavior or allergies leading to a higher complication rate after using geneX®. However, no definite reasons were found. In the group of 31 patients, there were 13 cigarette smokers with an average of 24 pack years (range, 1–74 pack years). Only one of these patients had a postoperative inflammation, therefore we think smoking behavior was not directly associated with the complications. However, a missing correlation could be the small number of patients enrolled in this series. Further, none of the patients with postoperative complications had allergies to synthetic materials.
The geneX® was prepared according to the manufacturer’s instructions during surgery and the bone windows were reapposed after injection if possible. One reason for inflammation and soft tissue cyst development could be that the defect side was overfilled and pressurized, especially when using allografts. Otherwise, there were patients with larger bone voids who had refilling without any postoperative complications.
In the current series, geneX® caused marked local aseptic inflammation damaging adjacent soft tissues such as muscle and skin, and inflammatory cystic formations developed in the soft tissues in two additional patients. All complications occurred after curettage of benign and low-grade malignant bone tumors. Saadoun et al. [11] also reported similar findings with geneX® in a small series of three patients after spinal surgery. In all cases, the bone graft substitute caused sterile pus in the soft tissues adjacent to the geneX®. Histologic examinations showed a marked infiltration of inflammatory cells damaging adjacent soft tissues [11]. We suspect that the local soft tissue reactions are caused by liquefaction of the calcium sulfate component during resorption resulting in a decrease of the local pH value and invasion of inflammatory cells.
Saadoun et al. [11] also reported that four of 10 orthopaedic surgeons in Great Britain using geneX® also experienced wound breakdown and purulent discharge [11]. This suggests that complications with geneX® may be more common but are being underreported in the literature. Saadoun et al. [11] reported geneX® caused significant muscle and skin necrosis next to the injection site in a mouse model with loss of normal tissue structure. However, Zhang et al. [18] and Yang et al. [15] reported that geneX® is a useful alternative to PMMA in vertebroplasty for vertebral compression fractures in a calf and a sheep model, respectively, but they also recommended additional studies be conducted in humans to evaluate its resorption in vivo. Zhan and Ye [17] reported good biocompatibility, strong bone inducibility, no complications, little loss of vertebrae height and Cobb angle, and satisfactory results after using geneX® for vertebroplasty in 38 patients.
Although we prepared the geneX® according to the manufacturer’s instructions, five of our patients experienced sterile inflammation with delayed wound healing and development of soft tissue cysts. One of these patients needed revision surgery owing to severe damage of the skin. These complications after such surgeries were unknown to us and based on the frequency, the ongoing prospective trial was halted prematurely. Therefore, we caution against the use of this bone graft substitute for such indications.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Biocomposites L. geneX®; Injectable Bone graft with ZPC. Biocomposites, Ltd. Available at: http://www.biocomposites.com/ortho/Genex2.asp. Accessed May 1, 2013.
- 2.Evaniew N, Tan V, Parasu N, Jurriaans E, Finlay K, Deheshi B, Ghert M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics. 2013;36:e216–e222. doi: 10.3928/01477447-20130122-25. [DOI] [PubMed] [Google Scholar]
- 3.Goslings JC, Gouma DJ. What is a surgical complication? World J Surg. 2008;32:952. doi: 10.1007/s00268-008-9563-3. [DOI] [PubMed] [Google Scholar]
- 4.Gupta MC, Theerajunyaporn T, Maitra S, Schmidt MB, Holy CE, Kadiyala S, Bruder SP. Efficacy of mesenchymal stem cell enriched grafts in an ovine posterolateral lumbar spine model. Spine (Phila Pa 1976). 2007;32:720–726; discussion 727. [DOI] [PubMed]
- 5.Johnson LJ, Clayer M. Aqueous calcium sulphate as bone graft for voids following open curettage of bone tumours. ANZ J Surg. 2013;83:564–570. doi: 10.1111/j.1445-2197.2012.06175.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CM, Wilkins RM. Treatment of benign bone lesions with an injectable calcium sulfate-based bone graft substitute. Orthopedics. 2004;27(1 suppl):S131–S135. doi: 10.3928/0147-7447-20040102-11. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Lun DX. Current application of β-tricalcium phosphate composites in orthopaedics. Orthop Surg. 2012;4:139–144. doi: 10.1111/j.1757-7861.2012.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuda T, Ioku K, Yonezawa I, Minagi H, Kawachi G, Gonda Y, Murayama H, Shibata Y, Minami S, Kamihira S, Kurosawa H, Ikeda T. The effect of the microstructure of beta-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials. 2007;28:2612–2621. doi: 10.1016/j.biomaterials.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Orii H, Sotome S, Chen J, Wang J, Shinomiya K. Beta-tricalcium phosphate (beta-TCP) graft combined with bone marrow stromal cells (MSCs) for posterolateral spine fusion. J Med Dent Sci. 2005;52:51–57. [PubMed] [Google Scholar]
- 10.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005;435:211–218. doi: 10.1097/01.blo.0000160024.06739.ff. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun S, Macdonald C, Bell BA, Papadopoulos MC. Dangers of bone graft substitutes: lessons from using GeneX. J Neurol Neurosurg Psychiatry. 2011;82:e3. doi: 10.1136/jnnp.2010.232371. [DOI] [PubMed] [Google Scholar]
- 12.Van Hoff C, Samora JB, Griesser MJ, Crist MK, Scharschmidt TJ, Mayerson JL. Effectiveness of ultraporous beta-tricalcium phosphate (vitoss) as bone graft substitute for cavitary defects in benign and low-grade malignant bone tumors. Am J Orthop (Belle Mead NJ). 2012;41:20–23. [PubMed] [Google Scholar]
- 13.Walsh WR, Langdown AJ, Auld JW, Stephens P, Yu Y, Vizesi F, Bruce WJ, Pounder N. Effect of low intensity pulsed ultrasound on healing of an ulna defect filled with a bone graft substitute. J Biomed Mater Res B Appl Biomater. 2008;86:74–81. doi: 10.1002/jbm.b.30989. [DOI] [PubMed] [Google Scholar]
- 14.Walsh WR, Morberg P, Yu Y, Yang JL, Haggard W, Sheath PC, Svehla M, Bruce WJ. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res. 2003;406:228–236. doi: 10.1097/00003086-200301000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Yang HL, Zhu XS, Chen L, Chen CM, Mangham DC, Coulton LA, Aiken SS. Bone healing response to a synthetic calcium sulfate/β-tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater. 2012;100:1911–1921. doi: 10.1002/jbm.b.32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Zhan BL, Ye Z. Significance of percutaneous vertebroplasty with Genex in the treatment of thoracolumbar burst fractures. Zhongguo Gu Shang. 2011;24:223–226. [PubMed] [Google Scholar]
- 18.Zhang S, Jiang J, Zhu Q, Huang Z. [Biomechanical study of vertebroplasty with geneX((R)) cement augmentation in a calf osteoporotic vertebral compression fracture model][in Chinese] Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:843–846. [PubMed] [Google Scholar]