Abstract
Background
Aseptic failure of massive endoprostheses used in the reconstruction of major skeletal defects remains a major clinical problem. Fixation using compressive osseointegration was developed as an alternative to cemented and traditional press-fit fixation in an effort to decrease aseptic failure rates.
Questions/purposes
The purpose of this study was to answer the following questions: (1) What is the survivorship of this technique at minimum 2-year followup? (2) Were patient demographic variables (age, sex) or anatomic location associated with implant failure? (3) Were there any prosthesis-related variables (eg, spindle size) associated with failure? (4) Was there a discernible learning curve associated with the use of the new device as defined by a difference in failure rate early in the series versus later on?
Methods
The first 50 cases using compressive osseointegration fixation from two tertiary referral centers were retrospectively studied. Rates of component removal for any reason and for aseptic failure were calculated. Demographic, surgical, and oncologic factors were analyzed using regression analysis to assess for association with implant failure. Minimum followup was 2 years with a mean of 66 months. Median age at the time of surgery was 14.5 years.
Results
A total of 15 (30%) implants were removed for any reason. Of these revisions, seven (14%) were the result of aseptic failure. Five of the seven aseptic failures occurred at less than 1 year (average, 8.3 months), and none occurred beyond 17 months. With the limited numbers available, no demographic, surgical, or prosthesis-related factors correlated with failure.
Conclusions
Most aseptic failures of compressive osseointegration occurred early. Longer followup is needed to determine if this technique is superior to other forms of fixation.
Introduction
Endoprosthetic reconstruction is frequently used as a means of limb salvage for primary malignant bone tumors. Endoprostheses offer superior functional outcomes when compared with amputation [22], and they have lower infection and fracture risks than bulk allograft reconstructions [16, 20]. Allograft-prosthetic composite reconstructions are used at many centers, but infection risk and failure to incorporate at the host bone junction remain limitations [5, 7, 8, 27]. Optimal fixation of the endoprosthetic constructs continues to be controversial with advocates of both cemented [23, 26] and noncemented fixation [9, 10]. Most noncemented implants use cylindrical, porous-coated stems for fixation in a manner similar to many of the noncemented stems used for hip arthroplasty. These implants rely on friction between the porous stem surface and the endosteal surface of the medullary canal for initial fixation. Compressive osseointegration is an alternative strategy for noncemented endoprosthetic fixation, which uses axial compression between the implant and the cut surface of the diaphyseal bone for initial implant fixation [18].
A potential advantage of compressive osseointegration is the ability to fix megaprostheses to very short diaphyseal segments to which traditional stem fixation would be tenuous if not impossible. Additionally, compressive osseointegration was shown to avoid the stress shielding of bone commonly seen with stemmed implants in an in vitro model [4]. The axial loading of compressive osseointegration has also been shown to induce bone hypertrophy and in-growth at the fixation interface in human retrieval studies [3, 15]. Finally, the small amount of bone length required to place the implant preserves diaphyseal bone stock, which may be useful if future revision is required in this generally young, active cohort of patients.
The purpose of this study is to review the outcomes of compressive osseointegration for the fixation of endoprostheses used in oncologic limb salvage at a minimum of 2 years. These implants are a relatively new technology. FDA clearance for use of the device in femoral reconstructions was obtained in 2003, and compressive osseointegration for other sites remains FDA off-label use. Consequently, there are few reports of this implant technique in the literature, and the largest of these comes from the developers of the device. We specifically sought to evaluate the following questions: (1) What is the survivorship of this technique at minimum 2-year followup? (2) Were patient demographic variables (age, sex) or anatomic location associated with implant failure? (3) Were there any prosthesis-related variables (eg, spindle size) associated with failure? (4) Was there a discernible learning curve associated with the use of the new device as defined by a difference in failure rate early in the series versus later on?
Patients and Methods
Patient Selection and Data Collection
All cases from the two participating centers in which compressive osseointegration was used for long bone endoprosthesis fixation were reviewed. Patients with less than 2-year followup were excluded. Selection of compressive osseointegration for fixation was determined at the discretion of the four participating surgeons (RLR, LDW, KBJ, JEC). An absolute indication for use of the device at both study sites during the period in question was short residual bone length for which standard stem fixation would not be possible. Age younger than 50 years was a relative indication for compressive osseintegration fixation. Metastatic bone disease requiring endoprosthetic replacement was an absolute contraindication. Routine followup was performed at 6 weeks, 3 months, and 6 months. Further followup was every 3 to 6 months depending on the surveillance requirements of each individual patient’s cancer diagnosis. All clinic charts, operative reports, implant records, and radiographs for each patient were reviewed for the study. Average followup for the entire group was 68 months (range, 31–113 months), and two patients were lost to followup. Demographic data, diagnoses at presentation, operative details, oncologic outcomes, and subsequent need for reoperation were recorded. Institutional review board approval for the study was obtained.
Patient Demographics and Diagnoses
The cohort included 25 males and 25 females. The mean age was 20.5 years and median age was 16 years. Average height, weight, and body mass index were 159 cm, 55 kg, and 21 kg/m2, respectively. Osteosarcoma was the diagnosis in 39 patients (78%). The remaining diagnoses included three chondrosarcomas, three Ewing’s sarcomas, two giant cell tumors, one malignant fibrous histiocytoma of bone, one malignant pleomorphic mesenchymal tumor, and one desmoplastic fibroma of bone. Distal femoral replacement was performed in 37 patients (74%). The remaining procedures included six proximal femoral replacements, three proximal humerus replacements, two proximal tibia replacements, and two intercalary femoral replacements.
Surgical Techniques
Surgical approach and resection length were determined individually by the characteristics of the specific tumors and the preferred techniques of the operating surgeons. Implantation of the Compress® (Biomet, Warsaw, IN, USA) devices was performed using the manufacturer’s recommended techniques (Fig. 1). Use of this technology for reconstruction at sites other than the femur was FDA off-label, and appropriate informed consent of off-label use was obtained in all such cases. A spindle-sizing guide provided by the manufacturer was used to select the appropriate diameter spindle in the femur, whereas custom spindles were used in the tibia and humerus. Compression force application was determined the thickness of the cortical bone per the manufacturer’s guidelines. Antirotation pins placed through the spindle to aid in the prevention of early torsional failure are an optional adjunct to the device. Use of these pins was at the discretion of the operating surgeons, and their use was recorded and analyzed. The authors routinely used two pins when using the antirotation pins. The same rotating hinge knee articulation was used for all distal femur and proximal tibia cases. Hemiarthroplasty components were used for the proximal femur and proximal humerus cases. Two cases were intercalary femur reconstructions in which two separate Compress® devices (one proximal and one distal) were used in the same bone. These patients were treated as single cases for all analyses except those in which individual characteristics of the Compress® junctions such as compression force and antirotation pin use might have influenced aseptic failure independently. The postoperative weightbearing protocol was 6 weeks of strict nonweightbearing followed by progression to weightbearing as tolerated.
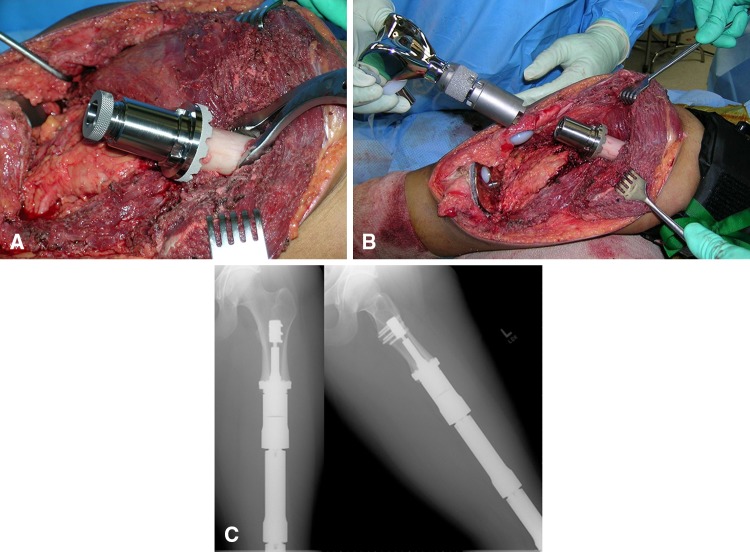
Fig. 1A–C.
(A) Intraoperative photograph of Compress® implantation is shown. (B) This is an intraoperative photograph of distal femoral replacement attachment to the Compress® device. (C) Two-year postoperative radiographs show the osseointegrated device.
Statistical Analysis
Descriptive statistics including means, medians, and percentages were calculated. Removal of components for any reason and aseptic failure for any reason were recorded and analyzed separately. The term aseptic failure is used rather than aseptic loosening because failures of this device typically result from initial failure of osseointegration rather than subsequent loosening as is more common with cemented designs. Planned reoperations for lengthenings in skeletally immature patients were not included in the analysis. Univariate analyses were performed to identify patient, surgical, and oncologic factors correlated with removal of components for any reason and aseptic failure. Time to removal for any reason and aseptic failure were measured from age at surgery. For each failure subgroup, survival was estimated using the Kaplan-Meier method starting on the date of the operation and ending on the date of removal, failure, or latest followup. Cox regression analyses were performed to determine whether demographic, oncologic, or operative factors were associated with survival until removal or failure. There were two intercalary reconstructions and therefore 52 Compress® junctions were placed in the 50 patients. When factors such as compression force and spindle size could be attributed to a specific junction, the data were analyzed as 52 individual junctions rather than as 50 patients.
To evaluate the learning curve for the procedure, survival of the first 10 cases at each institution (20 total cases) was compared with the remaining 30 cases using Cox regression analysis.
Sample Size Calculation
A post hoc power analysis was performed to assess the likelihood of detecting significant differences with the limited numbers available in this study. Using a standard power estimate of 0.80, we calculated hazard ratios that could be detected. With our sample size of 50 patients, the Cox proportional hazards regression models used to estimate the associations between demographic variables and component removal were only powered to detect hazard ratios between 3 and 4. The analyses of aseptic failure were similarly powered. To have detected hazard ratios of 2 or 1.75, we would have needed approximately 150 to 250 patients.
Results
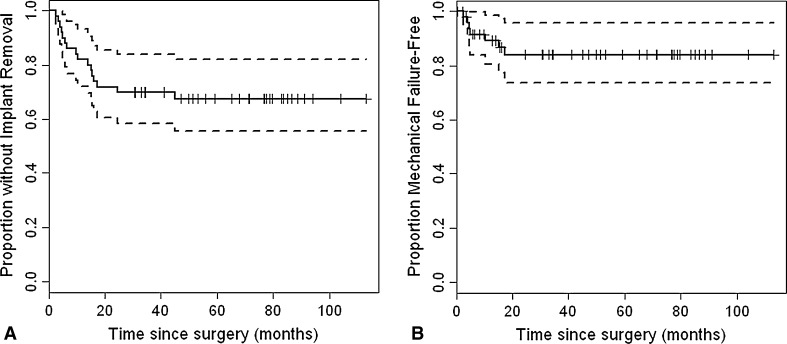
Survivorship of these reconstructions, using component removal for any reason as the end point, was 32% at minimum 24-month followup (Fig. 2). Sixteen patients (32%) had removal of components for reasons including aseptic failure (seven), infection (six), dislocation (two), and fracture distant to the fixation site (one). Average time to component removal was 12.4 months. Aseptic failure occurred in seven (14%) patients with six failures of osseointegration and one malrotation of components. Average time to revision for aseptic failure was 8.3 months with a median of 5 months.
Fig. 2A–B.
(A) Kaplan-Meier survival curves for all-cause failure is shown. (B) This is Kaplan-Meier survival curves for aseptic failure.
With the numbers available, no patient demographic variables were associated with failure. A total of 44% of males had component removal for any reason compared with 20% for females (p = 0.13), and aseptic failure percentages were 20% and 8%, for men and women, respectively (p = 0.42). The rates of component removal and aseptic failure for non-osteosarcoma patients were 45%, whereas patients with osteosarcoma underwent component removal after 28% of the procedures (p = 0.47); aseptic failure rates, likewise, were no different (Table 1). Distal femoral replacement and other types of reconstruction had roughly equivalent rates of all-cause failure. Neoadjuvant and adjuvant chemotherapy was given in 84% and 90% of patients, respectively (Table 2). Chemotherapy did not appear to negatively impact aseptic failure rates because two of seven aseptic failures occurred in patients not receiving neoadjuvant chemotherapy. One of these two failure cases received no chemotherapy. Only six patients (12%) received radiation therapy. There were no aseptic failures among these six patients. At latest followup, six of 50 (12%) patients had died of their oncologic disease. One of the patients who died was revised for infection and another was revised for aseptic failure. The others had well-functioning implants at the time of death. Cox regression analysis showed no association between implant survival rates and demographic or oncological factors.
Table 1.
Demographic and diagnosis-related factors
| Patient factor | All | Any revision | Mechanical failure |
|---|---|---|---|
| Total number of patients | 50 | 16 (32) | 7 (14) |
| Mean age (years) | 20.5 | 24.8 | 23.1 |
| p = 0.18 | p = 0.53 | ||
| Median age (years) | 16 | 17.5 | 18 |
| Body mass index (kg/m2) | 20.8 | 21.8 | 17.2 |
| p = 0.60 | p = 0.38 | ||
| Right side | 25 | 10 (40) | 4 (16) |
| p = 0.11 | p = 0.58 | ||
| Male sex | 25 | 11 (44) | 5 (20) |
| p = 0.14 | p = 0.29 | ||
| Site other than distal femur | 13 | 3 (23) | 1 (8) |
| p = 0.96 | p = 0.53 | ||
| Diagnosis other than osteosarcoma | 11 | 5 (45) | 2 (18) |
| p = 0.33 | p = 0.65 |
Percentages are listed in parentheses.
Table 2.
Oncologic factors
| Factor | Total | All removed | Mechanical failure |
|---|---|---|---|
| Number of patients | 50 | 16 (32) | 7 (14) |
| No evidence of disease | 44 | 14 (32) | 6 (14) |
| Died of disease | 6 | 2 (33) | 1 (17) |
| Preoperative chemotherapy | 42 | 11 (26) | 5 (12) |
| Postoperative chemotherapy | 45 | 14 (31) | 6 (13) |
| p = 0.39 | p = 0.72 | ||
| Radiation therapy | 6 | 4 (67) | 0 (0) |
| p = 0.39 | p = 0.30 |
Percentages are listed in parentheses.
With the numbers available, no prosthesis-related or surgical variables were associated with failure. The average bone resection length for the entire cohort was 17.8 cm, and the average resection length of patients requiring revision for aseptic failure was 15.9 cm (p = 0.43). Spindle size was small in 42%, large in 26%, and custom in 32% of the cohort. Aseptic failure occurred in 24% of the small spindles, 13% of the custom spindles, and none of the large spindles (p = 0.17). Antirotation pins were used in 30% of the cases. Patients in whom antirotation pins were used had an aseptic failure rate of 6.7% compared with 17.1% in reconstructions in which antirotation pins were not used; with the numbers available, this difference was not significant (p = 0.33). Six hundred pounds of compression force was used in 70% of cases, whereas 400 pounds and 800 pounds were used in 18% and 12%, respectively. The amount of compression force used was not significantly associated with failure (Table 3). Cox regression analysis showed no association between survival rates and surgical factors.
Table 3.
Surgical factors
| Factor | Total | All removed | Mechanical failure |
|---|---|---|---|
| Number of patients | 50 | 16 (32) | 7 (14) |
| Resection length | 17.8 | 16.6 | 15.9 |
| p = 0.17 | p = 0.30 | ||
| Derotation pins | 15 | 5 (33) | 1 (7) |
| No derotation pins | 35 | 11 (31) | 6 (17) |
| p = 0.79 | p = 0.40 | ||
| Early cases (first 10 at each center) | 20 | 6 (30) | 3 (15) |
| Later cases | 30 | 10 (33) | 4 (13) |
| p = 0.71 | p = 0.99 | ||
| Small spindle | 21 | 7 (33) | 5 (24) |
| Custom spindle | 18 | 6 (33) | 2 (11) |
| Large spindle | 13 | 3 (23) | 0 (0) |
| p = 0.42 | p = 0.99 | ||
| 400 PSI | 11 | 3 (27) | 1 (9) |
| 600 PSI | 35 | 12 (34) | 5 (14) |
| 800 PSI | 6 | 1 (17) | 1 (17) |
| p = 0.51 | p = 0.83 |
Percentages are listed in parentheses.
No learning curve effect was detected with the numbers available. Failure rates were roughly equivalent with a 15% aseptic failure rate among the early cases and a 13% aseptic failure rate among the later cases. Survival analysis of the first 10 patients at each institution and the final 30 patients showed no statistical difference in component removal rates (p = 0.61) or in aseptic failure rates (p = 0.96).
Discussion
Aseptic failure of massive endoprostheses used for oncologic reconstruction remains a significant and difficult problem. Compressive osseointegration was developed to potentially improve on the aseptic failure rates of traditional fixation methods. It also permits fixation to short diaphyseal segments not amenable to standard stem fixation. We studied this technique and found a 14% aseptic failure rate at a minimum followup of 2 years. No demographic, surgical, or oncologic predictors of failure were identified, and no learning curve effects were detected, although our small study sample was powered to detect only large differences (hazard ratios between 3 and 4).
Limitations of the present study include sample size, retrospective review, lack of functional outcomes data, and lack of a comparison cohort. Although limited to 50 patients, this study is the second largest oncologic series of compressive osseointegration cases of which we are aware (Table 4). Retrospective review introduces the potential for selection bias. Indications for use of compressive osseointegration were not uniform among the four participating surgeons or longitudinally throughout the series. We believe this lack of uniformity in indications is common in the adoption of new surgical technologies. Ideal candidates were sought for the earlier cases, and the indications were subsequently broadened as familiarity and experience with the device increased. This may be the reason that no learning curve was identified by our review. Despite its retrospective design, the series is consecutive and had minimal loss to followup. Functional outcome data were not uniformly collected between centers and therefore accurate analysis of these variables was not possible. Although such information will be important in future studies, our intent here was to analyze osseointegration rather than function. Another potential weakness is the inclusion of cases using the implant in different body sites. The four sites studied (proximal humerus, proximal femur, distal femur, and proximal tibia) have different biomechanics, which may potentiate different failure mechanisms. An advantage of presenting all sites in a consecutive series is that it reflects the spectrum of disease and modes of endoprosthesis use typically encountered in orthopaedic oncology practice. Additionally, the different long bones probably have similar biology at the osseointegration site.
Table 4.
Prior published series reporting compressive osseointegration
| First author | Date | Institution | Number of patients | Mean followup (months) | Followup Range (months) | Mean Age (years) | Mean body mass index (kg/m2) | Percent distal femur | Resect length | Compression 400/600/800 | Percent removed | Percent aseptic failure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avedian [1], chemotherapy | 2007 | UCSF | 30 | 16.6 | 3–24 | 19.5 | 14.8 | 100 | 18.8 | N/R | N/R | 10 |
| Avedian [1], nonchemotherapy | 2007 | UCSF | 24 | 16.6 | 3–24 | 30.2 | 18.8 | 100 | 20 | N/R | N/R | 0 |
| O’Donnell [19] | 2009 | UCSF | 16 | 54 | 24–124 | 18 | N/R | 0 | 17 | 5/10/1 | 12.5 | 6.3 |
| Farfalli [6] | 2009 | MSKCC | 41 | 45 | 3–97 | 27 | N/R | 100 | N/R | 9/21/11 | N/R | 12.2 |
| Bhangu [2], Pedtke [21] | 2006, 2012 | UCSF | 26 | 73 | 13–110 | 24.9 | N/R | 100 | N/R | N/R | 19.2 | 3.8 |
| Healey [12] | 2013 | MSKCC | 82 | 48 | 6–131 | 20.4 | N/R | 100 | N/R | N/R | N/R | 9.9 |
| Current study | 2012 | Current | 50 | 68 | 24–113 | 20.5 | 21 | 76 | 17.8 | 9/35/6 | 32 | 14 |
UCSF = University of California at San Francisco; MSKCC = Memorial Sloan Kettering Cancer Center; N/R = not reported.
A recent multicenter review of 2174 endoprostheses implanted between 1974 and 2008 identified a 25% rate of implant removal for any reason with a mean time to failure of 47 months. A 12% rate of aseptic failure was reported. The mean time to aseptic failure varied by mechanism: soft tissue (64 months), aseptic loosening (102 months), and structural failure (93 months) [13]. Cemented fixation was most commonly used, and the time to failure resulting from aseptic loosening averaged 88 months, which was the longest duration of any of the five failure modes studied. In contrast, the present study identified early aseptic failure resulting from lack of osseointegration as the major mode of implant-related failure. These failures occurred at an average of 8.3 months with only two failures occurring after 1 year. The largest series of 81 compressive osseointegration cases by Healey et al. [12] reported a similar trend with only one aseptic failure occurring at greater than 5 years. The comparatively small fixation surface of the device we used may predispose patients to early aseptic failure before osseointegration. This is likely to be especially significant in patients with poor bone quality or high early activity levels. Although bone quality can be assessed radiographically and intraoperatively, patient compliance with postoperative weightbearing restrictions is more difficult to evaluate and quantify. Despite this risk of early failure, avoidance of stress shielding and biologic osseointegration may mitigate the risks of longer-term aseptic loosening seen with cemented and traditional noncemented implants. Our 14% rate of revision for aseptic failure at a minimum of 2 years was similar to other published reports (Table 4). The previously mentioned study of 81 distal femoral compressive osseointegration cases found a 9.9% aseptic failure rate at mean of 48 months [12] Another reported a 10% aseptic failure rate among 30 oncologic cases and no failures among 24 nononcologic cases at an average 16.6-month followup [1]. The overall early aseptic failure rate from that study of 54 consecutive cases was 5.6%. A series of 16 consecutive proximal tibia compressive osseointegration cases reported a 6.3% aseptic failure rate at 54-month average followup [19]. Because this is a relatively new technique, there are no long-term series using these implants.
Others have demonstrated that chemotherapy has an adverse effect on compression-induced bone remodeling and a trend toward decreased prosthetic survival with use of the compressive osseointegration technique [1]. The current study could not confirm or refute this finding because 90% of the patients in the series received neoadjuvant chemotherapy, adjuvant chemotherapy, or both. Notably, two aseptic failures occurred in patients who did not receive neoadjuvant chemotherapy. Radiation therapy may also adversely influence bone remodeling and in-growth. Only six patients in this series received radiation, and none of these had aseptic failure.
Resection length has been shown to be a risk factor for aseptic failure in prior studies of stemmed implants. Kawai et al. found resection of greater than 40% of the femur to be an independent risk factor for failure of distal femoral replacements [14]. A study by Unwin et al. also found a higher aseptic failure rate among patients with greater than 40% of the femur resected during distal femoral replacement [25]. A more recent study by Guo et al. found resection length of greater than 14 cm independently predicted failure of cemented endoprostheses about the knee [11]. With the numbers available, we did not observe a difference in failure risk associated with resection length. In theory, the compressive osseointegration technique is insensitive to resection length because fixation occurs at the cut bone surface and not in the residual canal. The present series supports this theoretical advantage. The present series similarly failed to identify a correlation between compression force and failure. The influence of compression force on aseptic failure has been reported in only one prior study. Farfalli et al. [6] found no correlation between aseptic loosening and applied compression force in their series of 41 patients. The authors of this study concur with the Farfalli et al. study that the force applied should be tailored to the quality of each individual patient’s bone and in keeping with the manufacturer’s described technique. In this series, none of the 11 implants with large spindles failed. Prior studies have not analyzed this factor with respect to implant survival. Whether this relates to the spindle size itself or to the size and quality of the bones to which these larger spindles were compressed cannot be determined with the available data. Finally, antirotation pins were used in 15 of 50 cases in this series. Only one of 15 (6.7%) implants with antirotation pins failed aseptically. Use of these pins has not been analyzed in prior reports of implant survivorship. However, fracture through a pin site was reported in one case in a series using this implant [24]; thus, antirotation pin use entails risks as well as benefits.
Learning curve may influence the success of any new implant or technique. Henderson et al. proposed that institutional volume and experience with endoprostheses may have contributed to the relatively low aseptic failure rate of their series of 2174 cases [13]. O’Donnell analyzed FDA investigational device data and reported that early failure of the Compress® device was more common among surgeons who had performed fewer than five cases [18]. The current dual-center study failed to identify a learning curve for this implant. The failure rate among the first 10 cases at each participating institution did not differ significantly from the succeeding cases. This discrepancy may result from the fact that the centers in the present study are high volume and had extensive prior experience with endoprosthetic replacement.
Compressive osseointegration has been compared with both cemented [2, 21] and noncemented fixation [6] in prior studies. Bhangu et al. [2] reported early equivalence of a matched cohort study of 26 Compress® cases compared with 26 cemented Stanmore® (Stanmore Implants, Middlesex, UK) cases. Pedtke et al. [21] recently reported intermediate-term followup of these cohorts. Five-year implant survival with aseptic loosening as the end point was 83.5% in the Compress® group compared with 66.6% in the Stanmore® groups. Although a distinct trend was noted with three failures in the cementation group and one failure in the compressive osseointegration group, the difference was not statistically significant. Notably, the cemented implants in the comparison group are a modern design with some of the best cemented results reported in the literature [17]; therefore, this may be a best case comparison for the cemented group. The previously mentioned study by Farfalli et al. [6] retrospectively compared cohorts of Compress® and traditional noncemented stems using the same hinge knee component. They found no difference in survival at 5 years between the two groups (Table 4). However, the authors did find the compressive osseointegration failures occurred earlier (usually less than 1 year), whereas the traditional noncemented stem failures continued to occur throughout the followup period.
In conclusion, we identified a 14% aseptic failure rate in a consecutive series of compressive osseointegration cases followed for a minimum of 2 years. Consistent with prior reports, the aseptic failures in this series occurred early. Although the early reliance on compression rather than friction combined with the comparatively small contact area between implant and bone likely potentiates these early failures, the subsequent bone hypertrophy and avoidance of stress shielding inherent to compressive osseointegration may prevent late failures resulting from aseptic loosening. This technology is especially advantageous for endoprosthetic reconstruction when minimal residual bone is available for osseous purchase. The short deployment length of this device permits fixation in some cases where standard stem fixation is not possible. Improved patient selection, meticulous surgical technique, and careful progression of weightbearing postoperatively offer the possibility of decreasing early failures. Based on these data, we advocate the continued study of compressive osseointegration for the fixation of endoprostheses. Determining any long-term advantages and disadvantages of this technique over other contemporary fixation methods requires further followup.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed at the University of Utah, Salt Lake City, UT, USA, and Indiana University, Indianapolis, IN, USA.
References
- 1.Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clini Orthop Relat Res. 2007;459:48–53. doi: 10.1097/BLO.0b013e3180514c66. [DOI] [PubMed] [Google Scholar]
- 2.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress implant. Int Orthop. 2006;30:465–472. doi: 10.1007/s00264-006-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23:707–711. doi: 10.3928/0147-7447-20000701-18. [DOI] [PubMed] [Google Scholar]
- 4.Cristofolini L, Bini S, Toni A. In vitro testing of a novel limb salvage prosthesis for the distal femur. Clin Biomech (Bristol, Avon). 1998;13:608–615. doi: 10.1016/S0268-0033(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 5.Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466:459–465. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792–2799. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert NF, Yasko AW, Oates SD, Lewis VO, Cannon CP, Lin PP. Allograft-prosthetic composite reconstruction of the proximal part of the tibia. An analysis of the early results. J Bone Joint Surg Am. 2009;91:1646–1656. doi: 10.2106/JBJS.G.01542. [DOI] [PubMed] [Google Scholar]
- 9.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 10.Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79. doi: 10.1097/01.blo.0000180050.27961.8a. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Ji T, Yang R, Tang X, Yang Y. Endoprosthetic replacement for primary tumours around the knee: experience from Peking University. J Bone Joint Surg Br. 2008;90:1084–1089. doi: 10.1302/0301-620X.90B8.20240. [DOI] [PubMed] [Google Scholar]
- 12.Healey JH, Morris CD, Athanasian EA, Boland PJ. Compress® knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Relat Res. 2013;471:774–783. doi: 10.1007/s11999-012-2635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 14.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.1302/0301-620X.80B4.8216. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32:567–571. doi: 10.1007/s00264-007-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muscolo DL, Ayerza MA, Farfalli G, Aponte-Tinao LA. Proximal tibia osteoarticular allografts in tumor limb salvage surgery. Clin Orthop Relat Res. 2010;468:1396–1404. doi: 10.1007/s11999-009-1186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell RJ. Compressive osseointegration of modular endoprostheses. Curr Opin Orthop. 2007;18:590–603. doi: 10.1097/BCO.0b013e3282f0dafc. [DOI] [Google Scholar]
- 19.O’Donnell RJ. Compressive osseointegration of tibial implants in primary cancer reconstruction. Clin Orthop Relat Res. 2009;467:2807–2812. doi: 10.1007/s11999-009-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogilvie CM, Crawford EA, Hosalkar HS, King JJ, Lackman RD. Long-term results for limb salvage with osteoarticular allograft reconstruction. Clin Orthop Relat Res. 2009;467:2685–2690. doi: 10.1007/s11999-009-0726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedtke AC, Wustrack RL, Fang AS, Grimer RJ, O’Donnell RJ. Aseptic failure: how does the Compress® implant compare to cemented stems? Clin Orthop Relat Res. 2012;470:735–742. doi: 10.1007/s11999-011-2159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyler WK, Healey JH, Morris CD, Boland PJ, O’Donnell RJ. Compress periprosthetic fractures: interface stability and ease of revision. Clin Orthop Relat Res. 2009;467:2800–2806. doi: 10.1007/s11999-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 26.Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–250. doi: 10.1097/00003086-200403000-00034. [DOI] [PubMed] [Google Scholar]
- 27.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. doi: 10.1097/00003086-199601000-00026. [DOI] [PubMed] [Google Scholar]