Abstract
Background
As obesity becomes more prevalent, it becomes more common among patients considering orthopaedic surgery, including spinal surgery. However, there is some controversy regarding whether obesity is associated with complications, failed reconstructions, or reoperations after spinal surgery.
Questions/purposes
We wished to determine, in patients undergoing spine surgery, whether obesity is associated with (1) surgical site infection, (2) mortality and the need for revision surgery after spinal surgery, and (3) increased surgical time and blood loss.
Methods
A systematic literature search was performed to collect comparative or controlled studies that evaluated the influence of obesity on the surgical and postoperative outcomes of spinal surgery. Two reviewers independently selected trials, extracted data, and assessed the methodologic quality and quality of evidence. Pooled odds ratios (OR) and mean differences (MD) with 95% CIs were calculated using the fixed-effects model or random-effects model. Data were analyzed using RevMan 5.1. MOOSE criteria were used to ensure this project’s validity. Thirty-two studies involving 97,326 patients eventually were included.
Results
Surgical site infection (OR, 2.33; 95% CI, 1.94–2.79), venous thromboembolism (OR, 3.15; 95% CI, 1.92–5.17), mortality (OR, 2.6; 95% CI, 1.50–4.49), revision rate (OR, 1.43; 95% CI, 1.05–1.93) operating time (OR, 14.55; 95% CI, 10.03–19.07), and blood loss (MD, 28.89; 95% CI, 14.20–43.58), were all significantly increased in the obese group.
Conclusion
Obesity seemed to be associated with higher risk of surgical site infection and venous thromboembolism, more blood loss, and longer surgical time. Future prospective studies are needed to confirm the relationship between obesity and the outcome of spinal surgery.
Introduction
Obesity has become a global epidemic that is increasing in prevalence in adults and children. A BMI greater than 30 kg/m2 generally is categorized as obese. In 2008, the global prevalence of obesity was 9.8% in men and 13.0% in women, which is nearly twice the 1980 prevalence rates [7]. Obesity is a known risk factor for many chronic conditions including cardiovascular disease, diabetes, stroke, some forms of cancer, and musculoskeletal disorders such as knee osteoarthritis and low back pain [12, 18, 36].
More specifically, patients who are obese may have difficulties with surgical access and there have been reports of an increased risk of operative complications for surgical procedures such as spinal surgery [4, 6, 8, 17, 22, 23, 29, 35]. Some studies reported that obesity has been associated with unfavorable surgical outcomes such as longer operative times, greater operative blood loss, and a higher rate of revision for patients having spinal surgery [10, 14, 31, 32, 35, 39]. However, other studies did not find significant differences regarding surgical outcome and complications between patients who were obese or not obese [2, 5, 9, 26]. Djurasovic et al. [5] retrospectively reviewed clinical data of a total of 270 patients undergoing lumbar fusion and found that obese patients achieve similar benefits as nonobese patients. Gepstein et al. [9] also investigated the effect of obesity on patients undergoing lumbar decompressive spinal surgery and suggested that it was reasonable to operate on obese patients with appropriate indications. Consensus regarding the effect of obesity on spinal surgery appears to be lacking.
Accordingly, we sought to perform a meta-analysis to determine whether obesity is associated with (1) surgical site infection and venous thromboembolism, (2) mortality and the need for revision surgery after spinal surgery, and/or (3) increased surgical time and blood loss.
Materials and Methods
We did this meta-analysis in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [38] guidelines.
Search Strategy
The databases of PubMed, EMBASE, the Cochrane Library, Web of Science, and Chinese Biomedical Database were searched without language restrictions for reports published between 1970 and November 2012. The search terms were (obes* OR adiposity OR body mass index OR BMI) AND (cervical OR thoracic OR lumbar OR sacral OR spine surgery OR spinal surgery). Furthermore, the reference lists of review articles regarding this topic and included trials were checked manually to identify additional relevant citations. Two investigators (JJ, YT) independently reviewed the literature to identify relevant articles for full-text review. Disagreements regarding the search were resolved by discussion with a third author (YX).
Inclusion Criteria and Study Selection
Studies were included if they were controlled or comparative studies that focused on the influence of obesity on complication rate and outcome of spinal surgery. The outcomes we evaluated were operating time, postoperative blood loss, pain score, postoperative mortality, revision rate, length of hospital stay, postoperative complications including surgical site infection, wound complication, and venous thromboembolism. Studies involving at least one outcome were included; review articles, expert opinions, and trials without reporting the outcome measures of interest were excluded. Two authors (ZF, SK) independently reviewed all excluded citations and disagreements were resolved by a third reviewer (YX).
Data Abstraction and Quality Assessment
The data from the included studies were extracted using an Excel database (Microsoft Inc, Redmond, WA, USA) by two authors (JJ, YT) The available data from the selected studies were: country, study design, patient characteristics (age, sex, and other baseline characteristics), spinal level, and outcomes. Any disagreements in abstracted data were resolved by a third reviewer (YX).
Two authors (ZF, SK) independently assessed the methodologic quality of identified studies according to the Newcastle-Ottawa Scale [41] for observational studies. The Newcastle-Ottawa Scale assesses population selection, comparability of exposed (obese) and unexposed (nonobese), and adequacy of outcome assessment (including outcome ascertainment and attrition). Discrepancy or uncertainty was discussed with another author (YX).
The quality of the evidence for the overall outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [3]. The GRADE approach considers the results from the observational studies as low-quality evidence; however, there are criteria for rating up the quality level: a large effect (at least a twofold increase or reduction in risk), a dose-response gradient, and if all plausible confounding would decrease an apparent treatment effect or, in case of no effect, would create a spurious effect. The evidence quality for each outcome was generally low (Table 1).
Table 1.
Results of the meta-analysis
| Outcomes | Number of studies [references] | Number of patients | MD or OR (95% CI) | Heterogeneity | GRADE evidence | |
|---|---|---|---|---|---|---|
| Obese | Nonobese | |||||
| Surgical site infection | 24 [2, 5, 6, 8–10, 13–15, 17, 19, 20, 23, 24, 27, 28, 30–35, 39, 40] | 3493 | 89,690 | 2.33 (1.94, 2.79) | I2 = 49%, p = 0.007 | Moderate |
| Venous thromboembolism | 6 [2, 13, 15, 25, 27, 35] | 1643 | 83,442 | 3.15 (1.92, 5.17) | I2 = 0%, p = 0.63 | Moderate |
| Mortality | 6 [2, 9, 13, 15, 31, 32] | 2172 | 84,604 | 2.6 (1.50, 4.49) | I2 = 0%, p = 0.90 | Moderate |
| Revision | 9 [1, 2, 5, 21, 31–33, 39, 40] | 1039 | 1815 | 1.43 (1.05, 1.93) | I2 = 28%, p = 0.19 | Low |
| Operating time | 8 [10, 14, 31, 32, 34, 35, 39, 40] | 786 | 1658 | 14.55 (10.03, 19.07) | I2 = 44%, p = 0.09 | High |
| Blood loss | 7 [10, 14, 27, 31, 32, 35, 40] | 748 | 1548 | 28.89 (14.20, 43.58) | I2 = 38%, p = 0.14 | Moderate |
GRADE Working Group grades of evidence: High quality = further research is very unlikely to change our confidence in the estimate of effect; Moderate quality = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality = very uncertain about estimate.
Search Results
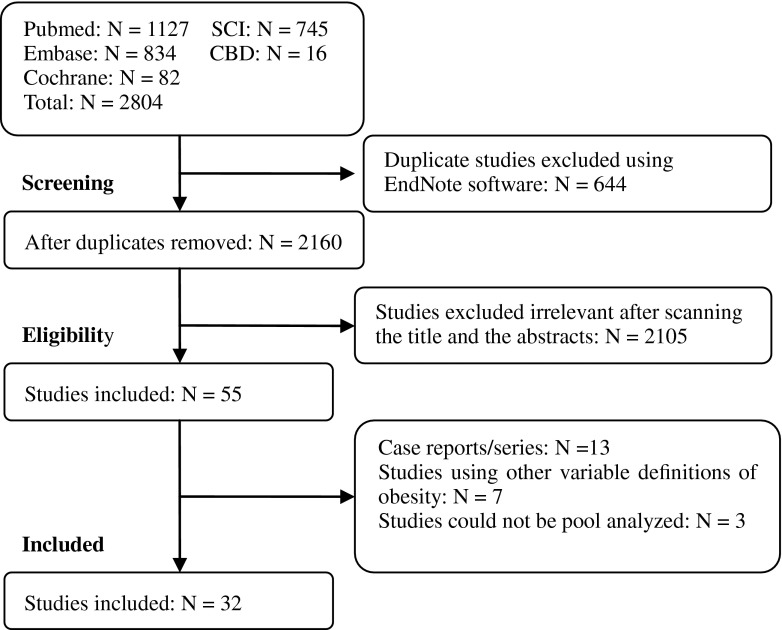
There were 2804 initial studies found after a comprehensive search. A total of 644 duplicates and 2105 citations were excluded based on screening the titles and abstracts, leaving 55 potentially relevant studies. After screening full texts, 23 citations were not in accordance with the inclusion criteria of our study and were excluded. Finally, 32 studies [1, 2, 5, 6, 8–11, 13–17, 19–28, 30–35, 39, 40, 43] involving 97,326 patients were included in our study (Figs. 1, 2).
Fig. 1.
The flow chart shows the article selection process we performed for this meta-analysis. SCI = Web of Science; CBD = Chinese Biomedical Database.
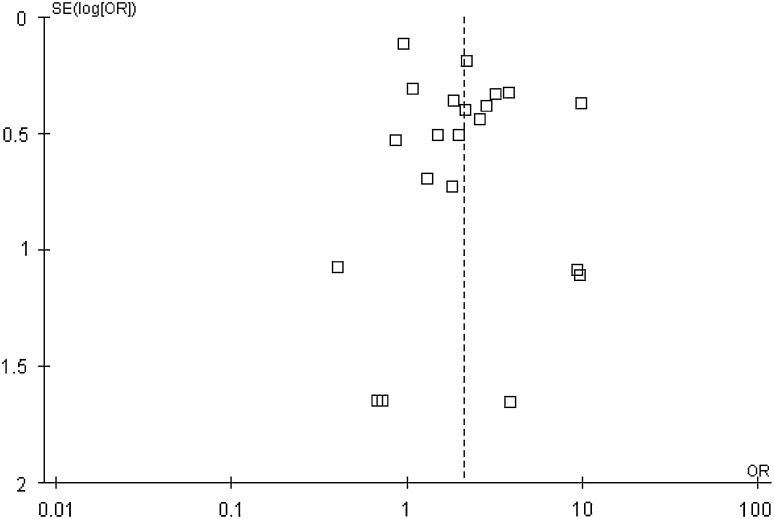
Fig. 2.
The funnel plot of the surgical site infection.
Characteristics of Included Studies
The articles were published between 1987 and 2013. Of all the included studies, eight [2, 8, 9, 13, 16, 27, 34, 43] were prospective and 24 were retrospective studies. All but five studies were conducted in the United States; two were from China [10, 11] and one each was from Israel [9], Sweden [16], and Austria [35]. All the studies reported the level of the spine in question: the cervical spine was involved in eight, the thoracic spine in 13, the lumbar spine in all included studies, and the sacral spine in five (Table 2).
Table 2.
Characteristics of included studies
| Study | Location | Years | Study design | Diagnosis | Spinal levels | Surgery | Number of patients | Mean age (years) | NOS score | Level of evidence | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obese | Nonobese | Obese | Nonobese | Combined | |||||||||
| Ain et al. [1] | USA | 1970–2003 | Retrospective | SS | L | Laminectomy | 32 | 17 | 39 | 8 | II | ||
| Andreshak et al. [2] | USA | NR | Prospective | SS, LDH | L | Laminectomy, discectomy, fusion | 55 | 95 | 47.9 | 46.8 | 5 | I | |
| Djurasovic et al. [5] | USA | 2001–2005 | Retrospective | SS, LDH | L | Fusion | 109 | 161 | 56.2 | 57.1 | 8 | II | |
| Fang et al. [6] | USA | 1991–1997 | Retrospective | SS, AIS | C, T, L | Laminectomy, discectomy, fusion | 47 | 92 | 42.5 | 5 | III | ||
| Friedman et al. [8] | USA | 1991–2001 | Prospective | DD | C, T, L | Laminectomy | 40 | 83 | 51.6 | 5 | III | ||
| Gepstein et al. [9] | Israel | 1990–2000 | Prospective | LDH | L | Laminectomy, discectomy | 67 | 231 | 69.6 | 71.9 | 8 | I | |
| Gu et al. [10] | China | 2010–2011 | Retrospective | SS | L | Fusion | 7 | 50 | 57 | 60.3 | 8 | II | |
| Gu et al. [11] | China | 1997–2000 | Retrospective | LDH | L | Discectomy | 80 | 368 | 45.4 | 7 | II | ||
| Hanigan et al. [13] | USA | 1984–1984 | Prospective | LDH | L | Discectomy | 17 | 88 | 42.7 | 40.4 | 8 | I | |
| Hardesty et al. [14] | USA | 1998–2010 | Retrospective | AIS | T, L | Fusion, segmental instrumentation | 55 | 181 | 14 | 14.5 | 8 | II | |
| Kalanithi et al.[15] | USA | 2003–2007 | Retrospective | DD | C, L | Fusion | 1455 | 83,152 | NR | NR | 8 | III | |
| Knutsson et al. [16] | Sweden | 2006–2008 | Prospective | SS | L | Fusion | 606 | 2027 | 67 | 69.4 | 8 | II | |
| Koutsoumbelis et al. [17] | USA | 2000–2006 | Retrospective | NR | L | Posterior fusion | 48 | 204 | 58.3 | 5 | III | ||
| Maragakis et al. [19] | USA | 2001–2004 | Retrospective | NR | L, S | Laminectomy, fusion | 64 | 144 | 55 | 5 | III | ||
| Mehta et al. [20] | USA | 2006–2008 | Retrospective | NR | L | Fusion | 122 | 176 | 59.7 | 5 | II | ||
| Meredith et al. [21] | USA | 2005–2008 | Retrospective | LDH | L | Microdiscectomy | 25 | 75 | 46 | 6 | II | ||
| Olsen et al. [22] | USA | 1996–1999 | Retrospective | NR | C, T, L, S | Laminectomy, fusion | 66 | 151 | 53.1 | 5 | III | ||
| Olsen et al. [23] | USA | 1998–2002 | Retrospective | DD | C, T, L, S | Laminectomy, discectomy, fusion | 92 | 181 | 52.4 | 5 | III | ||
| O’Neill et al. [24] | USA | 1991–2001 | Retrospective | AIS | T, L | Boston or custom-molded orthosis | 31 | 245 | 22.8 | 6 | II | ||
| Park et al. [25] | USA | 2006–2007 | Retrospective | SS | L | Laminectomy, discectomy, fusion | 56 | 21 | 54.1 | 43.7 | 7 | II | |
| Patel et al. [26] | USA | 2002–2005 | Retrospective | DD | T, L | Fusion | 39 | 50 | 58.8 | 6 | II | ||
| Peng et al. [27] | USA | 2007–2008 | Prospective | SS, LDH | L | Fusion | 33 | 41 | 47.3 | 44.7 | 8 | III | |
| Pull ter Gunne & Cohen [28] | USA | 1996–2005 | Retrospective | DD | C, T, L, S | Laminectomy, discectomy, fusion | 235 | 2939 | 55.6 | 5 | II | ||
| Rao et al. [30] | USA | Jan. 2008– Dec 2008 | Retrospective | DD, trauma | C, T, L | Fusion | 101 | 137 | 56 | 5 | III | ||
| Rihn et al. [31] | USA | 2000–2005 | Retrospective | DS, LSS | L | Laminectomy | 328 | 482 | 62.9 | 66.8 | 8 | II | |
| Rihn et al. [32] | USA | 2000–2005 | Retrospective | LDH | L | Discectomy | 336 | 854 | NR | NR | 8 | II | |
| Rodgers et al. [33] | USA | 2006–2008 | Retrospective | DD | T, L | Extreme lateral interbody fusion | 156 | 157 | 58.9 | 62.9 | 8 | II | |
| Rosen et al. [34] | USA | 2002–2006 | Prospective | DD | L | Fusion | 35 | 72 | 56 | 8 | I | ||
| Senker et al. [35] | Austria | 2010–2011 | Retrospective | DD | L | Fusion, laminotomy | 27 | 45 | 61.8 | 8 | II | ||
| Tomasino et al. [39] | USA | 2004–2007 | Retrospective | SS, LDH | L | Laminectomy, discectomy | 36 | 79 | 47.4 | 51.3 | 8 | II | |
| Upasani et al. [40] | USA | 1995–2004 | Retrospective | AIS | C, T, L, S | Fusion, thoracotomy | 48 | 193 | 14.3 | 8 | II | ||
| Yadla et al. [43] | USA | May 2008– Dec 2008 | Prospective | DD, trauma | T, L | Laminectomy, discectomy, fusion | 45 | 42 | 52.8 | 6 | I | ||
NOS = Newcastle-Ottawa scale; C = cervical; T: thoracic; L = lumbar; S: = sacral; DD = degenerative disease; SS = Spinal stenosis; DS = degenerative spondylolisthesis; LDH = lumbar disc herniation; AIS = adolescent idiopathic scoliosis; NR = not reported.
Quality of Included Studies
According to the Newcastle-Ottawa Scale for observational studies (Table 2), the overall quality was considered variable and the studies have some common problems. Only eight studies used a prospective design. Some studies used samples that could not represent the injured population, and some studies had a sample size smaller than 100 in some groups.
Data Analysis
If there were continuous variables, the weighted mean difference (MD) was recommended to assess the treatment, with 95% CI. For dichotomous outcomes, results were expressed as odds ratio (OR). Data were pooled using the fixed-effect model, but the random-effects model also was considered in the event that I2 index was less than 50%. Heterogeneity between trials was assessed by the I2 index, which measures the percentage of the variability in effect estimates that is attributable to heterogeneity. Subgroup analysis was conducted to investigate possible reasons for heterogeneity. A funnel plot based on the primary outcome was used to evaluate publication bias. The analysis for publication bias using the surgical site infection end point showed that there was little evidence for publication bias among the included studies.
Results
Twenty-three studies [2, 5, 6, 8–10, 13–15, 17, 19, 20, 22, 23, 27, 28, 30–35, 39, 40] including 8576 patients reported the incidence of surgical site infection. The pooled analysis showed that obesity was associated with higher rate of surgical site infection (relative risk [OR] = 1.87, 95% CI [1.53, 2.29]). A separate analysis of the Levels I and II studies also showed that a higher rate of surgical site infections was found in the obese group (MD = 1.83, 95% CI [1.38, 2.43]) and the result of Level III studies was consistent with those of Levels I and II studies (MD = 1.91, 95% CI [1.45, 2.52]). Six studies including 85,085 patients reported the risk of venous thromboembolism. The pooled analysis showed that obesity was associated with higher risk of venous thromboembolism (OR = 3.15, 95% CI [1.92, 5.17]). There was no heterogeneity among the studies with an I2 of 0%.
Five Level I or II studies [2, 9, 13, 31, 32] involving 2169 patients examined the risk of mortality in relation to obesity. The pooled analysis of data suggested that there was no significant difference in mortality between obese and nonobese groups (OR = 2.06, 95% CI [0.52, 8.09]). These studies had no heterogeneity, with an I2 of 0%.
Nine Level I or II studies [1, 2, 5, 21, 31–33, 39, 40] including 2854 patients reported the rate of revision for spinal surgery. The pooled analysis revealed that obesity was associated with high rate of revision (OR = 1.43, 95% CI [1.05, 1.93]).
Eight Level I or II studies [10, 14, 31, 32, 34, 35, 39, 40] including 2444 patients reported the results of operating time. The meta-analysis revealed that obesity was associated with longer operating time (OR = 14.55, 95% CI [10.03, 19.07]). Seven studies [10, 14, 27, 31, 32, 35, 40] involving 2296 patients reported the results of blood loss. The pooled analysis showed that obesity was associated with more blood loss (MD = 28.89, 95% CI [14.20, 43.58]). A separate analysis of the Levels I and II studies also showed that more blood loss was found in obese group (MD = 30.25, 95% CI [14.86, 45.65]), while analysis of Level III studies revealed no significant difference in blood loss between the two groups (MD = 15.00, 95% CI [−34.08, 64.08]).
Discussion
According to the WHO, at least 2.8 million people worldwide die every year because of being overweight or obese, and an estimated 35.8 million (2.3%) of global Disability Adjusted Life Years (DALYs) are caused by overweight [42]. Obesity has become more common among patients considering orthopaedic surgery, including spinal surgery. Results of the association between obesity and the outcome of spinal surgery remains a subject for debate. Our aim in this meta-analysis was to evaluate surgical site infection, mortality, revision surgery, surgical time, and blood loss in obese and nonobese patients undergoing spinal surgery.
The results of our meta-analysis showed that obesity was associated with longer operating time, more postoperative blood loss, higher risk of mortality, and more postoperative complications including surgical site infections and venous thromboembolisms.
Our study has several limitations. First, our meta-analysis incorporates observational studies that are low quality in design, and as such may have been sensitive to selection, detection, and performance biases. By conducting an evaluation for the possible presence of publication bias using a funnel plot on the surgical site infection end point, we observed that the plot was not symmetric, which suggests that publication bias might have affected the literature on this point. If it affected the literature on this point, it is conceivable that it could have affected the literature on our other end points as well. In general, publication bias results in the nonpublication of smaller studies that conclude no difference between groups, and so suggests that the findings with respect to obesity may have been overstated in our evaluation. Second, heterogeneity of included studies was induced by many factors such as different surgical procedures and different spine levels. However, as a result of limited data, we did not conduct subgroup analyses of different surgical procedures and different spine levels. Third, most of the studies were from the United States (n = 27); the results may or may not generalize well to other populations.
Like some previous studies [6, 8, 14, 17, 22, 23, 31–33, 35, 39] in this genre, our study found that obese patients had worse surgical outcomes including longer operating time, more postoperative blood loss, and a higher rate of revision than nonobese patients undergoing spinal surgery. Generally, patients with higher BMIs have a thicker layer of subcutaneous tissue, increasing the need for retraction and duration of surgery, consequently increasing the operating time [20]. The reason for more blood loss is presumably the larger corridor to the spine, which causes more tissue trauma. In addition, prolonged operative time may result in increased blood loss [14, 32, 35]. Length of hospital stay was not a statistical difference between obese and nonobese patients, which is consistent with previous studies [14, 27]. The rate of revision is greater in obese patients than in nonobese patients. Most of the revisions were the result of recurrent disc herniations. In obese patients, normal anatomy and physiology are altered to accommodate excess mass. When excess weight is carried, the spine is forced to sustain increased or altered stress, which may lead to advanced degeneration [43]. Meredith et al. [21] reviewed all cases of patients with a minimum followup of 6 months who had one- or two-level lumbar microdiscectomies from L2 to S1 performed by one surgeon, and reported that obesity was a strong and independent predictor of recurrent herniation of the nucleus pulposus after lumbar microdiscectomy.
Numerous authors have suggested that obesity is associated with a poor prognosis owing to associated comorbidities that might be linked to higher postoperative complication rates [9, 16, 26, 35, 43]. We found a higher risk of mortality and more postoperative complications including surgical site infections, wound complications, and venous thromboembolisms in obese patients than in normal-weight patients. The obese patients have thick subcutaneous adipose layers that form dead space after closure of the surgical wound. Therefore, the necrosis of local fat can result in a localized wound infection [20, 28]. In addition, this larger layer of subcutaneous tissue can lead to a potential dead space after closure, which may increase the risk of a complication such as a surgical site infection or wound complication. Venous thromboembolisms are a challenging problem for orthopaedic surgeons. Stein et al. [37] investigated the potential risk of obesity in patients with venous thromboembolism based on the database of the National Hospital Discharge Survey and indicated that obesity was a risk factor for men and women. Results of our meta-analysis were consistent with their study and showed that obesity was associated with a higher risk of venous thromboembolism.
Some outcomes we evaluated were influenced not only by minimally invasive surgeries or open procedures, but also the different levels of spinal surgery. Future studies need to investigate the influence of obesity, particularly in cervical or thoracic spine surgery. Because most of the studies were from the United States, whether the results of our meta-analyses could be applied to other countries was not clear. As a result, future studies conducted in other countries are needed. Most of the current studies were retrospective, which could introduce bias during data collection. In the future, studies should be conducted and data should be collected prospectively.
Based on the results of our meta-analysis, patients who were obese had a longer operation time, more postoperative blood loss, and higher risk of complications including surgical site infections and venous thromboembolisms than patients who were not obese.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ain MC, Chang TL, Schkrohowsky JG. Laminectomies and achondroplasia: does body mass index influence surgical outcomes? Am J Med Genet A. 2007;143A:1032–1037. doi: 10.1002/ajmg.a.31705. [DOI] [PubMed] [Google Scholar]
- 2.Andreshak TG, An HS, Hall J, Stein B. Lumbar spine surgery in the obese patient. J Spinal Disord. 1997;10:376–379. doi: 10.1097/00002517-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr, Zaza S, GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bederman SS, Rosen CD, Bhatia NN, Kiester PD, Gupta R. Drivers of surgery for the degenerative hip, knee, and spine: a systematic review. Clin Orthop Relat Res. 2012;470:1090–1105. doi: 10.1007/s11999-011-2004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djurasovic M, Bratcher KR, Glassman SD, Dimar JR, Carreon LY. The effect of obesity on clinical outcomes after lumbar fusion. Spine (Phila Pa 1976). 2008;33:1789–1792. doi: 10.1097/BRS.0b013e31817b8f6f. [DOI] [PubMed] [Google Scholar]
- 6.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30:1460–1465. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 7.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007;28:1060–1065. doi: 10.1086/519864. [DOI] [PubMed] [Google Scholar]
- 9.Gepstein R, Shabat S, Arinzon ZH, Berner Y, Catz A, Folman Y. Does obesity affect the results of lumbar decompressive spinal surgery in the elderly? Clin Orthop Relat Res. 2004;426:138–144. doi: 10.1097/01.blo.0000141901.23322.98. [DOI] [PubMed] [Google Scholar]
- 10.ZH GuGF, He SS, Ding Y, Jia JB, Zhou X. The effect of body mass index on the outcome of minimally invasive surgery for lumbar spinal stenosis complicated with lumbar instability. Chin J Spine Spinal Cord. 2012;22:313–317. [Google Scholar]
- 11.WH GuHL, Liu L, Zhou FH, Duan JZ. Correlation between surgical outcomes and body mass index in patients with lumbar disc herniation. Chin J Orthop. 2003;23:523–526. [Google Scholar]
- 12.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanigan WC, Elwood PW, Henderson JP, Lister JR. Surgical results in obese patients with sciatica. Neurosurgery. 1987;20:896–899. doi: 10.1227/00006123-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Hardesty CK, Poe-Kochert C, Son-Hing JP, Thompson GH. Obesity negatively affects spinal surgery in idiopathic scoliosis. Clin Orthop Relat Res. 2013;471:1230–1235. doi: 10.1007/s11999-012-2696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalanithi PA, Arrigo R, Boakye M. Morbid obesity increases cost and complication rates in spinal arthrodesis. Spine (Phila Pa 1976). 2012;37:982–988. doi: 10.1097/BRS.0b013e31823bbeef. [DOI] [PubMed] [Google Scholar]
- 16.Knutsson B, Michaelsson K, Sanden B. Obesity is associated with inferior results after surgery for lumbar spinal stenosis: a study of 2633 patients from the Swedish Spine Register. Spine (Phila Pa 1976). 2013;38:435–441. doi: 10.1097/BRS.0b013e318270b243. [DOI] [PubMed] [Google Scholar]
- 17.Koutsoumbelis S, Hughes AP, Girardi FP, Cammisa FP, Jr, Finerty EA, Nguyen JT, Gausden E, Sama AA. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93:1627–1633. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 18.Leach RE, Baumgard S, Broom J. Obesity: its relationship to osteoarthritis of the knee. Clin Orthop Relat Res. 1973;93:271–273. doi: 10.1097/00003086-197306000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Maragakis LL, Cosgrove SE, Martinez EA, Tucker MG, Cohen DB, Perl TM. Intraoperative fraction of inspired oxygen is a modifiable risk factor for surgical site infection after spinal surgery. Anesthesiology. 2009;110:556–562. doi: 10.1097/ALN.0b013e3181974be7. [DOI] [PubMed] [Google Scholar]
- 20.Mehta AI, Babu R, Karikari IO, Grunch B, Agarwal VJ, Owens TR, Friedman AH, Bagley CA, Gottfried ON. 2012 Young Investigator Award winner: The distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine (Phila Pa 1976). 2012;37:1652–1656. [DOI] [PubMed]
- 21.Meredith DS, Huang RC, Nguyen J, Lyman S. Obesity increases the risk of recurrent herniated nucleus pulposus after lumbar microdiscectomy. Spine J. 2010;10:575–580. doi: 10.1016/j.spinee.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, Fraser VJ. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149–155. [PubMed] [Google Scholar]
- 23.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62–69. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 24.O ’Neill PJ, Karol LA, Shindle MK, Elerson EE, BrintzenhofeSzoc KM, Katz DE, Farmer KW, Sponseller PD. Decreased orthotic effectiveness in overweight patients with adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87:1069–1074. [DOI] [PubMed]
- 25.Park P, Upadhyaya C, Garton HJ, Foley KT. The impact of minimally invasive spine surgery on perioperative complications in overweight or obese patients. Neurosurgery. 2008;62:693–699. doi: 10.1227/01.neu.0000317318.33365.f1. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Bagan B, Vadera S, Maltenfort MG, Deutsch H, Vaccaro AR, Harrop J, Sharan A, Ratliff JK. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine. 2007;6:291–297. doi: 10.3171/spi.2007.6.4.1. [DOI] [PubMed] [Google Scholar]
- 27.Peng CW, Bendo JA, Goldstein JA, Nalbandian MM. Perioperative outcomes of anterior lumbar surgery in obese versus non-obese patients. Spine J. 2009;9:715–720. doi: 10.1016/j.spinee.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34:1422–1428. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 29.Pull ter Gunne AF, van Laarhoven CJ, Cohen DB. Incidence of surgical site infection following adult spinal deformity surgery: an analysis of patient risk. Eur Spine J. 2010;19:982–988. doi: 10.1007/s00586-009-1269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao SB, Vasquez G, Harrop J, Maltenfort M, Stein N, Kaliyadan G, Klibert F, Epstein R, Sharan A, Vaccaro A, Flomenberg P. Risk factors for surgical site infections following spinal fusion procedures: a case-control study. Clin Infect Dis. 2011;53:686–692. doi: 10.1093/cid/cir506. [DOI] [PubMed] [Google Scholar]
- 31.Rihn JA, Kurd M, Hilibrand AS, Lurie J, Zhao W, Albert T, Weinstein J. The influence of obesity on the outcome of treatment of lumbar disc herniation: analysis of the Spine Patient Outcomes Research Trial (SPORT) J Bone Joint Surg Am. 2013;95:1–8. doi: 10.2106/JBJS.K.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rihn JA, Radcliff K, Hilibrand AS, Anderson DT, Zhao W, Lurie J, Vaccaro AR, Freedman MK, Albert TJ, Weinstein JN. Does obesity affect outcomes of treatment for lumbar stenosis and degenerative spondylolisthesis? Analysis of the Spine Patient Outcomes Research Trial (SPORT) Spine. 2012;37:1933–1946. doi: 10.1097/BRS.0b013e31825e21b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23:393–397. doi: 10.1097/BSD.0b013e3181b31729. [DOI] [PubMed] [Google Scholar]
- 34.Rosen DS, Ferguson SD, Ogden AT, Huo D, Fessler RG. Obesity and self-reported outcome after minimally invasive lumbar spinal fusion surgery. Neurosurgery. 2008;63:956–960. doi: 10.1227/01.NEU.0000313626.23194.3F. [DOI] [PubMed] [Google Scholar]
- 35.Senker W, Meznik C, Avian A, Berghold A. Perioperative morbidity and complications in minimal access surgery techniques in obese patients with degenerative lumbar disease. Eur Spine J. 2011;20:1182–1187. doi: 10.1007/s00586-011-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol. 2010;171:135–154. doi: 10.1093/aje/kwp356. [DOI] [PubMed] [Google Scholar]
- 37.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 39.Tomasino A, Parikh K, Steinberger J, Knopman J, Boockvar J, Hartl R. Tubular microsurgery for lumbar discectomies and laminectomies in obese patients: operative results and outcome. Spine (Phila Pa 1976). 2009;34:E664–E672. doi: 10.1097/BRS.0b013e3181b0b63d. [DOI] [PubMed] [Google Scholar]
- 40.Upasani VV, Caltoum C, Petcharaporn M, Bastrom T, Pawelek J, Marks M, Betz RR, Lenke LG, Newton PO. Does obesity affect surgical outcomes in adolescent idiopathic scoliosis? Spine (Phila Pa 1976). 2008;33:295–300. doi: 10.1097/BRS.0b013e3181624573. [DOI] [PubMed] [Google Scholar]
- 41.Wells GA Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/Programs/clinical_epidemiology/oxford.asp. Accessed Octber 10, 2013.
- 42.WHO. Global Health Observatory (GHO). Overweight. Available at: http://www.who.int/gho/ncd/risk_factors/overweight_text/en/index.html. Accessed October 10, 2013.
- 43.Yadla S, Malone J, Campbell PG, Maltenfort MG, Harrop JS, Sharan AD, Vaccaro AR, Ratliff JK. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10:581–587. doi: 10.1016/j.spinee.2010.03.001. [DOI] [PubMed] [Google Scholar]