Abstract
Background
Industrially preformed antibiotic-loaded cement spacers are useful to facilitate the second stage of two-stage exchange arthroplasty for infected THAs and TKAs. However, whether gentamicin alone or a combination of antibiotics (such as vancomycin and gentamicin) is more effective is not known.
Questions/purposes
We therefore sought to compare industrially prefabricated spacers containing either gentamicin or gentamicin and vancomycin with respect to (1) infection control, (2) complications, and (3) quality of life, pain, and patient satisfaction.
Methods
We performed a review of 51 patients with chronic infections treated at one center using either gentamicin or vancomycin and gentamicin-prefabricated spacers. The former were used exclusively from January 2006 until May 2009, and the latter from June 2009 until July 2011, and there was no overlap. We collected data on demographics, immunologic status (McPherson classification), prosthetic joint infection location, type of prosthesis, microbiologic results, and time between stages. We evaluated the primary outcome of infection control or recurrence after at least 12 months followup. We also recorded complications. Each patient completed a quality-of-life survey, VAS, and a self-administered satisfaction scale.
Results
The overall infection control rate was 83% after a mean followup of 35 months (range, 12.4–64.7 months). There were no differences between gentamicin and vancomycin and gentamicin spacers in terms of infection eradication (80 % versus 85 %, respectively; p = 0.73), nor in terms of complications, quality of life, pain, or satisfaction scores.
Conclusions
Prefabricated, antibiotic-loaded cement spacers has been proven effective for infection control in TKAs and THAs but with the numbers available, we did not find any differences between a gentamicin or vancomycin and gentamicin-prefabricated spacer, and therefore, we are unable to validate the superiority of the combination of vancomycin and gentamicin over gentamicin alone. Because of the higher costs involved with vancomycin and gentamicin spacers, and the potential risks of unselective use of vancomycin, further comparative studies are necessary to evaluate their role in the treatment of infected THAs or TKAs.
Level of Evidence
Level III, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Infection is a devastating complication after a TKA or THA, with an incidence of 1% to 2%. [41]. To our knowledge, Wilde and Ruth [40] and Booth and Lotke [7] were the first to use an antibiotic-impregnated spacer block after first-stage débridement and reported infection control rates of 80% and 96%, respectively, with improved function. Subsequently, use of antibiotic-impregnated bone cement spacers during the first stage has been considered the standard of care for patients with a chronic infection at the site of a joint infection [10, 19]. There are numerous types of mobile prosthesis-like spacers available [14, 25, 27, 30, 36, 37], one of which is an industrially preformed antibiotic-loaded cement spacer [25, 27]. These spacers are preformed at the factory and loaded with a fixed type and amount of antibiotic.
The theoretical advantages of using a prefabricated system [27] are: (1) the implant has been proven mechanically safe; (2) pharmacologically, such devices have proven reliably effective (that is, they provide standardized antibiotic release); (3) the improved joint geometry they offer can provide better function and quality of life; and (4) their use can save operative time during the first-stage procedure. In the initial models, the chosen antibiotic was gentamicin [27] owing to its wide spectrum of activity and favorable properties of release from bone cement. With the emergence of gentamicin-resistant bacteria, the addition of two potentially synergistic antibiotics to bone cement has become attractive [5]. Vancomycin and gentamicin often are combined [2] for their potential synergistic effect [33] and improved elution [5] from bone cement. However, whether adding vancomycin to prefabricated antibiotic spacers results in improved infection eradication, less pain, or better function is unknown.
We therefore sought to compare industrially prefabricated mobile cement spacers containing either gentamicin or gentamicin and vancomycin, with respect to (1) infection control, (2) complications, and (3) quality of life, pain, and patient satisfaction.
Patients and Methods
We performed a review of all patients with a chronic THA or TKA infection treated at one center using either gentamicin or vancomycin and gentamicin prefabricated spacers. Our center is a 900-bed tertiary university hospital which houses a national-reference musculoskeletal infection unit. The study was conducted as part of the routine work of our institution. Institutional review board approval was not required because patients were treated according to local standards of care; all patients signed an informed consent.
Gentamicin spacers were used exclusively from January 2006 until May 2009, and vancomycin and gentamicin spacers were used from June 2009 until July 2011. There was no overlap. A total of 51 patients were treated during the study period, 10 of whom were lost to followup (six from the gentamicin group and four from the vancomycin and gentamicin group), leaving 41 patients available for the study. A total of 46 spacers had been used in these 41 patients. The minimum followup was 12 months (range, 12.4–64.7 months) and the patient group included 20 men and 21 women ranging in age from 34 to 84 years old.
Both spacer types were manufactured by the same supplier (Tecres, Verona, Italy). We collected data on demographics, immunologic status (McPherson classification) [22], location of joint infection, type of prosthesis, microbiologic results, time between stages, adverse events, and clinical outcomes. All patients were classified following the Tsukayama system, which classifies joint infections based on time from prosthesis implantation [34]. Patients were divided into two groups according to the type of spacer used: gentamicin spacers or vancomycin and gentamicin spacers. Twenty spacers were implanted in the group of patients with gentamicin spacers (43.47%) and 26 (56.53%) in the group with vancomycin and gentamicin spacers (Table 1).
Table 1.
Demographic information
| Demographic | Gentamicin | Vancomycin and gentamicin | p value |
|---|---|---|---|
| Sample size | 20 spacers/19 patients | 26 spacers/22 patients | |
| Age of patients (years) (95% CI) | 68.21 (34.25–81.49) | 64.46 (35.16–84.19) | 0.388 |
| Sex | 9 male (47%)/10 female (53%) | 11 males (50%)/11 females (50%) | 1.000 |
| BMI (kg/m2) (95% CI) | 29.92 (18–38) | 29.26 (21–39) | 0.574 |
| McPherson Type A | 6 (32%) | 10 (45%) | 0.491 |
| McPherson Type B | 12 (63%) | 10 (45%) | |
| McPherson Type C | 1 (5%) | 2 (10%) | |
| Knee or hip | 11 (57.89%)/8 (42.11%) | 10 (45.45%)/12 (54.55%) | 0.536 |
| Primary or revision surgery | 14 (73.68%)/5 (26.32%) | 13 (59.09%)/9 (40.91%) | 0.510 |
| Time from first to second stage (95% CI) | 97.39 days (34–235 days) | 214.26 days (47–500 days) | 0.010 |
Using the systemic host grade of the McPherson classification, 16 patients were categorized as Type A uncompromised (39%), 22 as Type B compromised (54%), and three as Type C significantly compromised (7%). Twenty-one patients sustained TKA infections (51.22%) and 20 had THA infections (48.78%). In all patients, the onset of infectious signs occurred at least 4 weeks after implantation; that is, a late chronic Type IV infection. In 27 cases (66%), the failed septic implant was a primary arthroplasty prosthesis, and in 14 cases (34%), it was revision prosthesis (Table 1).
The final diagnosis of infection was made when a patient met at least one of the following criteria, as recommended by the Infectious Disease Society of America [24]: (1) presence of chronic sinus; (2) presence of purulent fluid in the joint observed during surgery; (3) at least two positive cultures of the same bacteria from intraoperative tissue samples; and (4) positive intraoperative histologic evaluation.
Spacer Descriptions
The gentamicin knee spacer SpacerK® (Tecres) is preformed at the factory with an ultracongruent condylar knee prosthesis design (Fig. 1) using gentamicin-impregnated acrylic cement and produced in three sizes. The three sizes contain, respectively, 0.8 g, 1.1 g, and 1.8 g active gentamicin. The vancomycin and gentamicin knee spacer Vancogenx® (Tecres) is loaded with a 1:1 concentration of antibiotics, containing a combined total of 0.9 g, 1.3 g, and 1.9 g antibiotics, respectively.
Fig. 1.
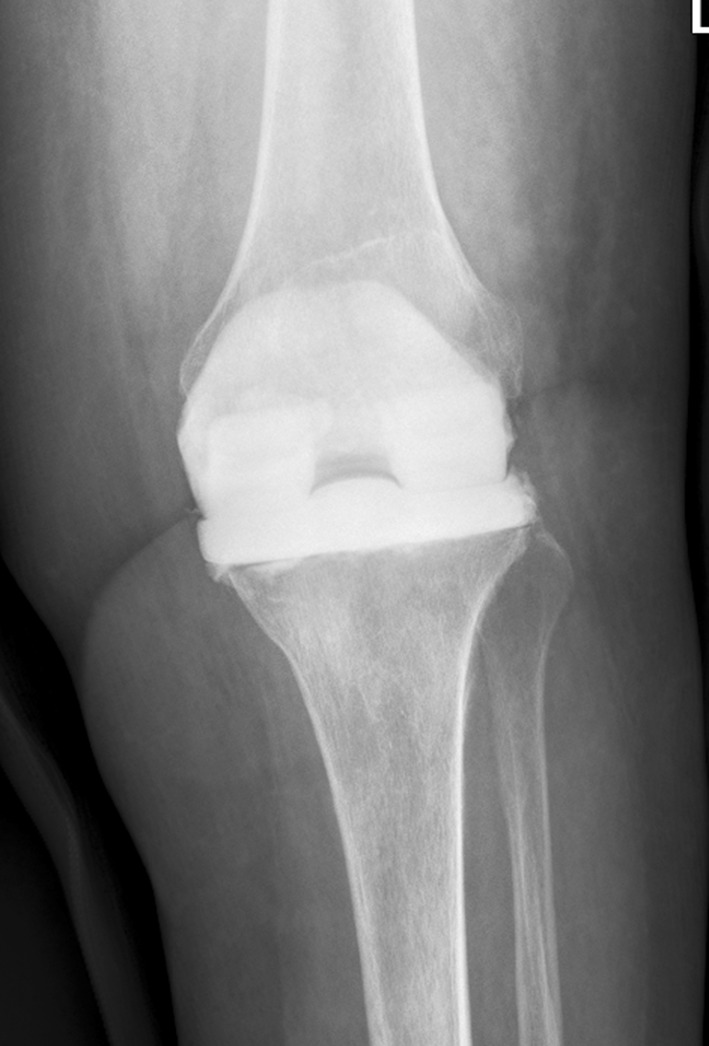
The radiograph shows an industrially premade knee spacer used during the spacer stage of a two-stage revision TKA.
The gentamicin hip spacer (SpacerG®) is preformed at the factory and resembles a femoral prosthesis (Fig. 2) made of gentamicin-impregnated acrylic cement. The inner part of the spacer consists of a stainless steel rod, which provides mechanical stability. These spacers are available in six versions: three head sizes (46, 54, and 60 mm), and in short-stem (153–168 mm) and long-stem (275–290 mm) versions. Depending on head size and stem length, the spacers contain from 1.2 g to 3.2 g active gentamicin.
Fig. 2.
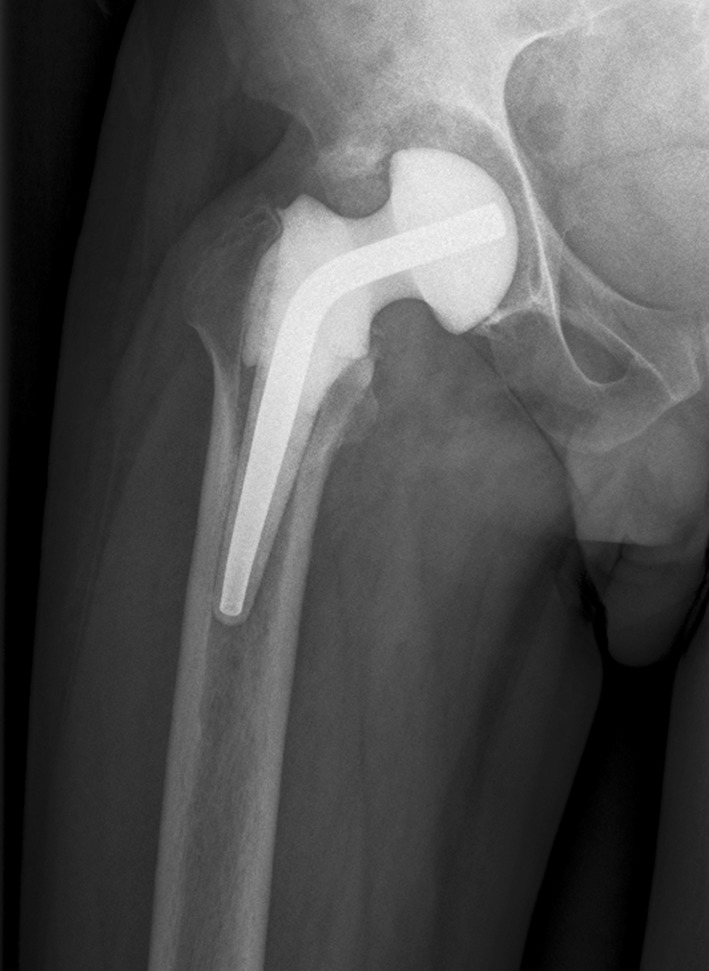
The radiograph shows an industrially premade hip spacer used during the spacer stage of a two-stage revision THA.
The vancomycin and gentamicin hip spacer (Vancogenx®) is loaded with a 1:1 concentration of antibiotics containing a combined total of 1.1 g to 3.2 g antibiotics, respectively.
Operative Technique
The same two-stage protocol was used in all cases. In the first stage we performed thorough débridement. Before administration of antibiotics, at least six specimens were taken for culture. All surgical fields were thoroughly irrigated with a low-pressure system followed by implantation of a prefabricated antibiotic-loaded cement spacer. According to our protocol, the second-stage procedure is done only after a minimum of 12 weeks of oral systemic antibiotic treatment and when C-reactive protein and erythrocyte sedimentation rate levels have returned to normal. Patients were discharged home and outpatient followup was performed in the office. Patients were assessed for presence of complications related to the spacer, including dislocation, breakage, infection recurrence, spacer-related bone loss, and drug-related complications. At the second stage, intraoperative analysis of frozen sections was used routinely for identification of infection at the time of revision arthroplasty. Feldman’s criterion was used, that is, at least five polymorphonuclear leukocytes in at least five high-power fields [4]. At least six tissue samples were collected at the time of the second-stage procedure [12]. All patients followed a similar antibiotic protocol after surgery under the guidance of an infectious diseases expert. In general, the antibiotic treatment was selected according the susceptibility profile of the bacteria. If an oral antibiotic (with high bioavailability) was available, a 12-week-long treatment was selected; if not, a course of intravenous antibiotics for a minimum of 6 weeks was the preferred treatment. With staphylococci infections, a combined treatment including rifampicin is the preferred antibiotic combination; with gram-negative infections fluoroquinolones are the preferred antibiotic.
Intraoperative cultures at the time of the first-stage procedure were available for all study patients. The most common infecting organisms were coagulase-negative staphylococci (Table 2). Operative cultures were negative in five patients; however, each of these patients had definitive evidence of infection [24].
Table 2.
Microorganisms isolated during first-stage surgery
| Single organism | Multiple organisms | Culture negative |
|---|---|---|
| Coagulase-negative Staphylococcus
aureus (9 methicillin-resistant) 14 Staphylococcus aureus (none methicillin-resistant) 4 Propionibacterium acnes 7 Pseudomonas stutzeri 1 Escherichia coli 1 Streptococcus pyogenes 1 Streptococcus agalactiae 1 Streptococcus oralis 1 Morganella morganii 1 Enterococcus faecalis 1 |
Coagulase-negative Staphylococcus aureus (sensitive) and Corynebacterium 1 Staphylococcus hominis and Staphylococcus costellatus 1 Coagulase-negative Staphylococcus (sensitive) and Propionibacterium acnes 1 Escherichia coli and Proteus mirabilis 2 Coagulase-negative Staphylococcus aureus (resistant) + Enterococcus faecium 1 P ropionibacterium acnes + Streptococcus viridans 1 Streptococcus viridans + Staphylococcus capitis 1 |
5 (10.42%)s |
Followup Outpatient Protocol
After the second-stage surgery, all patients were evaluated at least once within the first 6 weeks and then at approximately 3 months, 6 months, 1 year, and yearly thereafter.
We defined treatment failure [9] as the need for subsequent infection-related surgery for persistence or relapse of the infection, the need for prolonged suppressive antibiotic treatment, or the presence of infection symptoms observed at the outpatient followup.
At the final outpatient visit, each patient was asked to fill out three questionnaires. Pain was assessed in all patients with a VAS, which uses a simple numerical score of 0 to 10 [18]. The assessment of health-related quality of life after the procedure was measured used the SF-12 Health Survey version 2 (SF12v2) [31]. Finally, patients responded to a short, self-administered satisfaction scale [20] regarding their personal satisfaction with the surgical procedure. Items are scored on a 4-point Likert scale. The scale score is the unweighted mean of the scores from the individual items, ranging from 25 to 100 per item (with 100 being the most satisfied).
Statistical Analysis
All the recorded data were entered into an Excel® database (Microsoft, Redmond, WA, USA) and SPSS (SPSS 20.0, Student Version for Windows; SPSS Inc, Chicago, IL, USA). Differences between quantitative variables in the groups studied were analyzed with Student’s t-test for the comparison of means, and asymmetric samples were analyzed with the nonparametric Mann-Whitney U test. Comparison of medians was done with the nonparametric Gibbon test, and differences between qualitative variables were analyzed by the chi square test. A p value of 0.05 or less was considered statistically significant. A power analysis was performed with an alpha of 0.05 and the difference detected in our study.
Results
At final followup, there was no difference in the frequency of infection relapse between the two groups. In the gentamicin and vancomycin and gentamicin groups, at the end of followup after the two-stage replacement revision four of 20 (20%) and four of 26 (15.38%) patients experienced relapse, respectively (p = 0.73). We were unable to find any factors that were associated with an increased risk of infection recurrence (Table 1). Overall, relapse occurred in eight of the 46 patients with septic failed arthroplasties who had two-stage revisions using prefabricated articulating spacers, giving an overall infection control rate of 83%. All but three patients (all in the vancomycin and gentamicin spacers group) had reimplantation of prostheses. Two of these three patients who did not have a new prosthesis reimplanted had recurrence of infection with a discharging wound during the period without drugs. Both of these patients underwent another débridement with implantation of a new articulating spacer. At the time of the study, both were still awaiting the second-stage procedure. The other patient who did not have reimplantation of a new prosthesis was not considered suitable for reimplantation owing to her impaired medical status.
In three patients, two in the gentamicin group and one in the vancomycin and gentamicin group, it was necessary to repeat the first-stage surgery because of recurrence of infection before the infection could be considered controlled and the second-stage surgery could be scheduled (Table 3).
Table 3.
Operative variables
| Patient number | Sex | Procedure | Type of surgery | McPherson host type | Isolated bacteria | Spacer type | Reimplantation surgery | Complications | Interval between the two stages (months) | Followup (months) | Infection control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | TKA | Primary | A | Pseudomonas stutzeri | VG | Cemented hinge revision knee | Hematoma | 6.03 | 18.50 | Yes |
| 2 | F | TKA | Revision | A | Propionibacterium acnes + Streptococcus viridans | VG | Cemented hinge revision knee | None | 8.30 | 24.77 | Yes |
| 3 | F | TKA | Primary | A | Staphylococcus capitis + Streptococcus viridans | VG | Cemented hinge revision knee | None | 3.40 | 20.20 | Yes |
| 4 | M | TKA | Primary | B | Propionibacterium acnes | G | Uncemented revision knee | None | 4.93 | 46.80 | Yes |
| 5 | M | TKA | Primary | C | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | Cemented hinge revision knee | None | 9.20 | 23.20 | Yes |
| 6 | F | TKA | Primary | C | Escherichia coli | G | External fixation knee fusion | Skin necrosis | 0.93 | 21.73 | Yes |
| 7 | M | TKA | Primary | A | Propionibacterium acnes | G | Cemented hinge revision knee | None | 2.83 | 22.57 | Yes |
| 8 | M | TKA | Primary | B | Methicillin-sensitive coagulase-negative Staphylococcus aureus | G | Uncemented hinge revision knee | Hematoma | 7.57 | 64.67 | Yes |
| 9 | M | TKA | Primary | B | Negative cultures | G | Uncemented revision knee | None | 1.30 | 58.80 | Yes |
| 10 | F | TKA | Primary | B | Negative cultures | G | Uncemented hinge revision knee | None | 5.00 | 59.77 | Yes |
| 11 | F | TKA | Primary | A | Methicillin-sensitive Staphylococcus aureus | G | Uncemented hinge revision knee | Spacer dislocation | 0.70 | 61.73 | No |
| 12 | F | TKA | Revision | A | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | External fixation knee fusion | None | 1.57 | 43.13 | Yes |
| 13 | F | TKA | Primary | A | Methicillin-sensitive coagulase-negative Staphylococcus aureus | G | Uncemented revision knee | None | 4.93 | 47.03 | Yes |
| 14 | M | TKA | Primary | A | Methicillin-sensitive coagulase-negative Staphylococcus aureus + Corynebacterium | VG | Cemented hinge revision knee | None | 3.83 | 39.00 | Yes |
| 15 | F | TKA | Primary | A | Propionibacterium acnes | VG | Uncemented revision knee | None | 4.30 | 31.20 | Yes |
| 16 | F | TKA | Primary | A | Propionibacterium acnes + methicillin-sensitive coagulase-negative Staphylococcus aureus | VG | Uncemented revision knee | None | 7.43 | 33.53 | Yes |
| 17 | F | TKA | Primary | A | Propionibacterium acnes | G | Uncemented hinge revision knee | None | 2.07 | 35.63 | Yes |
| 18 | F | TKA | Revision | B | Staphylococcus hominis + Staphylococcus constellatus | VG | Uncemented hinge revision knee | None | 17.33 | 25.17 | Yes |
| 19 | M | TKA | Primary | B | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | Uncemented hinge revision knee | Skin necrosis | 18.90 | 23.47 | Yes |
| 20 | M | TKA | Primary | A | Methicillin-resistant coagulase-negative Staphylococcus aureus | G | Uncemented hinge revision knee | None | 38.97 | 14.17 | Yes |
| 21 | F | TKA | Primary | B | Methicillin-resistant coagulase-negative Staphylococcus aureus | G | Cemented hinge revision knee | None | 7.67 | 40.53 | Yes |
| 22 | F | TKA | Revision | B | Methicillin-sensitive coagulase-negative Staphylococcus aureus + Pseudomonas | VG | Cemented hinge revision knee | None | 0.80 | 25.33 | Yes |
| 23 | M | THA | Revision | B | Propionibacterium acnes | VG | Uncemented revision hip | None | 4.43 | 25.33 | Yes |
| 24 | M | THA | Primary | A | Streptococcus pyogenes | VG | Uncemented revision hip | None | 7.93 | 31.63 | No |
| 25 | M | THA | Primary | A | Methicillin-sensitive coagulase-negative Staphylococcus aureus | VG | Uncemented revision hip | None | 5.20 | 25.60 | Yes |
| 26 | F | THA | Revision | B | Negative cultures | G | Uncemented revision hip | None | 0.70 | 41.23 | Yes |
| 27 | M | THA | Revision | B | Streptococcus agalactie | VG | Uncemented revision hip | None | 3.57 | 20.70 | Yes |
| 28 | F | THA | Revision | C | Propionibacterium acnes | VG | Total femur arthroplasty | Spacer dislocation | 16.67 | 28.40 | Yes |
| 29 | M | THA | Primary | B | Methicillin-sensitive Staphylococcus aureus | VG | Uncemented revision hip | None | 3.73 | 30.03 | Yes |
| 30 | M | THA | Primary | A | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | Uncemented revision hip | Peroneal palsy | 16.00 | 32.80 | Yes |
| 31 | M | THA | Primary | B | Methicillin-resistant coagulase-negative Staphylococcus aureus | G | Uncemented revision hip | None | 6.07 | 48.00 | Yes |
| 32 | F | THA | Revision | A | Negative cultures | G | Uncemented revision hip | None | 0.77 | 50.30 | Yes |
| 33 | M | THA | Arthritis | B | Propionibacterium acnes | G | Uncemented revision hip | Spacer dislocation | 1.90 | 28.60 | Yes |
| 34 | F | THA | Revision | B | Methicillin-sensitive coagulase-negative Staphylococcus aureus | G | Uncemented revision hip | None | 7.73 | 42.83 | No |
| 35 | M | THA | Revision | B | Methicillin-sensitive Staphylococcus aureus | G | Uncemented revision hip | None | 6.63 | 47.77 | Yes |
| 36 | M | THA | Revision | B | Streptococcus oralis | VG | Uncemented revision hip | None | 10.97 | 32.23 | No |
| 37 | M | THA | Revision | B | Methicillin-sensitive Staphylococcus aureus | VG | None | Repeated first stage | 6.90 | 21.37 | No |
| 38 | F | THA | Revision | B | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | Total femur arthroplasty | None | 4.20 | 28.73 | Yes |
| 39 | F | THA | Moore | B | Escherichia coli + Proteus mirabilis | G | None | Repeated first stage | 1.87 | 45.87 | No |
| 40 | F | THA | Moore | B | Escherichia coli + Proteus mirabilis | G | Uncemented revision hip | None | 11.13 | 57.00 | Yes |
| 41 | M | THA | Primary | A | Negative cultures | VG | Uncemented revision hip | None | 5.77 | 30.60 | Yes |
| 42 | F | THA | Revision | B | Methicillin-resistant coagulase-negative Staphylococcus aureus | VG | Uncemented revision hip | None | 5.50 | 16.47 | Yes |
| 43 | F | THA | Moore | B | Methicillin-sensitive Staphylococcus aureus | VG | None | Not second stage | 0.00 | 25.17 | Yes |
| 44 | M | THA | Primary | B | Morganella morganii | G | None | Repeated first stage | 25.80 | 23.47 | No |
| 45 | M | THA | Primary | B | Methicillin-resistant coagulase-negative Staphylococcus + Enterococcus faecium | VG | Uncemented revision hip | None | 11.07 | 12.40 | Yes |
| 46 | F | THA | Primary | A | Enterococcus faecalis | VG | None | Repeated first stage | 10.47 | 14.17 | No |
G = gentamicin spacer; VG = vancomycin and gentamicin-spacer.
There were few complications associated with the spacers, and there were no differences between the groups in terms of complications. In the gentamicin group we observed two spacer dislocations; one involved a knee spacer and the other a hip spacer. In the vancomycin and gentamicin group, only one hip spacer dislocation was recorded. In the gentamicin group, one case of skin necrosis was observed in a patient with a knee spacer. No skin necrosis was observed in patients in the vancomycin and gentamicin group. No spacer breakage or reaction to the cement-on-cement articulation was recorded in either group, and no patients experienced drug-related complications.
There were no differences in quality of life, pain, or patient satisfaction between the two groups (Table 4).
Table 4.
Patient satisfaction, pain, and quality of life
| Questionnaires | Gentamicin spacers | Vancomycin-gentamicin spacers | p value |
|---|---|---|---|
| SAPS (95% CI) | 81.67 (25–100) | 70.63 (25–100) | 0.113 |
| SF-12-PSC (95% CI) | 29.75 (17.41–39.71) | 31.75 (19.67–48.21) | 0.722 |
| SF-12-MSC (95% CI) | 39.82 (15.35–18.46) | 52.59 (28.85–68.95) | 0.131 |
| VAS (95% CI) | 2.41 (0.00–8.00) | 2.81 (0.00–8.00) | 0.821 |
SAPS = Self-Administered Patient Satisfaction Scale; SF-12-PSC = SF-12 Physical Summary Component; SF-12-MSC = SF-12 Mental Summary Component.
Discussion
Infection is a devastating complication after TKA or THA. The two-stage exchange approach using an antibiotic-loaded cement spacer has become the preferred treatment for any chronically infected TKA or THA [10, 16, 19, 25, 27, 28, 36, 41]. The rationale for the choice of antibiotics to be included in such local delivery systems must follow several principles [10, 17], but the antimicrobial activity of the antibiotic at the infection site is of paramount importance, since drug selection depends on the microorganism(s) to be targeted. Because aminoglycosides meet all the requirements, they were considered the preferred antibiotics for this treatment approach [6, 10, 25, 27, 28, 41]. Staphylococcus species are the principal bacterial family related to TKA or THA infections [13, 23, 29], therefore a possible increase in aminoglycoside resistance in staphylococci causing an infection is a concern, and potentially might impact the utility of classic aminoglycoside-impregnated cement spacers [3, 5, 13, 29, 35]. This suggests that the use of other antibiotics or combinations of antibiotics in bone cement could be more effective for elimination of infection. The potential effectiveness of a combination of vancomycin and gentamicin in cement spacers has been suggested [5, 32, 33]. The vancomycin and gentamicin combination theoretically has a threefold advantage: (1) the potential synergy between vancomycin and gentamicin against gram-positive bacteria [17, 33, 39]; (2) the possibility of improved antibiotic elution from the spacer resulting from such a combination [5, 21, 26]; and (3) the possibility that such an antibiotic combination results in a decreased risk of bacterial growth on the surface of the cement spacer, that is, cement spacer colonization, which could be detrimental to curing the infection [1, 6]. However, to our knowledge, no comparative study has been published addressing this question. We therefore wanted to compare the efficacy of industrially prefabricated spacers containing either gentamicin or gentamicin and vancomycin, with respect to (1) infection control, (2) complications, and (3) quality of life, pain, and satisfaction. To our knowledge, there is no previously reported comparative study examining clinical outcomes using gentamicin and vancomycin and gentamicin industrially prefabricated cement spacers.
There were some limitations to our study. First, the study is a retrospective analysis with the inherent limitations of a retrospective design, specifically the inability to obtain all data that may be helpful. Second, our followup was limited to a minimum of 12 months because we have used these vancomycin and gentamicin spacers only in recent years. Future studies should include longitudinal followup of these patients. Third, there were numerous potential confounding factors, such as the use of varying antibiotic regimes and doses (even among the spacers used, owing to their different sizes), patient comorbidities, and the differences in interval between first and second surgeries among the groups. Fourth, the spacers were used unselectively, regardless of the susceptibility profile of the infecting bacteria. Finally, statistically significant results were not obtained and could be attributable to insufficient sample size and statistical power (Type II error; with the differences observed in our study only a power of 6% was achieved requiring a sample size of 1080 per group to detect statistically significant differences which is a large sample size that is not realistic for this field). The selected definition of infection could be considered a limitation as well, although we have used a standardized and accepted definition according the Infectious Diseases Society of America [24].
Regarding the infection control rate, although the combination of gentamicin and vancomycin in the cement spacers makes some intuitive sense, we found no clinical or statistical difference in terms of infection control between use of prefabricated cement spacers impregnated with gentamicin and those with vancomycin and gentamicin. With the data available we are not able to validate the superiority of the combination of vancomycin and gentamicin over gentamicin alone. From a clinical point of view, no obvious difference in infection eradication rates has been observed where antibiotic cement spacers with different antibacterial loads and compositions have been used [15]. Although a high rate of gentamicin resistance in staphylococci causing chronic joint infections could be suspected, one may argue that aminoglycosides alone are still effective because of the high local concentration achieved with the local antibiotic treatment.
The overall control rate (83%) is comparable to rates reported in other studies [11, 25, 27, 38]. An ongoing criticism of the industrially premade spacer concerns the limited selection of antibiotics offered and the use of dosages less than those recommended for treatment of infections [16, 38]. The data from our study support the usefulness of prefabricated, antibiotic-loaded cement spacers for effective infection control of TKAs or THAs. Although the antibiotic dosages in such devices are inferior to those of handmade spacers, antibiotic elution may be superior [27, 32].
There were no differences between the gentamicin-only and vancomycin and gentamicin spacers in terms of complications in our patients. These prefabricated spacers have proven to be mechanically safe [11, 25, 27], with a low number of complications.
Infection after a TKA or THA reduces patient satisfaction and impairs functional health status and health-related quality of life [8]. Our patients expressed a high degree of satisfaction with the results of their treatment in septic revision cases. The overall satisfaction rate was 76%. We found differences between the two types of spacers. Similarly, in terms of pain and of quality of life as measured with the SF-12 v2, we found no difference between the two spacer types. To our knowledge, information regarding quality of life and patient satisfaction after the use of industrially prefabricated spacers has not been published.
We found no differences in terms of rate of infection control, complications, health-related quality of life, pain, or patient satisfaction between groups treated with either a gentamicin-only spacer or a vancomycin and gentamicin-impregnated spacer. With our data we are not able to validate the superiority of the combination of vancomycin and gentamicin over gentamicin alone, and because of the higher costs involved with vancomycin and gentamicin spacers and the potential risks of unselective use of vancomycin, further comparative studies are necessary to evaluate their role in the treatment of infected THAs or TKAs.
Acknowledgments
We thank Russ Williams of RoundlyWorded.com for editorial recommendations. We also want to acknowledge the invaluable help of Carles Pigrau MD, PhD and Dolors Rodriguez MD, PhD, staff members of the Department of Infectious Diseases in our hospital. We also thank Santiago Pérez-Hoyos BSc, PhD for statistical and methodologic assistance, and we greatly appreciate the cooperation of Carles Amat MD, a member of our unit.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anagnostakos K, Hitzler P, Pape D, Kohn D, Kelm J. Persistence of bacterial growth on antibiotic-loaded beads: is it actually a problem? Acta Orthop. 2008;79:302–307. doi: 10.1080/17453670710015120. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80:193–197. doi: 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anguita-Alonso P, Hanssen AD, Osmon DR, Trampuz A, Steckelberg JM, Patel R. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin Orthop Relat Res. 2005;439:43–47. doi: 10.1097/01.blo.0000182394.39601.9d. [DOI] [PubMed] [Google Scholar]
- 4.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 5.Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–334. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 6.Bertazzoni Minelli E, Della Bora T, Benini A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe. 2011;17:380–383. doi: 10.1016/j.anaerobe.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Booth RE, Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60. [PubMed] [Google Scholar]
- 8.Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong). 2008;16:58–65. doi: 10.1177/230949900801600115. [DOI] [PubMed] [Google Scholar]
- 9.Corona Perez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, Pigrau Serrallach C, Guerra Farfan E, Amat Mateu C, Flores Sanchez X. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother. 2012;67:1749–1754. doi: 10.1093/jac/dks119. [DOI] [PubMed] [Google Scholar]
- 10.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 11.Degen RM, Davey JR, Davey JR, Howard JL, McCalden RW, Naudie DD. Does a prefabricated gentamicin-impregnated, load-bearing spacer control periprosthetic hip infection? Clin Orthop Relat Res. 2012;470:2724–2729. doi: 10.1007/s11999-012-2350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores X, Corona PS, Cortina J, Guerra E, Amat C. Temporary cement tectoplasty: a technique to improve prefabricated hip spacer stability in two-stage surgery for infected hip arthroplasty. Arch Orthop Trauma Surg. 2012;132:719–724. doi: 10.1007/s00402-012-1461-3. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson E, Della Valle CJ, Wise B, Walsh M, Preston C, Di Cesare PE. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006;88:1231–1237. doi: 10.2106/JBJS.E.00004. [DOI] [PubMed] [Google Scholar]
- 14.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements: prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 15.Iarikov D, Demian H, Rubin D, Alexander J, Nambiar S. Choice and doses of antibacterial agents for cement spacers in treatment of prosthetic joint infections: review of published studies. Clin Infect Dis. 2012;55:1474–1480. doi: 10.1093/cid/cis735. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356–368. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg. 2003;11:38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Klinger HM, Spahn G, Schultz W, Baums MH. Arthrodesis of the knee after failed infected total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2006;14:447–453. doi: 10.1007/s00167-005-0664-3. [DOI] [PubMed] [Google Scholar]
- 19.Mahmud T, Lyons MC, Naudie DD, Macdonald SJ, McCalden RW. Assessing the gold standard: a review of 253 two-stage revisions for infected TKA. Clin Orthop Relat Res. 2012;470:2730–2736. doi: 10.1007/s11999-012-2358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahomed N, Gandhi R, Daltroy L, Katz JN. The self-administered patient satisfaction scale for primary hip and knee arthroplasty. Arthritis. 2011;2011:591253. doi: 10.1155/2011/591253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13:331–338. doi: 10.1016/S0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 22.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. doi: 10.1097/00003086-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34:505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 25.Pattyn C, De Geest T, Ackerman P, Audenaert E. Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications. Int Orthop. 2011;35:1471–1476. doi: 10.1007/s00264-010-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–944. doi: 10.1016/S0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 27.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano CL, Romano D, Logoluso N, Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Hip Int. 2010;20:26–33. doi: 10.1177/112070001002000104. [DOI] [PubMed] [Google Scholar]
- 29.Sharma D, Douglas J, Coulter C, Weinrauch P, Crawford R. Microbiology of infected arthroplasty: implications for empiric peri-operative antibiotics. J Orthop Surg (Hong Kong). 2008;16:339–342. doi: 10.1177/230949900801600314. [DOI] [PubMed] [Google Scholar]
- 30.Sherman SL, Cunneen KP, Walcott-Sapp S, Brause B, Westrich GH. Custom total femur spacer and second-stage total femur arthroplasty as a novel approach to infection and periprosthetic fracture. J Arthroplasty. 2008;23:781–786. doi: 10.1016/j.arth.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Singh J, Sloan JA, Johanson NA. Challenges with health-related quality of life assessment in arthroplasty patients: problems and solutions. J Am Acad Orthop Surg. 2010;18:72–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Soffiatti R. The preformed spacers: from the idea to the realization of an industrial device. In: Calonego G, Meani E, Romano C, Crosby L, Hofmann G, editors. Infection and Local Treatment in Orthopedic Surgery. Heidelberg, Germany: Springer; 2007. pp. 112–120. [Google Scholar]
- 33.Streuli JC, Exner GU, Reize CL, Merkofer C, Scott CP, Zbinden R. In vitro inhibition of coagulase-negative staphylococci by vancomycin/aminoglycoside-loaded cement spacers. Infection. 2006;34:81–86. doi: 10.1007/s15010-006-5039-2. [DOI] [PubMed] [Google Scholar]
- 34.Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85(suppl 1):S75–S80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 35.Tunney MM, Ramage G, Patrick S, Nixon JR, Murphy PG, Gorman SP. Antimicrobial susceptibility of bacteria isolated from orthopedic implants following revision hip surgery. Antimicrob Agents Chemother. 1998;42:3002–3005. doi: 10.1128/aac.42.11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:994–1001. doi: 10.1007/s11999-010-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villanueva-Martinez M, Rios-Luna A, Pereiro J, Fahandez-Saddi H, Villamor A. Hand-made articulating spacers in two-stage revision for infected total knee arthroplasty: good outcome in 30 patients. Acta Orthop. 2008;79:674–682. doi: 10.1080/17453670810016704. [DOI] [PubMed] [Google Scholar]
- 38.Wan Z, Karim A, Momaya A, Incavo SJ, Mathis KB. Preformed articulating knee spacers in 2-stage total knee revision arthroplasty: minimum 2-year follow-up. J Arthroplasty. 2012;27:1469–1473. doi: 10.1016/j.arth.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Watanakunakorn C, Tisone JC. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1982;22:903–905. doi: 10.1128/AAC.22.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilde AH, Ruth JT. Two-stage reimplantation in infected total knee arthroplasty. Clin Orthop Relat Res. 1988;236:23–35. [PubMed] [Google Scholar]
- 41.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]


