Abstract
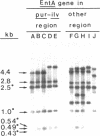
The genetic determinant of Staphylococcus aureus enterotoxin A (SEA) has been cloned in pBR322 in Escherichia coli and found to be expressed and secreted into the periplasmic space in that organism. The SEA gene (entA) is within a 2.5-kilobase-pair HindIII fragment that is part of a discrete genetic element 8-12 kilobase pairs in length. This entA element has a standard chromosomal location [between the purine (pur) and isoleucine-valine (ilv) markers] in most S. aureus strains. In some strains it is unlinked to pur-ilv. However, its internal structure is conserved at different locations. Some naturally occurring SEA-nonproducer (EntA-) strains lack the entire entA element, and one instance of its spontaneous loss is reported. Other naturally occurring strains have EntA- structural variants of the element at the same pur-ilv location at which the intact element is most commonly found. Some of these strains are EntA-, others are EntA+; the latter have a second, unlinked copy of the element containing their functional entA gene. These results suggest that entA is associated with a structurally unstable, possibly mobile, discrete genetic element.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKSDALE L., GARMISE L., RIVERA R. Toxinogeny in Corynebacterium diphtheriae. J Bacteriol. 1961 Apr;81:527–540. doi: 10.1128/jb.81.4.527-540.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casman E. P., Bennett R. W., Dorsey A. E., Issa J. A. Identification of a fourth staphylococcal enterotoxin, enterotoxin D. J Bacteriol. 1967 Dec;94(6):1875–1882. doi: 10.1128/jb.94.6.1875-1882.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELEK S. D., LEVY E. Distribution of haemolysins in pathogenic and non-pathogenic staphylococci. J Pathol Bacteriol. 1950 Oct;62(4):541–554. doi: 10.1002/path.1700620405. [DOI] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- Kasatiya S. S., Baldwin J. N. Nature of the determinant of tetracycline resistance in Staphylococcus aureus. Can J Microbiol. 1967 Aug;13(8):1079–1086. doi: 10.1139/m67-144. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl S., Guss B., Uhlén M., Philipson L., Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci U S A. 1983 Feb;80(3):697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallonee D. H., Glatz B. A., Pattee P. A. Chromosomal mapping of a gene affecting enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1982 Feb;43(2):397–402. doi: 10.1128/aem.43.2.397-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Batten P. L., Murray K. Restriction of bacteriophage lambda by Escherichia coli K. J Mol Biol. 1973 Dec 15;81(3):395–407. doi: 10.1016/0022-2836(73)90149-6. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Pattee P. A., Glatz B. A. Identification of a chromosomal determinant of enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1980 Jan;39(1):186–193. doi: 10.1128/aem.39.1.186-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R., Gould S., Bergdoll M. Detecting the enterotoxigenicity of Staphylococcus aureus strains. Appl Microbiol. 1974 Dec;28(6):946–950. doi: 10.1128/am.28.6.946-950.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Wüthrich K., Yamane T., Patel D. J., Blumberg W. E. Nuclear magnetic resonance determination of ligand-induced conformational changes in myoglobin. J Mol Biol. 1970 Oct 14;53(1):143–157. doi: 10.1016/0022-2836(70)90050-1. [DOI] [PubMed] [Google Scholar]
- So M., Heffron F., McCarthy B. J. The E. coli gene encoding heat stable toxin is a bacterial transposon flanked by inverted repeats of IS1. Nature. 1979 Feb 8;277(5696):453–456. doi: 10.1038/277453a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Pattee P. A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983 Apr;154(1):406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Pattee P. A. Genetic transformation in Staphylococcus aureus: demonstration of a competence-conferring factor of bacteriophage origin in bacteriophage 80 alpha lysates. J Bacteriol. 1981 Oct;148(1):294–300. doi: 10.1128/jb.148.1.294-300.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Weiss K. F., Robbins R. N. Relationship between staphylococcal antiserum titer and zone development on immune serum plates. Appl Microbiol. 1970 Jun;19(6):911–914. doi: 10.1128/am.19.6.911-914.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZABRISKIE J. B. THE ROLE OF TEMPERATE BACTERIOPHAGE IN THE PRODUCTION OF ERYTHROGENIC TOXIN BY GROUP A STREPTOCOCCI. J Exp Med. 1964 May 1;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]